Abstract
Background
Severe familial hypercholesterolemia (FH) individuals, refractory to conventional lipid-lowering medications are at exceptionally high risk of cardiovascular events. The established therapeutic option of last choice is lipoprotein apheresis (LA). Herein, it was sought to investigate the clinical usefulness of LA in a highly selected group of severe heterozygous FH (HeFH), as recently described by the International Atherosclerosis Society (IAS), for their efficacy in lipid reduction and safety.
Methods
Efficacy and safety of LA were investigated in 318 sessions of 7 severe HeFH females with cardiovascular disease, over a mean period of 26.9 ± 6.5 months. Relative reduction of low density lipoprotein cholesterol (LDL-C) ≥ 60%, clinical complications and vascular access problems were evaluated and compared between the direct adsorption of lipoproteins (DALI) and lipoprotein filtration (Membrane Filtration Optimized Novel Extracorporeal Treatment [MONET]). Additionally, lipoprotein (a) [Lp(a)], total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), triglycerides (TG) and fibrinogen concentrations were investigated.
Results
The relative reduction of LDL-C, TC, TG and Lp(a) were 69.4 ± 12.9%, 59.7 ± 9.1, 51.5 ± 14.2% and 71.3 ± 14.4%, respectively. A similar efficacy was found in both systems in LDL-C removal. DALI system led to larger depletions of Lp(a) (80.0 [76–83]% vs. 73.0 [64.7–78.8]%; p < 0.001). The frequency of clinical side effects and vascular access problems were low (8.5%).
Conclusions
Long-term LA in severe HeFH individuals is safe and efficiently reduces LDL-C and Lp(a). Higher efficacy of the DALI system than MONET in Lp(a) removal may indicate the need for individualized application of the LA system in severe HeFH individuals.
Keywords: lipoprotein apheresis, severe familial hypercholesterolemia, lipoprotein (a)
Introduction
Severe familial hypercholesterolemia (FH) patients are at exceptionally high risk of cardiovascular disease (CVD) [1]. Although some authors have suggested that individuals with heterozygous FH (HeFH) and untreated low-density lipoprotein cholesterol (LDL-C) greater than 309 mg/dL suffer from severe HeFH, the definition of severe phenotype of FH was not clear [2]. Recently, the International Atherosclerosis Society (IAS) considered not only initial LDL-C values, but also the presence of risk conditions, as well as clinical or advanced subclinical atherosclerotic CVD [2–7]. These high-risk conditions proposed by IAS include diabetes, arterial hypertension, smoking history, chronic kidney disease, positive family history of early CVD in first-degree relative, low high-density lipoprotein cholesterol (HDL-C) and elevated lipoprotein (a) [Lp(a)]. Among high risk features special attention should be directed to increased Lp(a) with its strong atherogenic and thrombogenic effect, and resistance to conventional lipid-lowering medications [5, 8]. Individuals with severe phenotype of FH often do not adequately respond to high-intensity lipid-lowering medications and do not achieve treatment goals [9]. The recommended target level for severe FH individuals for secondary prevention is LDL-C below 70 mg/dL [9]. Thus, more aggressive forms of therapy might be beneficial in severe FH to arrest the progression of atherosclerosis and reduce cardiovascular event rate [10–12]. The treatment option in severe FH widely used since the 80s has been long-term lipoprotein apheresis (LA) [13, 14]. LA is an extracorporeal technique of selective removal of lipoproteins. Various lipoprotein apheresis systems are routine in clinical use currently. Whole blood adsorption of lipoproteins includes direct adsorption of lipoproteins with polyacrylamide (DALI) and dextran sulfate cellulose adsorption. Atherogenic lipoproteins may also be eliminated in following primary plasma separation methods: lipoprotein filtration (MONET), heparin-induced extracorporeal LDL-C precipitation (HELP), silicate gel adsorption, immunoadsorption (IMA) and dextran sulfate cellulose [15]. LA procedures need to be repeated every 1–2 weeks due to LDL-C and Lp(a) level rebound effect. All LA techniques have been shown to effectively reduce LDL-C along with Lp(a) concentrations by more than 60%, while being well tolerated in long-term application. LDL-apheresis treatment also exerts a pleiotropic effect, improving rheological properties of the blood and reducing inflammatory markers [16, 17]. Regular apheresis sessions have been proved to slow the progression of atherosclerosis and reduce the incidence of cardiovascular events [12, 18–20]. The main goal of LA treatment in HeFH is to achieve an LDL-C reduction ≥ 60% at each therapeutic session [21]. Additionally, time-averaged LDL-C less than 100 mg/dL might be considered as a goal [21].
Although data confirming the effectiveness and safety of lipoprotein apheresis have been published, the studies often aggregated patients with undefined hypercholesterolemia, homozygous FH, HeFH and isolated increased Lp(a), treated with various apheresis systems and differing lipid-lowering medication regimens [22, 23]. This lack of stratification by type of dyslipidemia resulted in misleading findings. Therefore, the main purpose of the current research was to investigate the clinical usefulness of lipoprotein apheresis in a highly selected group of severe HeFH, according to the IAS definition, for their efficacy in lipid reduction and safety. Additionally, the safety and effectiveness of DALI and MONET systems were compared in the current study group.
Methods
The study was carried out prospectively in a large Polish lipoprotein apheresis center, established at the First Department of Cardiology, Medical University of Gdansk, following the Good Clinical Practice guidelines. Researchers obtained written informed consent before patient inclusion, in accordance with the Declaration of Helsinki. All treatment protocols and medical records for each patient undergoing LA were reviewed, except for initial sessions in the first month of treatment.
Patients
The primary indication to LA treatment was HeFH with symptomatic CVD and LDL-C concentration of more than 160 mg/dL despite maximally tolerated intensive lipid-lowering medications [14]. Seven female patients with definite FH according to the modified Dutch Lipid Network Criteria and confirmed a mutation in LDLR or APOB gene, fulfilling the criteria of severe FH by the IAS, were enrolled into the study [4, 24]. At the initiation of LA treatment, all patients had a history of a documented CVD and at least three additional high risk-features for severe FH. All individuals were treated with rosuvastatin in a dose of 40 mg daily with or without ezetimibe 10 mg daily by more than 12 months before starting LA and continued such treatment on apheresis. The mean age of patients at the start of LA therapy was 54.5 ± 5.5 years. Detailed clinical and biochemical characteristics of investigated patients are presented in Table 1.
Table 1.
Clinical and biochemical characteristics at lipoprotein apheresis initiation.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| Age [years] | 58 | 47 | 61 | 65 | 58 | 56 | 54 |
| FH-causing gene mutation | LDLR | LDLR | APOB | LDLR | LDLR | LDLR | LDLR |
| Body mass index [kg/m2] | 24 | 24 | 35 | 29 | 26 | 31 | 34 |
| Waist circumference [cm] | 90 | 80 | 98 | 95 | 95 | 103 | 117 |
| Hip circumference [cm] | 100 | 97 | 104 | 105 | 103 | 116 | 124 |
| High risk features for severe FH | |||||||
| Diabetes | − | + | + | IFG | − | + | − |
| Hypertension | + | + | + | + | + | + | + |
| Smoking history | + | + | + | + | − | − | + |
| Family history of early CVD in first-degree relative | + | + | + | + | + | + | + |
| Lp(a) max [g/L] | 1.37 | 0.8 | 0.54 | 0.2 | 1.18 | 0.22 | 0.6 |
| CKD (GFR < 60 mL/min/m2) | − | − | − | − | − | − | − |
| HDL-C < 40 mg/dL | − | − | − | − | − | − | + |
| Cardiovascular history | |||||||
| Coronary artery disease | + | + | + | + | + | + | + |
| ACS | 0 | 6 | 0 | 1 | 1 | 0 | 1 |
| ACS, age of first | NA | 41 | 49 | 57 | 51 | NA | 42 |
| PCI | 5 | 2 | 10 | 0 | 6 | 3 | 3 |
| PCI, age of first | 46 | 41 | 49 | NA | 51 | 49 | 42 |
| CABG | − | − | − | 1 | − | 1 | 1 |
| CABG, age | NA | NA | NA | 57 | NA | 45 | 42 |
| TIA | − | − | + | + | − | − | − |
| Stroke | − | 2 | − | − | − | − | − |
| Stroke, age of first | NA | 45 | NA | 63 | NA | NA | NA |
| Carotid artery stenosis | − | − | + | + | + | − | − |
| Peripheral artery disease | − | − | + | − | − | + | − |
| Revascularization of carotid or peripheral artery | − | − | + | + | − | − | − |
| Heart failure | − | + | − | − | − | − | + |
| LVEF [%] | 60 | 20 | 50 | 60 | 50–55 | 55 | 40 |
| Biochemical parameters | |||||||
| TC max [mg/dL] | 538 | 536 | 392 | 797 | 500 | 431 | 562 |
| LDL-C max [mg/dL] | 453 | 440 | 318 | 759 | 422 | 352 | 475 |
| HDL-C max [mg/dL] | 63 | 61 | 45 | 55 | 39 | 48 | 35 |
| TG max [mg/dL] | 111 | 182 | 145 | 195 | 196 | 146 | 258 |
| Apo A1 [mg/dL] | 1.91 | 1.91 | 1.07 | 1.5 | 1.87 | 1.38 | 1.46 |
| ApoB [mg/dL] | 0.86 | 1.37 | 1.17 | 1.21 | 1.85 | 2 | 1.22 |
| Hypolipidemic treatment | |||||||
| Statin, age of implementation | 46 | 35 | 49 | 45 | 40 | 34 | 38 |
| Intensive lipid-lowering treatment [years] | 12 | 12 | 10 | 8 | 18 | 7 | 16 |
| Statin, type and dose | rosuvastatin 40 mg | rosuvastatin 40 mg | rosuvastatin 40 mg | rosuvastatin 40 mg | rosuvastatin 40 mg | rosuvastatin 40 mg | rosuvastatin 40 mg |
| Ezetimibe 10 mg daily | − | + | + | + | + | + | + |
| Other lipid-lowering medications | − | − | − | − | − | − | − |
| Age at apheresis initiation [years] | 55 | 44 | 59 | 63 | 55 | 54 | 52 |
| Apheresis technique | MONET | MONET | MONET | MONET | DALI | DALI | DALI |
Conversion factors to SI units are as follows: glucose, 0.05551; cholesterol, 0.02586 and triglycerides 0.0114. TC-max, LDL-C-max, HDL-C max, TG max are the highest values before statin initiation. ACS — acute coronary syndrome; Apo — apolipoprotein; APOB — apolipoprotein B gene; CABG — coronary artery bypass graft; CKD — chronic kidney disease; CVD — cardiovascular disease; DALI — direct adsorption of lipoproteins; FH — familial hypercholesterolemia; GFR — glomerular filtration rate; HDL-C— high density lipoprotein cholesterol; IFG — impaired glucose tolerance; LDLR — low density lipoprotein receptor gene; LDL-C — low density lipoprotein cholesterol; Lp(a) — lipoprotein (a); LVEF — left ventricular ejection fraction; MONET — Membrane Filtration Optimised Novel Extracorporeal Treatment; NA — not applicable; PCI — percutaneous coronary intervention; TC — total cholesterol; TG — triglicerydes; TIA — transient ischemic attack
Lipoprotein apheresis
Lipoprotein apheresis sessions (n = 318) were performed in weekly or biweekly intervals using two techniques, according to patient characteristics over a period of 37 months. Concomitant angiotensin converting enzyme inhibitors (ACEI) therapy was a contraindication to DALI treatment. LA therapy was started 6.0 ± 1.5 years after the clinical diagnosis of FH. 162 DALI sessions with large adsorber configurations (DALI 1000, DALI 1250) and 156 MONET procedures were performed over a mean period of 26.9 ± 6.5 months [25]. To provide adequate effectiveness, at least 1.5 of blood volume was processed during DALI therapeutic sessions, and at least 45 mL of plasma volume/kg was treated during MONET sessions [26]. Both acid citrate dextrose (ACD-A) and heparin in the priming solution were used as an anticoagulant. Arteriovenous (AV) fistula was established as access in all patients due to the insufficiency of peripheral venous access. Initial sessions in the first months and procedures interrupted before expected blood/plasma volume purification were excluded from the final analysis of biochemical parameters.
Biochemical parameters
All biochemical parameters were measured in one laboratory at scheduled intervals. LDL-C levels were subsequently calculated using the Friedewald formula unless triglycerides (TG) were above 400 mg/dL. Acute reduction in total cholesterol (TC), LDL-C, HDL-C, TG, Lp(a) and fibrinogen were calculated from pre- and post-apheresis results. The time-averaged mean LDL-C level was calculated according to the formula devised by Kroon, where CMAX and CMIN are defined as the immediate pre- and post-treatment values: CAVG = CMIN + 0.73 × CMAX – CMIN [27]. The effectiveness of LA was expressed as an achievement of acute post-apheresis LDL-C reduction by more than 60%. Alternatively, the time-averaged LDL-C below 100 mg/dL was a goal of treatment. Additionally, HDL-C, TC, TG, Lp(a) and fibrinogen reductions were investigated.
Side-effects
Clinical complications and vascular access problems were investigated at each therapeutic apheresis session. Clinical complications were specified as follows: hypotension with systolic blood pressure < 90 mmHg and accompanying symptoms (paleness, nausea), hypocalcemia, oedema, severe bleeding, anemia, and thrombocytopenia. Vascular complications included puncture problems, hematoma, bleeding and stenosis of AV fistula.
Statistical analysis
Continuous data were presented as a mean value and standard deviation (SD) or as a median and interquartile range (IQR) or as a median and minimum and maximum value. Categorical data were presented as percentages. Normal distribution was verified by the Kolmogorov-Smirnov test. Continuous data were compared by the Student t-test or U-Mann Whitney test depending on the distribution. Categorical data were compared by the χ2 test and Fisher exact test. P value less than 0.05 was considered statistically significant. Data were analyzed using SPSS software v.21 (IBM, Chicago, Illinois, USA).
Results
Lipoprotein apheresis
The mean duration time of the procedure was 140.0 (60–240) min. ACD-A was used in a mean volume of 329.8 ± 134.9 mL, with the ratio of citrate: blood ranging from 1:20 to 1:40 in both DALI and MONET. DALI sessions were significantly shorter than MONET (130.0 [120–140] vs. 170.0 [158.0–183.7]; p < 0.001) with less ACD-A consumption (244.0 [215–302] vs. 431.0 (374.5–486.5); p < 0.001). Average blood volume processed during DALI sessions was 8540 ± 155 mL. During MONET sessions average plasma volume 2903.5 ± 867.1 mL was achieved.
Cholesterol, lipoprotein (a) and fibrinogen
Laboratory parameters before and during chronic LA treatments are summarized in Table 2. Mean pre-apheresis values of TC and LDL-C were high (308.9 ± 94.1 and 222.8 ± 89.5 mg/dL, respectively). Apheresis reduced both lipids acutely to 121.6 ± 39.3 mg/dL and 68.8 ± 37.6 mg/dL, respectively. Mean pre-apheresis values of HDL-C were below the normal range for females (42.5 ± 10.1 mg/dL). Apheresis reduced HDL-C to a lesser extent than other lipids (22.7 ± 10.9%). Apheresis sessions removed TG by 51.5 ± 14.2% in the mean, starting from 220.8 ± 162.7 mg/dL. Apheresis session led to acute depletion of Lp(a) from pre-apheresis concentration of 0.5 ± 0.4 g/L to 0.12 ± 0.09 g/L. Fibrinogen was reduced by 39.7 ± 21.2% starting from the pre-apheresis level of 3.25 ± 0.63 mg/dL (Table 2).
Table 2.
Lipoprotein apheresis — biochemical parameters.
| Mean ± SD | Median | Minimum | Maximum | |
|---|---|---|---|---|
| TC pre-apheresis [mg/dL] | 308.9 ± 94.1 | 294.0 | 153.0 | 569.0 |
| TC post-apheresis [mg/dL] | 121.6± 39.3 | 114.0 | 62.0 | 354.0 |
| TC [% reduction] | 59.7 ± 9.1 | 61.5 | 32.4 | 78.9 |
| LDL-C pre-apheresis [mg/dL] | 222.8 ± 89.5 | 207.5 | 54.0 | 490.0 |
| LDL-C post-apheresis [mg/dL] | 68.8 ± 37.6 | 66.0 | 1.0 | 177.0 |
| LDL-C [% reduction] | 69.4 ± 12.9 | 71.8 | 17.3 | 98.3 |
| Interval LDL-C [mg/dL] | 181.4 ± 72.8 | 154.9 | 40.0 | 395.8 |
| HDL-C pre-apheresis [mg/dL] | 42.5 ± 10.1 | 41.0 | 15.0 | 75.0 |
| HDL-C post-apheresis [mg/dL] | 32.4 ± 6.8 | 32.0 | 11.0 | 53.0 |
| HDL-C [% reduction] | 22.7 ± 10.9 | 21.7 | 0.0 | 62.16 |
| TG pre-apheresis [mg/dL] | 220.8 ± 162.7 | 157.0 | 46.0 | 1121.0 |
| TG post-apheresis [mg/dL] | 103.8 ± 84.8 | 72.0 | 23.0 | 616.0 |
| TG [% reduction] | 51.5 ± 14.2 | 53.2 | 10.8 | 83.6 |
| Lp(a) pre-apheresis [g/L] | 0.5 ± 0.4 | 0.39 | 0.08 | 1.37 |
| Lp(a) post-apheresis [g/L] | 0.12 ± 0.09 | 0.09 | 0.01 | 0.57 |
| Lp(a) [% reduction] | 71.3 ± 14.4 | 76.1 | 21.4 | 94.1 |
| Fibrinogen pre-apheresis [mg/dL] | 3.25 ± 0.63 | 3.25 | 2.2 | 6.47 |
| Fibrinogen post-apheresis [mg/dL] | 1.96 ± 0.77 | 2.02 | 0.63 | 3.96 |
| Fibrinogen [% reduction] | 39.7 ± 21.2 | 45.6 | 2.8 | 76.0 |
Data are presented as mean ± standard deviation (SD) and median with minimum and maximum. Abbreviations — see Table 1.
DALI vs. MONET
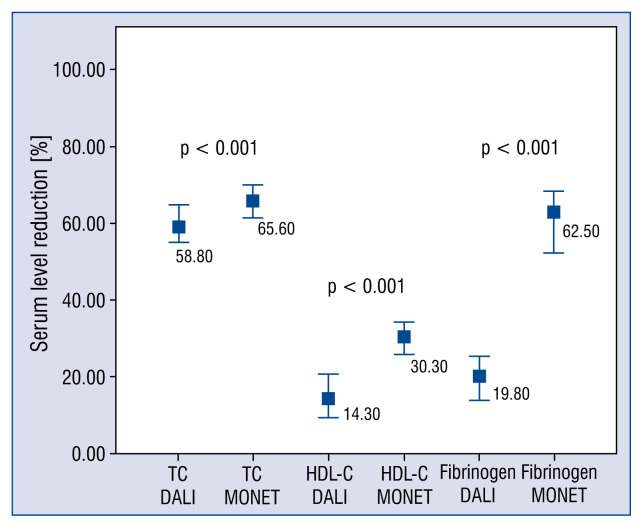
Higher pre- and post-apheresis TC, LDL-C, HDL-C and Lp(a) concentrations in MONET sessions vs. DALI (Table 3) were observed. In contrast, the pre- and post-apheresis TG levels were higher in patients treated by DALI system, compared to MONET (Table 3). MONET system led to higher TC and HDL-C reductions, compared to DALI (Fig. 1).
Table 3.
Biochemical parameters in DALI versus MONET system.
| DALI | MONET | P | |
|---|---|---|---|
| TC pre-apheresis [mg/dL] | 238.0 (207–295) | 372.0 (254.5–409.5) | < 0.001 |
| TC post-apheresis [mg/dL] | 100.0 (83–121) | 114.0 (101.5–134.5) | 0.003 |
| TC [% reduction] | 58.8 (54.8–64.5) | 65.6 (61.2–69.7) | < 0.001 |
| LDL-C pre-apheresis [mg/dL] | 141.0 (122–170) | 286.0 (191.5–326.5) | < 0.001 |
| LDL-C post-apheresis [mg/dL] | 37.5 (26–57) | 69.0 (49.5–88.0) | < 0.001 |
| LDL-C [% reduction] | 72.6 (66.5–83.5) | 74.1 (69.9–77.9) | 0.8 |
| Interval LDL-C [mg/dL] | 113.4 (96.9–137.5) | 228.5 (155.6–260.3) | < 0.001 |
| HDL-C pre-apheresis [mg/dL] | 36.0 (34–39) | 50.0 (42.5–58.0) | < 0.001 |
| HDL-C post-apheresis [mg/dL] | 31.0 (28.2–34.0) | 36.0 (30.0–40.0) | < 0.001 |
| HDL-C [% reduction] | 14.3 (9.5–20.6) | 30.3 (25.7–34.2) | < 0.001 |
| TG pre-apheresis [mg/dL] | 285.0 (184–383) | 132.0 (104.0–185.0) | < 0.001 |
| TG post-apheresis [mg/dL] | 126.50 (73–198) | 63.0 (49.5–81.5) | < 0.001 |
| TG [% reduction] | 51.81 (45.2–62.2) | 54.4 (44.3–62.6) | 0.9 |
| Lp(a) pre-apheresis [g/L] | 0.43 (0.22–0.72) | 0.77 (0.35–1.09) | 0.001 |
| Lp(a) post-apheresis [g/L] | 0.08 (0.04–0.13) | 0.17 (0.12–0.24) | < 0.001 |
| Lp(a) [% reduction] | 80.0 (76–83) | 73.0 (64.7–78.8) | < 0.001 |
Data are presented as median (interquartile range [IQR]). Abbreviations — see Table 1.
Figure 1.
Reduction of total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and fibrinogen in DALI vs. MONET. Data are presented as median and interquartile range.
The pre-apheresis concentrations of fibrinogen were similar in DALI and MONET groups. In comparison to MONET, DALI treatment led to a lower removal of fibrinogen (62.5 [52.1–68.0]% vs. 19.8 [13.9–25.2]%; p < 0.001) (Fig. 1). Post-apheresis fibrinogen concentration in MONET sessions was below normal range (1.15 [1.00–1.52] mg/dL).
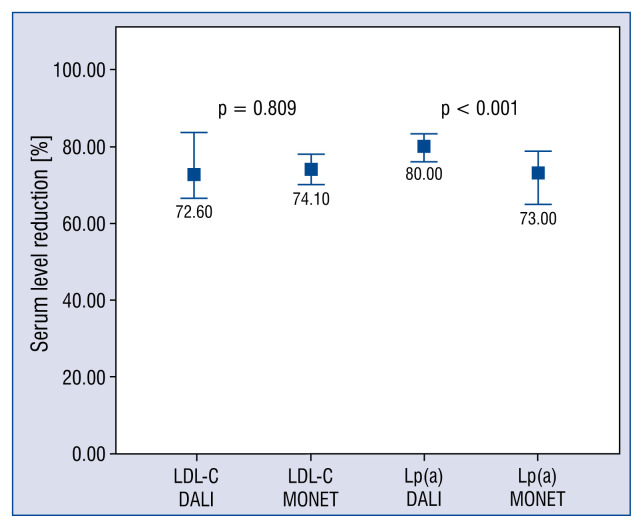
Analyzing all LA therapeutic sessions, the relative reduction of LDL-C up to 69.4 ± 12.9% (71.8 [17.3–98.3]%) was achieved. Comparing both systems, their similar efficacy was found (72.6 [66.5–83.5]% vs. 74.1 [69.9–77.9]%; p = 0.809) (Fig. 2). A large number of LA sessions resulted in at least a 60% reduction of LDL-C (82% of DALI treatments and 78% of MONET treatments).
Figure 2.
Reduction of low density lipoprotein cholesterol (LDL-C) and lipoprotein (a) [Lp(a)] in DALI vs. MONET. Data are presented as a median and interquartile range.
Calculated time-averaged LDL-C was 181.4 ± 72.8 (154.9 [40–395.8]) mg/dL. The DALI system resulted in the achievement of a lower time-averaged LDL-C, than MONET (113.4 vs. 228.5 mg/dL; p < 0.001).
The acute reduction of Lp(a) of 71.3 ± 14.4% (76.1 [21.4–94.1]%) was achieved. It was observed that DALI system was more efficient in relative removal of Lp(a) than MONET (80.0 [76–83]% vs. 73.0 [64.7–78.8]%; p < 0.001) (Fig. 2).
Additional analysis revealed that 54% of all sessions resulted in a post-apheresis LDL-C of less than 70 mg/dL. A higher percent of DALI vs. MONET sessions resulted in decreasing LDL-C below 70 mg/dL (88% vs. 32%).
Side effects
The total incidence of clinical side effects was low (8.5%). Major complications were observed in 2 cases of DALI treatment. One episode of bradykinin syndrome (hypotension, flush, bradycardia and dyspnea) with Quincke odema and lumbar pain was observed. The patient was switched to MONET system. Heparin-induced thrombocytopenia (HIT) with thrombosis in extracorporeal system appeared in another individual. Heparin was replaced by fondaparinux and DALI treatment was continued.
The total incidence of hypotension, vascular problems, and hypocalcemia was low (7.5%, 6.12%, 1.7%, respectively). However, the frequency of complications related to vascular access was higher in MONET sessions vs. DALI (10.5% vs. 1.4%, p = 0.001). Mild, transient hypotension occurred with similar frequency in DALI and MONET (4.9% vs. 9.9%, p = 0.12). Administration of crystalloid infusion before and during apheresis substantially reduced the frequency of symptomatic hypotension. Blood flow at the start and the end of procedures was not related with the incidence of hypotension (Table 4). Hypocalcemia incidence rate was similar during MONET sessions (3.3% vs. 0%, p = 0.06). Anemia occurred in one individual undergoing DALI and one treated by MONET system. Angina episodes were not recorded, as well as abdominal pain.
Table 4.
Influence of crystalloid infusion on hypotension during lipoprotein apheresis (LA) treatment.
| Symptomatic hypotension during apheresis (n = 22) | Apheresis session without hypotension (n = 272) | P | |
|---|---|---|---|
| Crystalloid infusion | 14 (63.63%) | 54 (19.85%) | < 0.001 |
| Crystalloid infusion before procedure | 8 (36.36%) | 39 (14.34%) | 0.01 |
| Crystalloid infusion during procedure | 9 (40.91%) | 20 (7.35%) | < 0.001 |
| Blood flow at start of LA [mL/min] | 52.86 ± 9.16 | 50.81 ± 8.71 | 0.3 |
| Blood flow 2 at the end of LA [mL/min] | 96.00 ± 29.00 | 101.17 ± 20.25 | 0.4 |
Data for blood flow are presented as mean ± standard deviation.
Discussion
The study was undertaken to assess the efficacy and safety of lipoprotein apheresis in a highly selected group of severe FH individuals, by IAS definition. Investigated patients presented a pattern of severe HeFH with an advanced CVD, high LDL-C at initial presentation (> 310 mg/dL) and at least three additional high-risk features. Increased Lp(a) level greater than 50 mg/dL was present in more than half of them (4/7) [28]. Administration of high-intensity oral lipid-lowering agents failed to reduce LDL-C below 160 mg/dL. Although cardiovascular risk in HeFH is largely driven by chronic exposure to elevated LDL-C, cardiovascular risk factors in HeFH are additive, indicating very high CVD risk in the present cohort. Therefore, treatment strategy should be aggressive, targeting an ideal goal of LDL-C below 70 mg/dL. In the current study, LDL-C was acutely reduced from 223 mg/dL to 69 mg/dL, which corresponded to a relative reduction of 69%. Relative decreases of LDL-C greater than 60% was reached in a large number of treatments (close to 80%) indicating a good quality of treatment. In LDL-Apheresis Atherosclerosis Regression Study (LAARS) the achieved acute 63% reduction in LDL-C led to the angiographic arrest of the progression of coronary artery disease in a majority of patients treated with simvastatin and biweekly LA [29]. Other studies carried out in HeFH, on various systems of apheresis reported 55–70% mean LDL-C reduction [21, 30, 31]. Nevertheless, it was found that post-apheresis LDL-C values were below 70 mg/dL only in 54% of LA sessions. When DALI and MONET systems were compared, single DALI sessions achieved LDL below 70 mg/dL more often when compared to MONET (88% vs. 32%). These results may be easily explained by higher pre-apheresis LDL-C observed in patients treated with MONET as compared to DALI. Premature discontinuation of MONET sessions due to adverse events decreased the percent of efficacious LA sessions, compared to other reports [31]. Last but not least, the staff and site experience are a known factor influencing the course of LA sessions [32].
Considering the achievement of a time-averaged LDL-C below 100 mg/dL as a goal of LA therapy, it was out of range in the present study (181 mg/dL). However, it agrees with previous studies. In a large study of 118 patients treated by LA in Dresden, the time-averaged LDL-C was 119.8 mg/dL, similar to the present DALI patients. It may be explained by lower pre-apheresis LDL-C in the Dresden group, than the cohort herein (148.8 mg/dL vs. 223 mg/dL) [33].
The mean reduction of Lp(a) observed in this study was close to 70%. DALI treatment led to larger depletion of Lp(a) than MONET. Ramlow et al. [31] showed equal Lp(a) removal and slightly better efficacy of LDL-C removal for DALI treatment than MONET. It is suspected that a more efficient removal of Lp(a) in the present DALI system might be an effect of an application of larger adsorbers (DALI 1000 and 1250) or higher blood volume processed. However, there are other factors determining the acute lipoprotein reduction in DALI-apheresis such as weight, height, preapheresis lipid levels, as well as blood flow rate through the adsorber.
The present findings also confirmed that direct adsorption and lipoprotein filtration varied in selectivity. MONET substantially reduced fibrinogen concentration, which may improve blood viscosity and its rheological properties. Bleeding complications were not reported, even though 2 patients were administrated with oral anticoagulants. MONET system led to slightly higher HDL-C reduction compared to DALI. However, the reduction was lower, than other lipids. Thus, results agree with previous reports [31].
A mean rate of side effects of 8.5% was observed, which is in the line with data from a large study by Dittrich-Riediger et al. [33]. Serious AE were incidental as in previous reports [34]. Despite ACEI cessation before DALI initiation, bradykinin syndrome was reported in the present study. Another patient treated by DALI was affected by HIT. DALI system is known to cause bradykinin release with peaks at 1000–2000 mL of treated blood volume and ACEI block bradykinin degradation into inactive metabolites. Thus, they are contraindicated in patients treated by DALI system. Angiotensin receptor blockers may be administrated, as in the present case. HIT is an extremely rare complication of LA. However, some authors reported thrombocytopenia previously. The most frequently observed complications of lipoprotein apheresis in the present study were vascular access problems and hypotension, as previously reported by other authors [33, 35]. A higher incidence of vascular access problems was found compared to other studies [23]. In a large multicenter, prospective study of German patients undergoing DALI and MONET apheresis, Kozik-Jaromin et al. [35] reported 27 puncture problems in 3451 sessions [35]. However, hematoma and bleeding as problems with vascular access were also reported herein. Otherwise, some data indicate an increased rate of venous puncture problems were found in female vs. male patients [33]. All investigated individuals in the present cohort were females. Secondly, the type of the vascular access determined issues with its maintenance. Accessing peripheral veins might be the best option for lipoprotein apheresis treatment [36]. In the United Kingdom analysis of peripheral vein cannulation represented even 79% of initial vascular access strategies with AV fistula use accounting for 15%, with a trend to AV cannulation [37]. Unfortunately, due to unavailability of large veins for repeated puncture, arteriovenous fistulas were established in all patients of the present study. Detailed analysis showed that 1 patient undergoing MONET suffered from recurrent stenosis and thrombosis of arteriovenous fistula. Kozik-Jaromin et al. [35] excluded the first 3 months of treatment from analysis, not only the first month of treatment as in the present study. Increased rate of AE during all 12 months of treatment was observed in previous reports.
Mild, transient hypotension, mainly caused by initial “blood donation” into the extracorporeal circuit, occurred with 7.5% frequency. The German Registry of Lipoprotein Apheresis (GLAR) showed a lower rate of hypotension of 1.09–1.28% [21]. However, as demonstrated herein, hypotension may be avoided by crystalloid infusion to the contralateral vein before and during an apheresis session. Some authors have described routine intravenous administration of saline or HAES at apheresis session initiation, and this may have been the reason for this difference. Symptomatic hypocalcemia caused by citrate infusion was rare in the present study. Oral supplementation of calcium prior to a DALI session was introduced in susceptible patients, as well as routine optimization of ACD-A.
Long-term LA with efficient LDL-C and Lp(a) removal was a consequence to improvement of the CVD course in the patients studied. The incidence of major adverse cardiac events (MACE; defined as cardiovascular death, non-fatal acute coronary syndrome and repeat coronary revascularization) decreased after LA initiation. MACE rate was reduced from 41 events before LA inception to 8 during the period of LA treatment. The positive impact of LDL apheresis on cardiovascular morbidity in individuals with hypercholesterolemia has been previously confirmed in several observational trials [38]. Sampietro et al. [39] reported a significant reduction of adverse cardiac or vascular events in 30 individuals with FH or familial combined hypercholesterolemia and CVD. Adverse cardiac or vascular events incidences occurred prior and after LA treatment inception, which were 86 and 15 events, respectively [39].
According to available research, this is the first report focused on the most severe phenotype of HeFH refractory to an equal regimen of statin (rosuvastatin 40 mg daily) at particularly high cardiovascular risk. All patients were female. Thus, gender influence on cardiovascular risk can be omitted. LA in the present study was carried out and documented by one physician at a specialized apheresis center. The treatment and observation period were long.
Study results highlight the importance of more aggressive forms of treatment such as LA in severe HeFH individuals with advanced CVD, additional high-risk features, and LDL-C greater than 160 mg/dL, despite high-intensity statin therapy. The present findings also point to the fact that despite LA there was a substantial unmet need for novel schedules of treatment to control LDL-C in those individuals [4]. In further studies on cardiovascular outcome in real-world practice, it might be interesting to clarify if severe HeFH individuals with CVD and increased Lp(a) concentration benefit from DALI treatment with large adsorbers (1000 or 1250) in combination with novel drugs [40].
Limitations of the study
The present study is small in size, which was caused by a low number of HeFH patients treated by lipoprotein apheresis in Poland. Despite an increase in the proportion of patients treated with strong statins in recent years, treatment goals in hypercholesterolemia are not being achieved [41]. Based on the prevalence of severe FH eligible to LA of 2.4% and approximately 1000 FH individuals with molecular confirmation in Poland (unpublished data), it is estimated that there are 24 severe HeFH, of which 7 are currently being treated with LA [42].
Conclusions
Long-term LA in severe HeFH individuals is safe and efficiently reduces LDL-C and Lp(a). Higher efficacy of DALI system vs. MONET in Lp(a) removal may indicate a need for individualized application of LA system in severe HeFH individuals.
Acknowledgements
This research did not receive any grants from funding agencies in public, commercial, or not-for-profit sectors. We thank all the nurses who participated in lipoprotein apheresis treatment.
Footnotes
This paper was guest edited by Prof. Krzysztof J. Filipiak
Conflict of interest: None declared
References
- 1.Neil A, Cooper J, Betteridge J, et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. 2008;29(21):2625–2633. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besseling J, Kindt I, Hof M, et al. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: a study of a cohort of 14,000 mutation carriers. Atherosclerosis. 2014;233(1):219–223. doi: 10.1016/j.atherosclerosis.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Tada H, Kawashiri Ma, Okada H, et al. Assessment of coronary atherosclerosis in patients with familial hypercholesterolemia by coronary computed tomography angiography. Am J Cardiol. 2015;115(6):724–729. doi: 10.1016/j.amjcard.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 4.Santos R, Gidding S, Hegele R, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 2016;4(10)(16):850–861. 30041–9. doi: 10.1016/s2213-8587. [DOI] [PubMed] [Google Scholar]
- 5.Alonso R, Andres E, Mata N, et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol. 2014;63(19):1982–1989. doi: 10.1016/j.jacc.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 6.Jansen ACM, van Aalst-Cohen ES, Tanck MW, et al. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J Intern Med. 2004;256(6):482–490. doi: 10.1111/j.1365-2796.2004.01405.x. [DOI] [PubMed] [Google Scholar]
- 7.Chan DC, Pang J, Hooper AJ, et al. Elevated lipoprotein(a), hypertension and renal insufficiency as predictors of coronary artery disease in patients with genetically confirmed heterozygous familial hypercholesterolemia. Int J Cardiol. 2015;201:633–638. doi: 10.1016/j.ijcard.2015.08.146. [DOI] [PubMed] [Google Scholar]
- 8.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez de Isla L, Alonso R, Watts GF, et al. Attainment of LDL-Cholesterol Treatment Goals in Patients With Familial Hypercholesterolemia: 5-Year SAFEHEART Registry Follow-Up. J Am Coll Cardiol. 2016;67(11):1278–1285. doi: 10.1016/j.jacc.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Leebmann J, Roeseler E, Julius U, et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128(24):2567–2576. doi: 10.1161/CIRCULATIONAHA.113.002432. [DOI] [PubMed] [Google Scholar]
- 11.Heigl F, Hettich R, Lotz N, et al. Clinical benefit of long-term lipoprotein apheresis in patients with severe hypercholesterolemia or Lp(a)-hyperlipoproteinemia with progressive cardiovascular disease. Clin Res Cardiol Suppl. 2015;10:8–13. doi: 10.1007/s11789-015-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabuchi H, Koizumi J, Shimizu M, et al. Long-term efficacy of low-density lipoprotein apheresis on coronary heart disease in familial hypercholesterolemia. Hokuriku-FH-LDL-Apheresis Study Group. Am J Cardiol. 1998;82(12):1489–1495. doi: 10.1016/s0002-9149(98)00692-4. [DOI] [PubMed] [Google Scholar]
- 13.Thompson GR, Catapano A, Saheb S, et al. Severe hypercholesterolaemia: therapeutic goals and eligibility criteria for LDL apheresis in Europe. Curr Opin Lipidol. 2010;21(6):492–498. doi: 10.1097/MOL.0b013e3283402f53. [DOI] [PubMed] [Google Scholar]
- 14.Ito MK, McGowan MP, Moriarty PM. Management of familial hypercholesterolemias in adult patients: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S38–S45. doi: 10.1016/j.jacl.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Klingel R, Fassbender T, Fassbender C, et al. From membrane differential filtration to lipidfiltration: technological progress in low-density lipoprotein apheresis. Ther Apher Dial. 2003;7(3):350–358. doi: 10.1046/j.1526-0968.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 16.Stefanutti C, Mazza F, Steiner M, et al. Relationship between Sustained Reductions in Plasma Lipid and Lipoprotein Concentrations with Apheresis and Plasma Levels and mRNA Expression of PTX3 and Plasma Levels of hsCRP in Patients with HyperLp(a) lipoproteinemia. Mediators Inflamm. 2016;2016:4739512. doi: 10.1155/2016/4739512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamai O, Matsuoka H, Itabe H, et al. Single LDL apheresis improves endothelium-dependent vasodilatation in hypercholesterolemic humans. Circulation. 1997;95(1):76–82. doi: 10.1161/01.cir.95.1.76. [DOI] [PubMed] [Google Scholar]
- 18.Aengevaeren WR, Kroon AA, Stalenhoef AF, et al. Low density lipoprotein apheresis improves regional myocardial perfusion in patients with hypercholesterolemia and extensive coronary artery disease. LDL-Apheresis Atherosclerosis Regression Study (LAARS) J Am Coll Cardiol. 1996;28(7):1696–1704. doi: 10.1016/s0735-1097(96)00388-9. [DOI] [PubMed] [Google Scholar]
- 19.Leebmann J, Roeseler E, Julius U, et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128(24):2567–2576. doi: 10.1161/CIRCULATIONAHA.113.002432. [DOI] [PubMed] [Google Scholar]
- 20.van Wijk DF, Sjouke B, Figueroa A, et al. Nonpharmacological lipoprotein apheresis reduces arterial inflammation in familial hypercholesterolemia. J Am Coll Cardiol. 2014;64(14):1418–1426. doi: 10.1016/j.jacc.2014.01.088. [DOI] [PubMed] [Google Scholar]
- 21.Schettler VJJ, Neumann CL, Peter C, et al. The German Lipoprotein Apheresis Registry (GLAR) - almost 5 years on. Clin Res Cardiol Suppl. 2017;12(Suppl 1):44–49. doi: 10.1007/s11789-017-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klingel R, Heibges A, Fassbender C. Lipoprotein apheresis for Lp(a)-hyperlipoproteinemia with progressive cardiovascular disease--Additional particular aspects of the Pro(a)LiFe multicenter trial. Atheroscler Suppl. 2015;18:35–40. doi: 10.1016/j.atherosclerosissup.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Heigl F, Hettich R, Lotz N, et al. Efficacy, safety, and tolerability of long-term lipoprotein apheresis in patients with LDL- or Lp(a) hyperlipoproteinemia: Findings gathered from more than 36,000 treatments at one center in Germany. Atheroscler Suppl. 2015;18:154–162. doi: 10.1016/j.atherosclerosissup.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Mickiewicz A, Chmara M, Futema M, et al. Efficacy of clinical diagnostic criteria for familial hypercholesterolemia genetic testing in Poland. Atherosclerosis. 2016;249:52–58. doi: 10.1016/j.atherosclerosis.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Bosch T, Schmidt B, Kleophas W, et al. LDL hemoperfusion — a new procedure for LDL apheresis: first clinical application of an LDL adsorber compatible with human whole blood. Artif Organs. 1997;21(9):977–982. doi: 10.1111/j.1525-1594.1997.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 26.Bosch T, Gahr S, Belschner U, et al. Direct adsorption of low-density lipoprotein by DALI-LDL-apheresis: results of a prospective long-term multicenter follow-up covering 12,291 sessions. Ther Apher Dial. 2006;10(3):210–218. doi: 10.1111/j.1744-9987.2006.00336.x. [DOI] [PubMed] [Google Scholar]
- 27.Kroon AA, van’t Hof MA, Demacker PN, et al. The rebound of lipoproteins after LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis. 2000;152(2):519–526. doi: 10.1016/s0021-9150(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 28.Lewandowski P, Romanowska-Kocejko M, Węgrzyn A, et al. Noninvasive assessment of endothelial function and vascular parameters in patients with familial and nonfamilial hypercholesterolemia. Pol Arch Med Wewn. 2014;124(10):516–524. doi: 10.20452/pamw.2458. [DOI] [PubMed] [Google Scholar]
- 29.Kroon AA, Aengevaeren WR, van der Werf T, et al. LDL-Apheresis Atherosclerosis Regression Study (LAARS). Effect of aggressive versus conventional lipid lowering treatment on coronary atherosclerosis. Circulation. 1996;93(10):1826–1835. doi: 10.1161/01.cir.93.10.1826. [DOI] [PubMed] [Google Scholar]
- 30.Emmrich U, Hohenstein B, Julius U. Actual situation of lipoprotein apheresis in Saxony in 2013. Atheroscler Suppl. 2015;18:215–225. doi: 10.1016/j.atherosclerosissup.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Ramlow W, Röseler E, Heigl F, et al. Efficacy of lipid reduction with DALI and MONET. Atheroscler Suppl. 2017;30:217–224. doi: 10.1016/j.atherosclerosissup.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 32.Julius U, Fischer S, Schatz U, et al. Why an apheresis center should offer more than one lipoprotein apheresis method. Ther Apher Dial. 2013;17(2):179–184. doi: 10.1111/j.1744-9987.2012.01129.x. [DOI] [PubMed] [Google Scholar]
- 33.Dittrich-Riediger J, Schatz U, Hohenstein B, et al. Adverse events of lipoprotein apheresis and immunoadsorption at the Apheresis Center at the University Hospital Dresden. Atheroscler Suppl. 2015;18:45–52. doi: 10.1016/j.atherosclerosissup.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Koziolek MJ, Hennig U, Zapf A, et al. Retrospective analysis of long-term lipid apheresis at a single center. Ther Apher Dial. 2010;14(2):143–152. doi: 10.1111/j.1744-9987.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- 35.Kozik-Jaromin J, Röseler E, Heigl F, et al. Safety aspects of lipidapheresis using DALI and MONET — Multicenter observational study. Atheroscler Suppl. 2017;30:225–231. doi: 10.1016/j.atherosclerosissup.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Kalantari K. The choice of vascular access for therapeutic apheresis. J Clin Apher. 2012;27(3):153–159. doi: 10.1002/jca.21225. [DOI] [PubMed] [Google Scholar]
- 37.Doherty DJ, Pottle A, Malietzis G, et al. Vascular access in lipoprotein apheresis: a retrospective analysis from the UK’s largest lipoprotein apheresis centre. J Vasc Access. 2018;19(1):52–57. doi: 10.5301/jva.5000755. [DOI] [PubMed] [Google Scholar]
- 38.Leebmann J, Roeseler E, Julius U, et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128(24):2567–2576. doi: 10.1161/CIRCULATIONAHA.113.002432. [DOI] [PubMed] [Google Scholar]
- 39.Sampietro T, Sbrana F, Bigazzi F, et al. The incidence of cardiovascular events is largely reduced in patients with maximally tolerated drug therapy and lipoprotein apheresis. A single-center experience. Atheroscler Suppl. 2015;18:268–272. doi: 10.1016/j.atherosclerosissup.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 40.Saborowski M, Dölle M, Manns MP, et al. Lipid-lowering therapy with PCSK9-inhibitors in the management of cardiovascular high-risk patients: Effectiveness, therapy adherence and safety in a real world cohort. Cardiol J. 2018;25(1):32–41. doi: 10.5603/CJ.a2017.0137. [DOI] [PubMed] [Google Scholar]
- 41.Kapłon-Cieślicka A, Michalak M, Kołtowski Ł, et al. How has the treatment of hypercholesterolemia in Poland changed over the last six years? Cardiol J. 2017;24(3):266–275. doi: 10.5603/CJ.a2017.0047. [DOI] [PubMed] [Google Scholar]
- 42.Vishwanath R, Hemphill LC. Familial hypercholesterolemia and estimation of US patients eligible for low-density lipoprotein apheresis after maximally tolerated lipid-lowering therapy. J Clin Lipidol. 2014;8(1):18–28. doi: 10.1016/j.jacl.2013.11.002. [DOI] [PubMed] [Google Scholar]