Abstract
Pulmonary Embolism Response Team (PERT) is a multidisciplinary team established to stratify risk and choose optimal treatment in patients with acute pulmonary embolism (PE). Established for the first time at Massachusetts General Hospital in 2013, PERT is based on a concept combining a Rapid Response Team and a Heart Team. The growing role of PERTs in making individual therapeutic decisions is identified, especially in hemodynamically unstable patients with contraindications to thrombolysis or with co-morbidities, as well as in patients with intermediate-high risk in whom a therapeutic decision may be difficult. The purpose of this document is to define the standards of PERT under Polish conditions, based on the experience of teams already operating in Poland, which formed an agreement called the Polish PERT Initiative. The goals of Polish PERT Initiative are: improving the treatment of patients with PE at local, regional and national levels, gathering, assessing and sharing data on the effectiveness of PE treatment (including various types of catheter-directed therapy), education on optimal treatment of PE, creating expert documents and supporting scientific research, as well as cooperation with other communities and scientific societies.
Keywords: pulmonary embolism, pulmonary embolism response team, catheter-directed therapy, embolectomy
Introduction. PERT definition. Polish PERT Initiative
Acute pulmonary embolism (PE) is one of the most common diseases of the cardiovascular system. It is estimated that PE occurs at a frequency of 39–115/100,000 population/year and causes over 400,000 deaths in Europe every year [1–3]. It is the third most frequent vascular disease, after myocardial infarction and stroke [1, 2]. The clinical presentation of PE is heterogenous: from mild impairment of exercise tolerance (low-risk PE), through severe dyspnoe with symptoms of right ventricular overload (intermediate-risk PE) to hemodynamic collapse, “obstructive” shock and death (high-risk patients) most often related to acute insufficiency of the right ventricle (RV) and respiratory failure [1–3]. Although most patients with PE can be successfully treated with anticoagulants, hemodynamically unstable patients require urgent systemic thrombolysis (ST). Thrombolytic therapy, however, has significant limitations. First of all, it is associated with a significant increase in the risk of major bleeding (13%), including up to 3% of patients treated with ST, who have dangerous intracranial bleeding, mainly cerebral hemorrhagic stroke [4]. In the group of patients of the ZATPOL — Polish national prospective registry, it has been shown that the occurrence of major bleeding in acute PE significantly worsened the prognosis [5]. In high-risk (hemodynamically unstable) patients, the clinical benefit of thrombolysis exceeds the risk of bleeding, but in intermediate-risk patients the potential clinical benefit of ST does not balance the risk of major bleeding and does not reduce mortality [6]. On the other hand, about 5–10% of patients who are initially hemodynamically stable may experience a sudden and unexpected clinical deterioration [7]. According to the guidelines of the European Society of Cardiology, in the case of contraindications to thrombolysis or its failure, surgical embolectomy is recommended [3]. However, a severe preoperative condition and high incidence of comorbidities, as well as limited availability of cardiac surgery result in high mortality in this group. Furthermore, it has been shown that thrombolytic therapy is often not used in high-risk patients, even though there are no contraindications [8]. Moreover, in intermediate-high-risk patients, the decision on thrombolysis or surgical therapy is often taken too late, and hemodynamic deterioration in this group of patients is associated with high mortality [9]. Consequently, new techniques for transcatheter invasive treatment of PE (catheter-directed therapy [CDT]) have been developed, which can remove thrombi from the pulmonary arteries without the additional risks posed by systemic thrombolysis or cardiac surgery. In the last decade, many new devices and techniques have been proposed for transcatheter treatment of PE [10]. There is also a growing number of scientific data from clinical observational studies and registries confirming the clinical effectiveness of interventional treatment with a reduction in the number of patients with significant bleeding. However, only few randomized trials comparing CDT with standard anticoagulant and thrombolytic therapy are available in the literature [11, 12]. Nonetheless, quick and correct diagnosis, proper risk stratification and selection of optimal therapy from a constantly growing pharmacological and intervention armamentarium, seems to be crucial in patients with acute PE.
In 2013, in Massachusetts General Hospital in Boston (United States), the world’s first multispecialist team was created for quick consultation and selection of the therapy in patients with PE [13]. This team was named the Pulmonary Embolism Response Team (PERT). The PERT concept was based on a combination of two other proven clinical practice models: The Heart Team and the Rapid Response Team [14, 15]. The growing role of PERTs in making individual therapeutic decisions is pointed out, especially in hemodynamically unstable patients with contraindications to thrombolysis (e.g. with active bleeding shortly after surgery) or with significant co-morbidities (including cancers), as well as in patients with intermediate-high risk patients in whom this treatment may accelerate clinical improvement and improve prognosis [13–15]. Treatment methods should also include various methods of CDT as an alternative to ST and surgical embolectomy. The current guidelines of the European Society of Cardiology recommended set-up (class recommendations IIa/level of evidence C) of in-hospital PERTs adapted to local resources and access to specialists [3].
The purpose of this document is to define the standards of PERT under Polish conditions, based on the experience of teams already operating in Poland, which was formed in April 2019 an agreement called the Polish PERT Initiative (PPI). The goals of PPI are improving the treatment of patients with PE at local, regional and national levels, involving gathering, assessing and sharing of data on the effectiveness of PE treatment (including various types of CDT), disseminating the knowledge about optimal treatment of PE, optimizing financing of procedures in this area, creating expert documents and supporting scientific research, as well as cooperating with other communities and scientific societies.
PERT models, interdisciplinary cooperation, minimal organizational and institutional framework
A clear organization model of PERT has yet to be defined. The world’s first PERT from Massachusetts General Hospital involved specialists from various fields, serving for immediate consultation and selection of therapy in patients with PE [16]. The first PERT served as a model for other medical centers and soon these centers combined their experience within the National PERT Consortium [17]. The data presented by the PERT Consortium shows a vast diversity in the organization of teams and the patient population they are dealing with [18, 19]. The essence of PERT’s activity is to coordinate the diagnostic and therapeutic process of patients with PE of severe or atypical course by choosing the optimal management strategy based on the expert knowledge of a multidisciplinary team of specialists of whom it is comprised.
The PERT coordinator should be continuously available by phone (24/7) on a dedicated phone number and after accepting the application must be able to organize quick consultations (< 30 min) with relevant specialists. These consultations may be a teleconference, during which all participants have access to a patient’s medical data and imaging tests. The PERT should include specialists with practical experience in the treatment of acute PE using various methods, as well as experts to assist in case of complications or the presence of comorbidities that require modification of standard methods of acute PE. Among the physicians directly involved in the process of treating a patient upon PERT care there are usually specialists who have experience in the field of intensive cardiac therapy, echocardiography and interventional cardiology, as well as cardiac surgeons, specialists in emergency medicine, anesthesiologists and radiologists, including interventional radiologists. The second group of specialists whose consultations may be necessary in selected cases should include neurologists, neurosurgeons, oncologists, vascular surgeons, hematologists and specialists in lung disease. It seems that the specialists listed in the first group should constitute a permanent PERT team. However, the final composition of PERT varies between centers and depends on resources and experience. The most important tasks of PERT in the acute phase of PE include choosing optimal pharmacotherapy (including determining indications or contraindications for thrombolysis), interventional treatment (thrombus fragmentation, CDT, venous filter implantation) or cardiac surgery (pulmonary embolectomy). For those reasons, the ideal organizational solution is to create PERT in hospitals with all available treatment options in one location. If PERT is located in a hospital without a cardiac surgery department, it should have the formal cooperation with a cardiac surgery center ensuring the possibility of immediate transfer of patients for further treatment. Optimally, every PERT should have access to treatment with extra corporeal membrane oxygenation (ECMO) device, which is the equipment of cardiac surgery departments in Poland. In addition to the acute phase of PE treatment, the role of PERT may be to support the optimization of management in subsequent months, including determining the method and duration of chronic anticoagulation, possible implantation of the inferior vena cava filter and patient monitoring for the occurrence of chronic thromboembolic pulmonary hypertension (CTEPH).
Risk stratification in PE, qualification to treatment
Hemodynamically unstable patients, i.e. with systemic hypotension, in shock, requiring infusion of catecholamines or cardiopulmonary resuscitation constitute a group of patients with high risk of early death with early mortality exceeding 15% and contain about 5% of patients with PE. Hypotonia is defined as systolic blood pressure < 90 mmHg or a decrease in systolic blood pressure of at least 40 mmHg for > 15 min if it is not associated with new arrhythmias, hypovolemia or sepsis. Confirmation of the diagnosis of high-risk PE is not only the finding of RV dysfunction in echocardiographic or tomographic assessment [3].
Patients with high-risk PE require immediate reperfusion treatment. Systemic thrombolysis in high-risk PE patients is recommended as class I (evidence level B), surgical embolectomy as class I recommendation (evidence level C), and catheter-directed therapy as class IIa recommendation (evidence level C) [3]. Among initially hemodynamically stable patients with the presence of RV overload and positive markers of myocardial overload, 5–10% will develop hemodynamic instability despite anticoagulation [6]. Therefore, patients with intermediate-high-risk PE at an early stage of hospitalization require monitoring, preferably in an intensive care unit. In the case of hemodynamic instability, it is advisable to implement thrombolytic treatment (class I recommendation/evidence level B), for which the alternative is surgical embolectomy or CDT (class IIa recommendation/evidence level C) [3]. The Bova scale allows the identification of patients at risk of decompensation and death dependent on PE from among initially stable hemodynamically stable patients (Table 1) [20].
Table 1.
Bova score — risk stratification scale in medium-risk acute pulmonary embolism.
| Parameter | Points |
|---|---|
| Systolic pressure 90–100 mmHg | 2 |
|
| |
| Elevated troponin concentration (above the cutoff level) | 2 |
|
| |
| Right ventricle dysfunction: | 2 |
| Echocardiography: ≥ 1 of the following: RV/LV > 0.9, sPAP > 30 mmHg, RV diameter > 30 mm, or RV hypokinesis; | |
| Multidetector computed tomography: RV/LV > 1 | |
|
| |
| Tachycardia > 110/min | 1 |
|
| |
| ≥ 5 points – 30-day risk: | |
| 42% complications (death of PE, decompensation, recurrence of acute PE); | |
| 10% mortality in PE | |
LV — left ventricle; PE — pulmonary embolism; RV — right ventricle; sPAP — systolic pulmonary artery pressure.
Interventional therapy in PE
At the beginning of transcatheter therapy for a patient with PE, a pulmonary artery angiography from the femoral or internal jugular vein should be performed initially using 5–7 F diameter pigtail catheters [21]. Pulmonary angiography enables visualization of thrombi not only in the main pulmonary, lobar or segmental arteries but even in smaller (subsegmental) vessels. During pulmonary angiography, hemodynamic measurements should be made in the right atrium and right ventricle, and the pulmonary trunk to assess the severity of PE and exclude the overlapping of acute PE on CTEPH. Mechanical reperfusion involves the introduction of a catheter into the pulmonary arteries from the femoral or internal jugular vein to remove thrombi and reduce pulmonary resistance, facilitate the return of RV function, improve the patient’s clinical condition and prognosis [22]. Percutaneous embolectomy involves a variety of methods, from mechanical thrombus fragmentation, to thrombus aspiration and a pharmacomechanical approach of mechanical or ultrasound-assisted thrombus fragmentation with local administration of reduceddose thrombolysis (Table 2). Before the procedure, one should perform echocardiographic examination not only to assess RV function, but also to exclude thrombi in the right heart cavities and exclude thrombosis in the punctured femoral vein. In the published meta-analysis of Bajaj et al. [23], periprocedural success, defined as hemodynamic stabilization, reduction of hypoxia and discharge from hospital, was achieved in 87% of patients treated with endovascular treatment. The first Polish experiences with transcatheter methods (AngioJet system, Indigo Penumbra aspiration thrombectomy, EKOS system and Cleaner system) have recently been published [24–29].
Table 2.
Selected methods of catheter-directed therapy in patients with acute pulmonary embolism.
| Transcatheter therapies with local thrombolysis | Transcatheter therapies without thrombolysis | ||
|---|---|---|---|
|
|
|
||
| Method | Catheter/device | Method | Catheter/device |
| Rheolytic thrombectomy with local thrombolysis | AngioJet PE® catheter 6 F with the local thrombolysis application system Power Pulse™ (Boston Scientific, Minneapolis, MN USA) |
Rheolytic thrombectomy | 6 F AngioJet PE® catheter (Boston Scientific, Minneapolis, MN, USA) |
| Ultrasound assisted catheter-directed thrombolysis | EkoSonic® 5.2 F (EKOS, Boston Scientfic, Minneapolis, MN, USA) | Aspiration therombectomy | Aspirex® 8 F, 10 F catheters |
| (Straub Medical, Switzerland): Angiovac cannula — veno-venous bypass (26 F – 16–20 F access) (AngioDynamics, Latham, NY, USA): Continuous aspiration catheter Indigo® (Penumbra, Alameda, CA, USA): 8 F catheter connected to the suction pump Aspiration using vacuum (40–60 mL syringe) with guiding catheter (e.g. 8–9 F multi-purpose catheter) |
|||
| Mechanical thrombectomy | Flowtriever® (Inari Medical, Irvine, CA, USA): 20 F catheter and the device made by three self-expanding nitinol disks | ||
| Thrombus fragmentation with local thrombolysis | Pigtail catheters (5–6 F) or balloon catheters (5–10 mm) | Thrombus fragmentation | Pigtail catheters (5–6 F) or balloon catheters (5–10 mm) |
Another interventional technique supporting the treatment of acute PE is the implantation of vascular filters into the inferior vena cava. Venous filters protect the patient’s pulmonary arteries from subsequent embolism from deep veins of the lower extremities or the pelvic venous plexus. The results of PREPIC studies indicate that venous filters reduce the incidence of PE, while slightly increasing the incidence of venous thrombosis, without reducing overall mortality [30, 31]. In a recent analysis of the database of an American health fund, which included 16,950 patients with PE and concomitant cancer, a venous filter was used in 19% of patients. A reduction of mortality was demonstrated in a group of patients > 60 years of age in whom the filter was implanted, in relation to the group of conservatively treated patients [32]. Current European Society of Cardiology guidelines recommend the implantation of venous filters in patients with PE and absolute contraindications to anticoagulation or in patients who have recurrent PE despite adequate treatment (class IIa recommendation/evidence level C) [3]. Currently, the standard is an application of retrievable filters, which can be used in patients with PE or venous thrombosis before extensive surgery requiring temporary cessation of anticoagulation. After stabilization of the patient’s condition and resolution of contraindications to anticoagulation, removal of the filter should always be considered. Depending on the type of filter, it can be removed up to 6 months after implantation [33].
Methods of surgical treatment of PE. Application of ECMO
In high-risk PE, surgical pulmonary embolectomy should be used in patients with absolute contraindications to thrombolytic therapy or if it is ineffective and should be considered in selected patients at intermediate risk [3, 34, 35]. A separate group consists of patients with thrombi passing from the right atrium to the left side through the patent foramen ovale.
Pulmonary embolectomy can be performed in any center equipped with an extracorporeal circulation device. Under Polish conditions, mainly cardiac surgery centers with their personnel and equipment are accessible.
An alternative and attractive tool can also be mobile devices that are extracorporeal techniques (ECLS/ECMO), in which, by using quick access through peripheral vessels, it is possible to stabilize the patient in shock or hypotension. The prior mentioned devices for extracorporeal perfusion techniques are more and more often used in areas of modern intensive therapy of critical states.
In the Wielkopolska region, the PERT program was created in parallel with the program of universal access to extracorporeal techniques, including ECMO, as part of an organizational program “ECMO for Wielkopolska” (ECMO for Greater Poland) [36–38]. Such organizational cooperation gives a real chance to use a wide spectrum of applications in extracorporeal techniques in PE therapy, particularly for high risk associated with cardiogenic shock [38].
Extracorporeal techniques with the use of ECMO in PE therapy with potential use in the treatment of PE are as follows:
-
Veno-arterial (VA) ECMO — as a partial RV bypass in cardiogenic shock with hypotension:
As part of extended cardiopulmonary resuscitation when PE is the cause of in-hospital cardiac arrest or out-hospital cardiac arrest;
As a bridge to surgical embolectomy or transcatheter therapy;
As RV support after surgical embolectomy in extracorporeal circulation;
As a support during parenteral heparin anticoagulation.
Veno-arteriovenous (VA-V) ECMO — incidentally in the treatment of shock with concomitant RV failure as a RV assist device.
Veno-venous (VV) ECMO — incidentally after pulmonary embolism therapy with concomitant refractory respiratory failure with hypoxia and hypercapnia.
Partial VV Angiovac system with the possibility of conversion to VA ECMO.
PERT: Proposed operating model
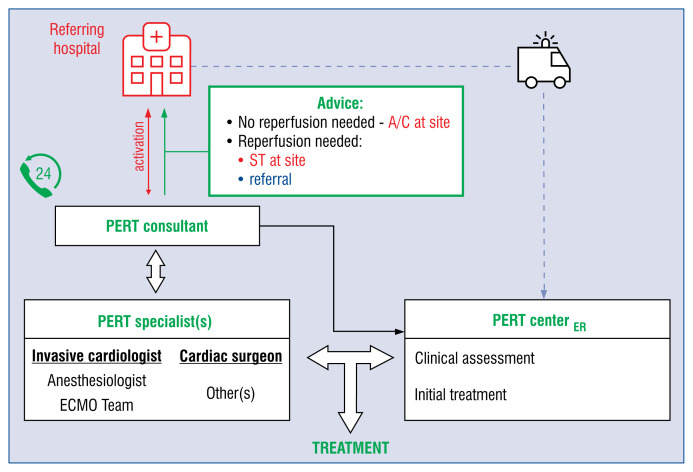
As PERT is modeled on a philosophy of rapid response, it is crucial to constitute a clear and sound operating protocol [13–16]. This protocol should accommodate PERT structure, activation pathways and operating mode. There are two basic elements of any PERT operating model. First of all, the PERT activation should be accessible via a commonly known telephone number. A dedicated on-call PERT consultant should be ready to answer a PERT activation call. The activation call may come from any healthcare provider in the region (district hospital, outpatient clinic, ambulance) networked with the PERT center in a hub-and-spoke model. The role of the PERT consultant is to collect necessary information on the patient consulted from a referring physician, including: clinical status and duration of symptoms, PE burden and hemodynamic significance, RV function and adverse outcome risk factors, comorbidities, contraindications to specific treatments like thrombolysis, surgical embolectomy or CDT. To facilitate the process of data collection, a standardized form should be in use (see the next paragraph). Depending on the PERT center institutional policies, structure and personnel resources, the PERT consultant may be an intentionally dedicated individual or any other on-call physician, who is capable of executing the PERT operating model upon activation.
The second key element of the PERT operating model is cooperation between PERT specialists. The on-call PERT specialists including, at least, an interventional cardiologist and cardiac surgeon who should be ready to enter cooperation upon a PERT consultant request. There are several modes of PERT specialist mobilization, among which a staged approach seems to be the most resource efficient and practical (Fig. 1). In contrast to activation of all PERT members upfront, in the staged model the PERT consultant initially contacts one on-call PERT specialist to determine whether reperfusion therapy is needed and discuss treatment options. If a decision is not possible, another PERT specialist(s) may be asked for an opinion. After reaching a final conclusion it is communicated back to the referring physician as a PERT therapeutic recommendation. The goal should be to complete this process within 30 min from activation.
Figure 1.
Proposed Pulmonary Embolism Response Team (PERT) operating model. A dedicated PERT consultant is ready 24/7 to respond to an activation call from a regional healthcare provider, collect necessery information on the patient consulted and contact an on-call PERT specialist(s) to determine whether reperfusion therapy is needed, and discuss treatment options. Their conclusion is communicated back to the referring physician as PERT therapeutic advice, which may include: (1) continue anticoagulation at the referring site, in cases where no reperfusion is required; (2) start systemic thrombolysis at the site immediately if shock or cardiac arrest is present; (3) transfer the patient to a PERT center, if reperfusion is required and there are contraindications to systemic thrombolysis, risk of major bleeding is high or the patient needs surgical intervention. ECMO — extracorporeal membrane oxygenation; A/C — anticoagulation; ST — systemic thrombolysis; ER — emergency room.
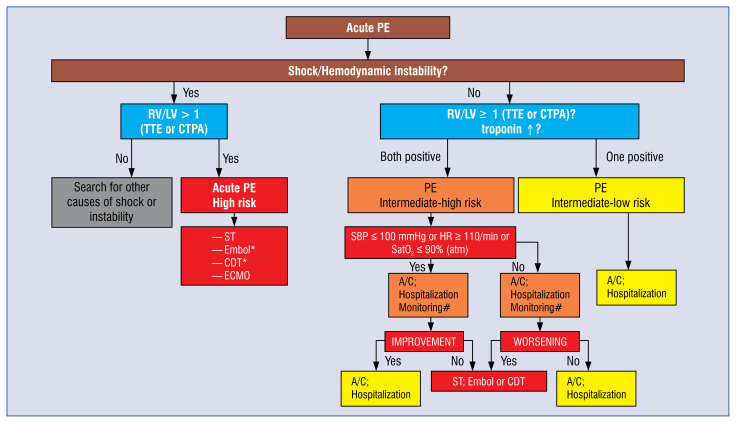
If no reperfusion therapy is required at the time of consultation, the patient may continue anticoagulation treatment at the referring hospital. The referring physician should stay in touch with the PERT center in case a patient deteriorates. If, in contrast, reperfusion therapy is indicated at the site, systemic thrombolysis should primarily be considered, or if contraindications to ST are present, risk of major bleeding is high or surgical intervention is needed, the patient should be transferred to the PERT center. To support the decision-making process a therapeutic algorithm may be adapted (Fig. 2).
Figure 2.
Proposed therapeutic algorithm in acute pulmonary embolism for the use of Pulmonary Embolism Response Teams. A/C — anticoagulation; CDT — catheter-directed therapy; CTPA — computed tomography pulmonary angiography; ECMO — extracorporeal membrane oxygenation; Embol — surgical embolectomy; HR — heart rate; PE — pulmonary embolism; RV/LV — right-to-left ventricular dimeter index; SatO2 — arterial blood oxygen saturation; SBP — systolic blood pressure; ST — systemic thrombolysis; sPESI — simplified Pulmonary Embolism Severity Index; TTE — transthoracic echocardiography; *If ST is contraindicated or has failed; #Monitoring and observing period of the deterioration/improvement of the patient’s condition should be individualized depending on clinical conditions and should not exceed 6–12 hours to decide on intensification of treatment.
Communication and data collection tools
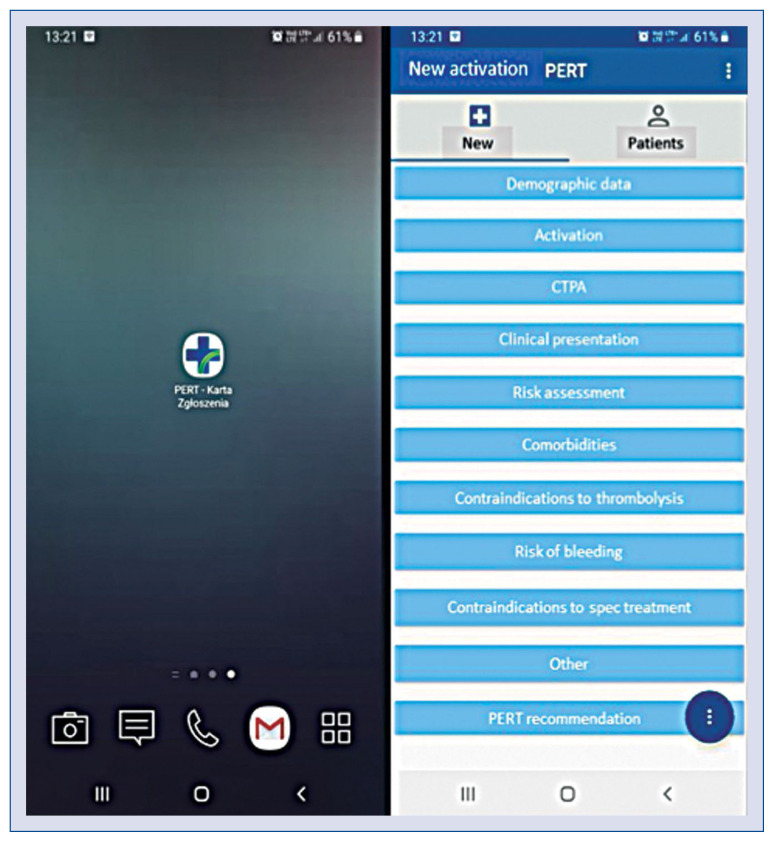
To promote rapid and efficient course of PERT consultations, it may be useful to formulate PERT activation and decision cards. These documents should i.e. contain referral center contact details, patient demographic data, duration of symptoms, distribution of thrombi in computed tomography, clinical status and risk of death defined by contemporary algorithms (e.g. Bova score), comorbidities, risk factors of venous thromboembolism, contraindications to thrombolysis and anticipated risk of bleeding complications. Said forms may then serve as a background for the PERT decision-making process. With the advent of modern technologies, it has become available to acquire PERT activation documents in the form of mobile applications (Fig. 3). Use of mobile technologies is expected to make the process of data collection easier and more universal and to facilitate sharing information between PERT specialists during consultations.
Figure 3.
Mobile application developed by the Pulmonary Embolism Response Team (PERT) of the John Paul II Hospital in Krakow, Poland and is used to collect pulmonary embolism patient data and to conduct PERT consultations.
It is also desirable to record data on activations, operating modes, PERT decisions and patient follow-up in the form of a registry. A regular evaluation of accumulated data should provide insight in general PE quality measures such as mortality and morbidity, but also PERT — specific measures such as: time from activation to decision, time from decision to therapeutic anticoagulation or to reperfusion therapy, methods and effects of reperfusion treatment, PERT structure and activation modes, application of contemporary guidelines, etc. Such data may also help to improve knowledge on the role of PERT in acute PE care.
Conclusions
Organization of multidisciplinary PERT in reference centers for management of high- and intermediate-high risk PE is recommended depending on local resources and available expertise.
The most important tasks of PERT in the acute phase of PE include choosing optimal pharmacotherapy (including determining indications or contraindications for thrombolysis), interventional treatment (catheter-directed therapy, venous filter implantation) or cardiac surgery (pulmonary embolectomy).
It is crucial to constitute a clear and sound operating protocol. Such protocol should accommodate the PERT structure, activation pathways and operating mode.
A PERT coordinator should continuously be available by phone (24/7) on a dedicated phone number and after accepting the application must be able to organize a quick consultation (< 30 min) with relevant specialists.
Among the physicians directly involved in the process of treating a patient under PERT care there are usually specialists who have experience in the field of intensive cardiac therapy, echocardiography and interventional cardiology, as well as cardiac surgeons, specialists in emergency medicine, anesthesiologists and radiologists including interventional radiologists.
The second group of specialists whose consultations may be necessary in selected cases should include neurologists, neurosurgeons, oncologists, vascular surgeons, hematologists and specialists in lung diseases.
To promote rapid and efficient course of PERT consultations, it may be useful to formulate PERT activation and decision cards (if possible, in the form of mobile applications).
PERTs operating in Poland
Centrum Interwencyjnego Leczenia Zatorowości Płucnej (CELZAT); Department and Faculty of Cardiology, Medical University of Warsaw; Banacha 1a, tel: 691 520 108; Department of Pulmonary Circulation, Thromboembolic Diseases and Cardiology European Health Center, Otwock, tel: 22 710 30 58
POZ-PERT — Pulmonary Embolism Response Team at Lord’s Transfiguration University Hospital, Poznan University of Medical Sciences, Poznań, tel: 608 574 375
DJ-PERT — Pulmonary Embolism Response Team at the Infant Jesus University Hospital; Lindleya 4, Warsaw, Department of Internal Medicine and Cardiology, Medical University of Warsaw, Poland, tel: 507 121 347/507 121 367
Pulmonary Embolism Response Team at the John Paul II Hospital in Krakow (PERTJPII), tel: 606 762 306
Footnotes
Conflict of interest: None declared
References
- 1.Cohen AT, Agnelli G, Anderson FA, et al. VTE Impact Assessment Group in Europe (VITAE) Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–764. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber S, Bounameaux H. Pulmonary embolism and deep vein thrombosis. The Lancet. 2012;379(9828)(11):1835–1846. 61904–1. doi: 10.1016/s0140-6736. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinides S, Meyer G Task Force for the Management of Acute Pulmonary Embolism of the European Society of C. ESC guidelines for the diagnosis and management of acute pulmonary embolism developed with collaboratin with European Respiratory Society (ERS) Eur Heart J. 2019;2019;40(42):3453–3455. doi: 10.1093/eurheartj/ehz726. [DOI] [PubMed] [Google Scholar]
- 4.Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341(8844):507–511. doi: 10.1016/0140-6736(93)90274-k. [DOI] [PubMed] [Google Scholar]
- 5.Budaj-Fidecka A, Kurzyna M, Fijałkowska A, et al. In-hospital major bleeding predicts mortality in patients with pulmonary embolism: an analysis of ZATPOL Registry data. Int J Cardiol. 2013;168(4):3543–3549. doi: 10.1016/j.ijcard.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Meyer G, Vicaut E, Danays T, et al. PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311(23):2414–2421. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 8.Stein PD, Matta F. Thrombolytic therapy in unstable patients with acute pulmonary embolism: saves lives but underused. Am J Med. 2012;125(5):465–470. doi: 10.1016/j.amjmed.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Grifoni S, Olivotto I, Cecchini P, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101(24):2817–2822. doi: 10.1161/01.cir.101.24.2817. [DOI] [PubMed] [Google Scholar]
- 10.Schultz J, Andersen A, Kabrhel C, et al. Catheter-based therapies in acute pulmonary embolism. EuroIntervention. 2018;13(14):1721–1727. doi: 10.4244/eij-d-17-00437. [DOI] [PubMed] [Google Scholar]
- 11.Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 12.Tapson VF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018;11(14):1401–1410. doi: 10.1016/j.jcin.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Provias T, Dudzinski DM, Jaff MR, et al. The Massachusetts General Hospital Pulmonary Embolism Response Team (MGH PERT): creation of a multidisciplinary program to improve care of patients with massive and submassive pulmonary embolism. Hosp Pract (1995) 2014;42(1):31–37. doi: 10.3810/hp.2014.02.1089. [DOI] [PubMed] [Google Scholar]
- 14.Kabrhel C, Jaff MR, Channick RN, et al. A multidisciplinary pulmonary embolism response team. Chest. 2013;144(5):1738–1739. doi: 10.1378/chest.13-1562. [DOI] [PubMed] [Google Scholar]
- 15.Dudzinski DM, Piazza G. Multidisciplinary pulmonary embolism response teams. Circulation. 2016;133(1):98–103. doi: 10.1161/CIRCULATIONAHA.115.015086. [DOI] [PubMed] [Google Scholar]
- 16.Kabrhel C, Rosovsky R, Channick R, et al. A multidisciplinary pulmonary embolism response team: initial 30-month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150(2):384–393. doi: 10.1016/j.chest.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Barnes GD, Kabrhel C, Courtney DM, et al. Diversity in the Pulmonary Embolism Response Team Model: An Organizational Survey of the National PERT Consortium Members. Chest. 2016;150(6):1414–1417. doi: 10.1016/j.chest.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Rosovsky R, Chang Y, Rosenfield K, et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis. 2019;47(1):31–40. doi: 10.1007/s11239-018-1737-8. [DOI] [PubMed] [Google Scholar]
- 19.Schultz J, Giordano N, Zheng H, et al. EXPRESS: A Multidisciplinary Pulmonary Embolism Response Team (PERT) - Experience from a national multicenter consortium. Pulm Circ. 2019 doi: 10.1177/2045894018824563. [Epub ahead of print] 2045894018824563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bova C, Sanchez O, Prandoni P, et al. Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J. 2014;44(3):694–703. doi: 10.1183/09031936.00006114. [DOI] [PubMed] [Google Scholar]
- 21.Kurzyna M, Araszkiewicz A, Błaszczak P, et al. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the Polish Cardiac Society’s Working Group on Pulmonary Circulation and Association of Cardiovascular Interventions. Kardiol Pol. 2015;73(1):63–68. doi: 10.5603/KP.2015.0011. [DOI] [PubMed] [Google Scholar]
- 22.Engelberger RP, Kucher N, Engelberger RP, et al. Catheterbased reperfusion treatment of pulmonary embolism. Circulation. 2011;124(19):2139–2144. doi: 10.1161/CIRCULATIONAHA.111.023689. [DOI] [PubMed] [Google Scholar]
- 23.Bajaj NS, Kalra R, Arora P, et al. Catheter-directed treatment for acute pulmonary embolism: Systematic review and single-arm meta-analyses. Int J Cardiol. 2016;225:128–139. doi: 10.1016/j.ijcard.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 24.Roik M, Wretowski D, Łabyk A, et al. Initial experience of pulmonary embolism response team with percutaneous embolectomy in intermediate-high- and high-risk acute pulmonary embolism. Kardiol Pol. 2019;77(2):228–231. doi: 10.5603/KP.a2018.0239. [DOI] [PubMed] [Google Scholar]
- 25.Roik M, Wretowski D, Machowski M, et al. Successful treatment of intermediate-high-risk pulmonary embolism with aspiration thrombectomy: first experience in Poland. Kardiol Pol. 2018;76(9):1381. doi: 10.5603/KP.2018.0190. [DOI] [PubMed] [Google Scholar]
- 26.Latacz P, Simka M, Brzegowy P, et al. Treatment of high- and intermediate-risk pulmonary embolism using the AngioJet percutaneous mechanical thrombectomy system in patients with contraindications for thrombolytic treatment - a pilot study. Wideochir Inne Tech Maloinwazyjne. 2018;13(2):233–242. doi: 10.5114/wiitm.2018.75848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stępniewski J, Kopeć G, Magoń W, et al. Ultrasound assisted, catheter directed, lowdose thrombolysis for the treatment of acute intermediatehigh risk pulmonary embolism. Pol Arch Intern Med. 2018;128(6):394–395. doi: 10.20452/pamw.4272. [DOI] [PubMed] [Google Scholar]
- 28.Kurzyna M, Pietrasik A, Opolski G, et al. Contemporary methods for the treatment of pulmonary embolism - is it prime-time for percutaneous interventions? Kardiol Pol. 2017;75(11):1161–1170. doi: 10.5603/KP.a2017.0125. [DOI] [PubMed] [Google Scholar]
- 29.Araszkiewicz A, Jankiewicz S, Sławek-Szmyt S, et al. Rapid clinical and haemodynamic improvement in a patient with intermediate-high risk pulmonary embolism treated with transcatheter aspiration thrombectomy. Adv Interv Cardiol. 2019;15(4):497–498. doi: 10.5114/aic.2019.90229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. NEJM. 1998;338(7):409–416. doi: 10.1056/nejm199802123380701. [DOI] [PubMed] [Google Scholar]
- 31.PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416–422. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- 32.Stein PD, Matta F, Lawrence FR, et al. Inferior vena cava filters in patients with acute pulmonary embolism and cancer. Am J Med. 2018;131(4):442.e9–442.e12. doi: 10.1016/j.amjmed.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 33.Charalel RA, Durack JC, Mao J, et al. Statewide inferior vena cava filter placement, complications, and retrievals: epidemiology and recent trends. Med Care. 2018;56(3):260–265. doi: 10.1097/MLR.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 34.Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest. 2006;129(4):1043–1050. doi: 10.1378/chest.129.4.1043. [DOI] [PubMed] [Google Scholar]
- 35.Myers PO, Bounameaux H, Panos A, et al. Impending paradoxical embolism: systematic review of prognostic factors and treatment. Chest. 2010;137(1):164–170. doi: 10.1378/chest.09-0961. [DOI] [PubMed] [Google Scholar]
- 36.Stefaniak S, Puślecki M, Ligowski M, et al. Venoarterial extracorporeal membrane oxygenation in massive pulmonary embolism. Kardiol Pol. 2018;76(5):931. doi: 10.5603/KP.2018.0107. [DOI] [PubMed] [Google Scholar]
- 37.Puślecki M, Ligowski M, Dąbrowski M, et al. „ECMO for Greater Poland”: a unique regional program for extracorporeal life support. Pol Arch Intern Med. 2017;127(7–8):567–568. doi: 10.20452/pamw.4082. [DOI] [PubMed] [Google Scholar]
- 38.Puślecki M, Ligowski M, Stefaniak S, et al. „Extracorporeal Membrane Oxygenation for Greater Poland” Program: how to save lives and develop organ donation? Transplant Proc. 2018;50(7):1957–1961. doi: 10.1016/j.transproceed.2018.02.159. [DOI] [PubMed] [Google Scholar]