Abstract
Background
Coronary artery disease (CAD) affects milions of people and can result in myocardial infarction (MI). Previously, mast cells (MC) have been extensively investigated in the context of hypersensitivity, however as regulators of the local inflammatory response they can potentially contribute to CAD and/or its progression. The aim of the study was to assess if serum concentration of MC proteases: carboxypeptidase A3, cathepsin G and chymase 1 is associated with the extension of CAD and MI.
Methods
The 44 patients with angiographically confirmed CAD (23 subjects with non-ST-segment elevation MI [NSTEMI] and 21 with stable CAD) were analyzed. Clinical data were obtained as well serum concentrations of carboxypeptidase A3, cathepsin G and chymase 1 were also measured.
Results
Patients with single vessel CAD had higher serum concentration of carboxypeptidase than those with more advanced CAD (3838.6 ± 1083.1 pg/mL vs. 2715.6 ± 442.5 pg/mL; p = 0.02). There were no significant differences in levels of any protease between patients with stable CAD and those with NSTEMI. Patients with hypertension had ≈2-fold lower serum levels of cathepsin G than normotensive individuals (4.6 ± 0.9 pg/mL vs. 9.4 ± 5.8 pg/mL; p = 0.001). Cathepsin G levels were also decreased in sera of the current smokers as compared with non-smokers (3.1 ± 1.2 ng/mL vs. 5.8 ± 1.2 ng/mL, p = 0.02).
Conclusions
Decreased serum level of carboxypeptidase is a hallmark of more advanced CAD. Lower serum levels of carboxypeptidase A3 and catepsin G are associated with risk factors of blood vessel damage suggesting a protective role of these enzymes in CAD.
Keywords: mast cells, carboxypeptidase A3, cathepsin G, chymase 1, coronary artery disease
Introduction
Chronic inflammation plays an important role in the pathogenesis of coronary artery disease (CAD) and acute myocardial infarction (AMI).The link between mast cell (MC) proteases and a local inflammation process is an attractive research area. After activation, MC releases a wide range of proteases that have a potential pro or anti-inflammatory effect [1–3]. The major MC proteases include tryptase, chymase 1, carboxypeptidase A3 and cathepsin G.
In previously published work, it was shown that tryptase and endothelin-1 released from activated MC are elevated in patients with an AMI [4]. Therefore, in the current study theaim was to analyze if other MC proteases chymase 1, carboxypeptidase A3 and cathepsin G play also have a role in CAD and AMI.
Chymase 1 is a serine protease stored in MC. It is released after stimulation during an inflammatory or ischemic injury that is known to be a hallmark of AMI. The protein release is associated with activation of matrix metalloproteinase-9, which was shown to increase infarct size in an experimental model [5]. Oyamada et al. [6] have shown, that chymase 1 inhibition results in myocardial protection and attenuates fibrosis after AMI. In addition, chymase plays a crucial role in transformation of angiotensin I to angiotensin II independent from angiotensin converting enzyme [7].
Cathepsin G is a serine protease also synthesized and stored in MC. However, MC are not the only source of the enzyme, it is also released from activated neutrophils and macrophages [8–10]. Despite that cathepsin G may promote early atherogenesis as it is an elastase [11] and collagenase activator [12], Wang et al. [13] suggested that cathepsin G promotes early atherogenesis through its elastinolytic activity, but at the same time suppresses late progression of atherosclerosis. In their study, patients with atherosclerosis had significantly reduced plasma levels of cathepsin G that were in negative correlation with total cholesterol and low density lipoprotein (LDL), but not high density lipoprotein (HDL) or triglycerides, suggesting a role of cathepsin G in degradation of LDL without affecting HDL or triglycerides [13].
A carboxypeptidase A3 (CPA3) is a zinc metalloprotease that is released from MCs and basophils as well. This enzyme degrades proteins and peptides, including the apolipoprotein B; a component of LDL particles [14]. Upon MC activation and degranulation, CPA3 with the chymases and tryptases interacts with heparin proteoglycans [15]. It was shown to play a role in the inactivation of endothelin [16, 17] and degradation of angiotensin II [18], which suggests its antihypertensive activity.
The aim of the study was to check if a concentration of MC derived proteases is elevated in sera of patients with different extensions of CAD, and thus to assess the role of MC in its pathogenesis.
Methods
This was a prospective and single-center study. The study was conducted according to the Declaration of Helsinki and the protocol was reviewed and approved by the local ethics committee. All patients gave written informed consent.
Patients
Between November 2012 and May 2013, 44 consecutive patients were prospectively screened who underwent diagnostic coronary angiography because of non-ST-segment elevation myocardial infarction (NSTEMI) or stable angina with a positive stress test. All the procedures were performed at the Department of Invasive Cardiology, Pomeranian Cardiology Centers, Wejherowo, Poland. Patients with renal failure, malignancy, and acute or chronic inflammatory disease were excluded from the study. Finally, 44 patients (23 NSTEMI and 21 with stable angina) were included. Complete demographic and clinical data were obtained. Accordingly to the extension of CAD, patients were divided into two groups: subjects with one vessel CAD and those with two or three vessel CAD.
Blood sampling and laboratory tests
The blood samples were obtained after puncture of a radial or a femoral artery and they were drawn from the vascular sheath during a coronary angiography.
Then, blood samples were centrifuged at 1000 × g for 15 min to obtain serum. Subsequently, standard clinical parameters were measured and the remaining serum was apportioned into 0.5 mL aliquots, and stored at −80°C until analysis of CPA3, cathepsin G and chymase 1.
Measurement of CPA3, cathepsin G and chymase 1
Carboxypeptidase A3, cathepsin G and chymase 1 concentrations were measured with ELISA (Cloud-Clone Corp., Houston, TX, USA) according to manufacturer instructions. The lower limit of detection of CPA3, cathepsin G and chymase 1 was < 3.0 pg/mL, < 0.065 ng/mL and < 13.5 pg/mL, respectively. Protein concentration was measured spectrophotometrically (Perkin Elmer VICTOR X4) at a wavelength of 450 ± 10 nm and was determined by comparing the O.D. of samples to a standard curve.
Statistical analysis
The results are expressed as mean ± standard deviation. Comparisons between groups were performed using the t-Student test for continuous variables and χ2 test for categorical variables. Wizard Statistics 1.8.16 software was used for analysis, p-value < 0.05 was considered statistically significant.
Results
The demographic and biochemical data of 44 patients with significant CAD are presented in Table 1. Patients with more advanced CAD had more often had a history of previous myocardial infarction (MI).
Table 1.
Demographic and biochemical data of 44 patients with significant coronary artery disease (CAD) according to the extension of CAD.
| 1 vessel CAD (n = 11) | 2–3 vessel CAD (n = 33) | P | |
|---|---|---|---|
| Age [years] | 68.3 ± 7.1 | 67.5 ± 2.6 | 0.7 |
| Male/female | 5/6 | 19/14 | 0.5 |
| Body mass index [kg/m2] | 30.7 ± 3.7 | 29.9 ± 1.5 | 0.6 |
| Total cholesterol [mg/dL] | 220.1 ± 48.1 | 171.3 ± 17 | 0.01 |
| LDL cholesterol [mg/dL] | 158.6 ± 46.8 | 98.4 ± 16 | 0.002 |
| HDL cholesterol [mg/dL] | 45.7 ± 9.3 | 44.6 ± 4.2 | 0.8 |
| Serum creatinine [mg/dL] | 1.7 ± 1.3 | 1.2 ± 0.1 | 0.1 |
| eGFR | 45.9 ± 11.4 | 53.5 ± 4.4 | 0.1 |
| LVEF [%] | 48.2 ± 5.5 | 47.6 ± 4.3 | 0.9 |
| Arterial hypertension | 11 | 13 | 0.2 |
| Diabetes | 4 | 15 | 0.6 |
| History of previous MI | 2 | 24 | 0.005 |
| History of stroke/TIA | 3 | 7 | 0.7 |
| Current smoker | 4 | 18 | 0.7 |
eGFR — estimated glomerular filtration rate; HDL — high density lipoprotein; LDL — low density lipoprotein; LVEF — left ventricular ejection fraction; MI — myocardial infarction; TIA — transient ischemic attack
The major clinical and biochemical data of 23 NSTEMI patients were compared to subjects with stable CAD are listed in Table 2. Patients with stable CAD had more often had a history of MI in the past and presented more often with three vessel CAD compared to NSTEMI patients. On the other hand, subjects with NSTEMI had significantly higher levels of C-reactive protein and lower glomerular filtration rate.
Table 2.
Demographic, biochemical and clinical data of 23 patients with non-ST-elevation myocardial infarction (NSTEMI) and 21 patients with stable coronary artery disease (CAD).
| NSTEMI (n = 23) | Stable CAD (n = 21) | P | |
|---|---|---|---|
| Age [years] | 68.5 ± 3.8 | 66.8 ± 3.3 | 0.5 |
| Body mass index [kg/m2] | 30 ± 1.7 | 30.3 ± 2.3 | 0.8 |
| Total cholesterol [mg/dL] | 198.1 ± 28 | 167.5 ± 20.9 | 0.08 |
| LDL cholesterol [mg/dL] | 124.9 ± 27.3 | 100.9 ± 22.5 | 0.2 |
| HDL cholesterol [mg/dL] | 44.3 ± 5.3 | 45.6 ± 5.6 | 0.7 |
| Serum creatinine [mg/dL] | 1.5 ± 0.6 | 1.1 ± 0.1 | 0.2 |
| eGFR | 47.3 ± 6.6 | 56.4 ± 4.8 | 0.03 |
| CRP | 33.2 ± 25.6 | 5.3 ± 2.8 | 0.03 |
| Arterial hypertension | 21 | 18 | 0.5 |
| Diabetes | 9 | 10 | 0.6 |
| History of previous MI | 7 | 17 | 0.001 |
| History of stroke/TIA | 3 | 0 | 0.09 |
| Current smoker | 6 | 4 | 0.6 |
| 3 vessel CAD/1–2 vessel CAD | 3/20 | 9/12 | 0.03 |
| LVEF [%] | 45.9 ± 4.2 | 49.7 ± 5.8 | 0.3 |
eGFR — estimated glomerular filtration rate; CRP — C-reactive protein; HDL — high density lipoprotein; LDL — low density lipoprotein; LVEF — left ventricular ejection fraction; MI — myocardial infarction; TIA — transient ischemic attack
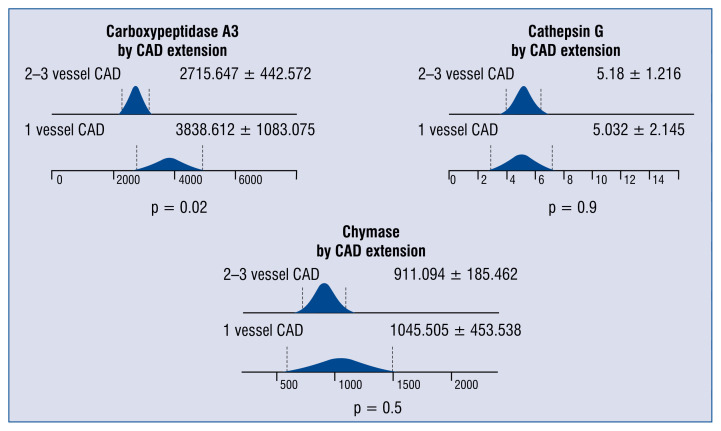
Patients with one vessel CAD presented a significantly higher level of carboxypeptidase than those with more advanced CAD. Neither the cathepsin G nor chymase differed between the two groups (Fig. 1).
Figure 1.
The differences in serum concentrations of carboxypeptidase A3 (pg/mL), chymase 1 (pg/mL) and cathepsin G (ng/mL) in patients with single vessel coronary artery disease (CAD) and 2 or3 vessel CAD.
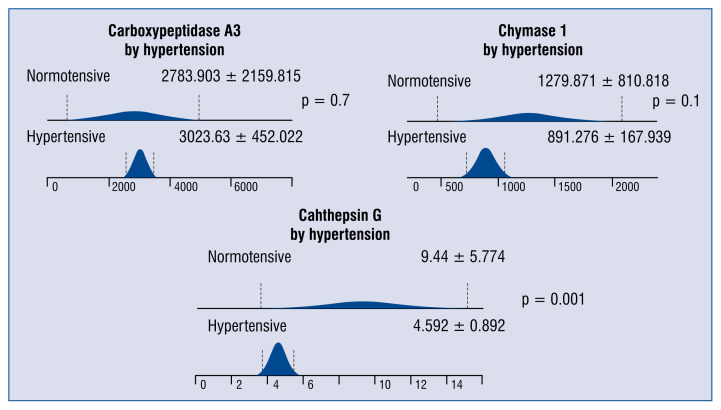
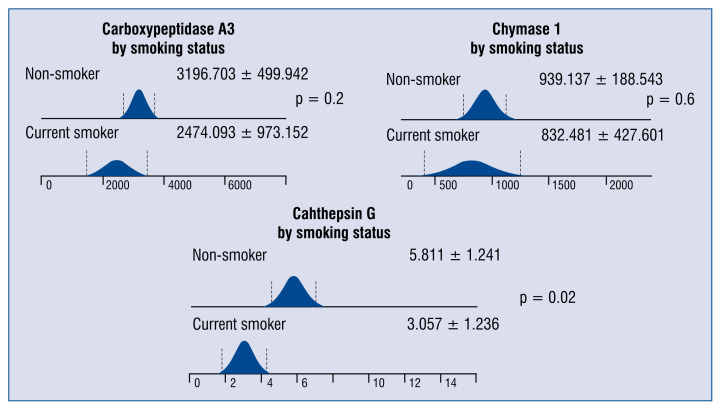
Significantly lower levels of cathepsin G were observed among patients with CAD and hypertension as compared with normotensive CAD subjects (Fig. 2). Lower serum concentrations of cathepsin G in individuals with CAD were also associated with smoking (Fig. 3).
Figure 2.
The differences in serum concentrations of carboxypeptidase A3 (pg/mL), chymase 1 (pg/mL) and cathepsin G (ng/ml) among patients with or without hypertension.
Figure 3.
The differences in serum concentrations of carboxypeptidase A3 (pg/mL), chymase 1 (pg/mL) and cathepsin G (ng/ml) among patients with coronary artery disease (CAD) according to their smoking status.
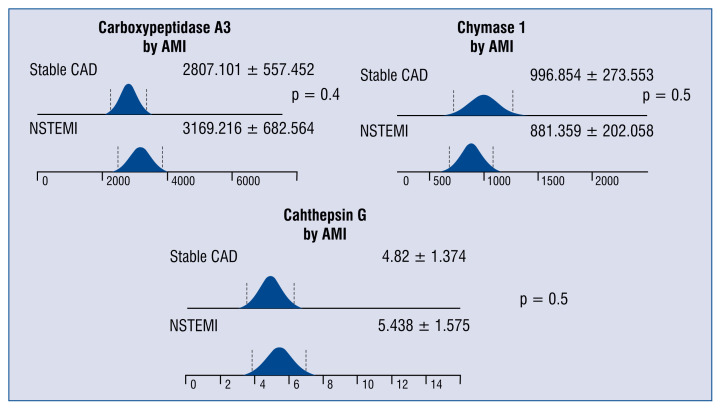
There were no statistically significant differences in levels of any protease between patients with stable CAD and those with NSTEMI (Fig. 4).
Figure 4.
The differences in serum concentrations of carboxypeptidase A3 (pg/mL), chymase 1 (pg/mL) and cathepsin G (ng/ml) in patients with stable coronary artery disease (CAD) and non-ST-segment elevation myocardial infarction (NSTEMI); AMI — acute myocardial infarction.
Discussion
Mast cells have been extensively investigated in the context of hypersensitivity [19] and little is known about their influence on the human body apart from allergic inflammation. However, in the present study it was shown that serum concentration of MC derived enzyme CPA3 was decreased in patients with more advanced CAD. In addition, higher levels of cathepsin G were found in normotensive CAD patients and in those who were non-smokers. This data has shed new light on MC and the role of their activation in cardiovascular diseases.
Previously, Xiang et al. [20] reported increased chymase concentration in patients with MI. However, there is no data concerning a possible association of carboxypeptidase and presence of CAD. In the current study, significantly higher levels of CPA3 were observed in patients with angiographically confirmed single vessel CAD as compared with subjects with advanced two or three vessel disease.
Carboxypeptidase A3 and chymase 1 play a key role in the formation as well as degradation of angiotensin II [18]. Whereas chymase is the main tissue converter of angiotensin I to angiotensin II, CPA3 creates peptides Ang-(1–9) and Ang-(1–7) that antagonize angiotensin II [18]. The peptide angiotensin II is not only a strong vasoconstrictor, but it also can promote atherosclerosis via several biologic activities such as: an increased expression of adhesion molecules on endothelial cells, activation of macrophages and upregulation of matrix metalloproteinases and proinflammatory cytokines [21, 22]. Therefore, as an indirect antagonist of angiotensin II, CPA3 exerts a protective effect in CAD. CPA3 was also found to degrade another vasoconstrictor — endothelin-1 and was found to play a cardioprotective effect during ischemia-reperfusion injury [23]. In addition, the present study revealed that lower serum concentration of the peptide was characteristic for patients with more advanced CAD and also for those who were active smokers. As smoking is one of the most widely known risk factors of cardiovascular diseases [24], this data suggests that one of the mechanisms of the deleterious impact of smoking on blood vessels might be an inhibitor of CPA3 production. Thus, all these data together confirms a protective effect of this enzyme in CAD.
When NSTEMI patients were compared with those having stable CAD, nosignificant differences were found in the levels of any of the proteases studied. Previously Xiang et al. [20] reported that serum chymase levels were higher in patients with AMI compared to subjects with stable CAD, however this difference was not statistically significant [20]. The lack of significant differences in serum levels of chymase between patients with MI and stable CAD in the cited study and chymase and carboxypeptidase of the present research may reflect the fact that an underlying mechanism of angiotensin II regulation influences chronic atherosclerosis rather than a formation of unstable coronary plaque and consequently AMI.
The concentration of cathepsin G was significantly lower in current smokers and hypertensive patients than in non-smokers and normotensive individuals, respectively. Wang et al. [13] has shown, that cathepsin G promoted early atherogenesis through its elastinolytic activity, but on the other hand it suppressed late progression of atherosclerosis by degrading LDL. In their study patients with CAD were characterized with significantly lower levels of cathepsin G and higher levels of LDL, than individuals without CAD [13]. In the present study, decreased serum concentration of cathepsin G in CAD patients was associated with tabacco smoking or hypertension. Cathepsin G was found to exert an anti-inflammatory effect by reducing biological activity of inflammatory cytokines [25]. As both hypertension and smoking are associated with inflammation and increased production of pro-inflammatory cytokines [26], lower levels of protease in these patients may reflect an exhaustion of its production in chronic inflammation.
Conclusions
Decreased serum concentration of CPA3 is a hallmark of more advanced CAD.
Lower serum levels of CPA3 and catepsin G are associated with risk factors of blood vessel damage suggesting a protective role of these enzymes in CAD.
Acknowledgements
This study was supported with funds from the Polish National Science Center on the basis of Decision no. DEC-2012/07/B/NZ5/00017. The founders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest: None declared
References
- 1.Kaartinen M, Penttilä A, Kovanen PT. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation. 1994;90(4):1669–1678. doi: 10.1161/01.cir.90.4.1669. [DOI] [PubMed] [Google Scholar]
- 2.Kaartinen M, Penttilä A, Kovanen PT. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNF-alpha. Circulation. 1996;94(11):2787–2792. doi: 10.1161/01.cir.94.11.2787. [DOI] [PubMed] [Google Scholar]
- 3.Zhao W, Oskeritzian CA, Pozez AL, et al. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175(4):2635–2642. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 4.Lewicki L, Siebert J, Marek-Trzonkowska N, et al. Elevated Serum Tryptase and Endothelin in Patients with ST Segment Elevation Myocardial Infarction: Preliminary Report. Mediators Inflamm. 2015;2015:395173. doi: 10.1155/2015/395173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oyamada S, Bianchi C, Takai S, et al. Impact of acute myocardial ischemia reperfusion on the tissue and blood-borne renin-angiotensin system. Basic Res Cardiol. 2010;105(4):513–522. doi: 10.1007/s00395-010-0093-4. [DOI] [PubMed] [Google Scholar]
- 6.Oyamada S, Bianchi C, Takai S, et al. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. J Pharmacol Exp Ther. 2011;339(1):143–151. doi: 10.1124/jpet.111.179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei CC, Tian B, Perry G, et al. Differential ANG II generation in plasma and tissue of mice with decreased expression of the ACE gene. Am J Physiol Heart Circ Physiol. 2002;282(6):H2254–H2258. doi: 10.1152/ajpheart.00191.2001. [DOI] [PubMed] [Google Scholar]
- 8.Helske S, Syväranta S, Kupari M, et al. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur Heart J. 2006;27(12):1495–1504. doi: 10.1093/eurheartj/ehi706. [DOI] [PubMed] [Google Scholar]
- 9.Owen CA, Campbell EJ. Angiotensin II generation at the cell surface of activated neutrophils: novel cathepsin G-mediated catalytic activity that is resistant to inhibition. J Immunol. 1998;160(3):1436–1443. [PubMed] [Google Scholar]
- 10.Lindstedt KA, Mäyränpää MI, Kovanen PT. Mast cells in vulnerable atherosclerotic plaques--a view to a kill. J Cell Mol Med. 2007;11(4):739–758. doi: 10.1111/j.1582-4934.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudier C, Godeau G, Hornebeck W, et al. The elastolytic activity of cathepsin G: an ex vivo study with dermal elastin. Am J Respir Cell Mol Biol. 1991;4(6):497–503. doi: 10.1165/ajrcmb/4.6.497. [DOI] [PubMed] [Google Scholar]
- 12.Chatham WW, Blackburn WD, Heck LW. Additive enhancement of neutrophil collagenase activity by HOCl and cathepsin G. Biochem Biophys Res Commun. 1992;184(2):560–567. doi: 10.1016/0006-291x(92)90626-v. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Sjöberg S, Tang TT, et al. Cathepsin G activity lowers plasma LDL and reduces atherosclerosis. Biochim Biophys Acta. 2014;1842(11):2174–2183. doi: 10.1016/j.bbadis.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokkonen JO, Vartiainen M, Kovanen PT. Low density lipoprotein degradation by secretory granules of rat mast cells. Sequential degradation of apolipoprotein B by granule chymase and carboxypeptidase A. J Biol Chem. 1986;261(34):16067–16072. [PubMed] [Google Scholar]
- 15.Schwartz LB, Riedel C, Schratz JJ, et al. Localization of carboxypeptidase A to the macromolecular heparin proteoglycan-protein complex in secretory granules of rat serosal mast cells. J Immunol. 1982;128(3):1128–1133. [PubMed] [Google Scholar]
- 16.Irani AM, Goldstein SM, Wintroub BU, et al. Human mast cell carboxypeptidase. Selective localization to MCTC cells. J Immunol. 1991;147(1):247–253. [PubMed] [Google Scholar]
- 17.Dougherty RH, Sidhu SS, Raman K, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010;125(5):1046–1053.e8. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundequist A, Tchougounova E, Abrink M, et al. Cooperation between mast cell carboxypeptidase A and the chymase mouse mast cell protease 4 in the formation and degradation of angiotensin II. J Biol Chem. 2004;279(31):32339–32344. doi: 10.1074/jbc.M405576200. [DOI] [PubMed] [Google Scholar]
- 19.Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol. 2011;31(6):475–529. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang M, Sun J, Lin Y, et al. Usefulness of serum tryptase level as an independent biomarker for coronary plaque instability in a Chinese population. Atherosclerosis. 2011;215(2):494–499. doi: 10.1016/j.atherosclerosis.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sata M, Fukuda D. Crucial role of renin-angiotensin system in the pathogenesis of atherosclerosis. J Med Invest. 2010;57(1–2):12–25. doi: 10.2152/jmi.57.12. [DOI] [PubMed] [Google Scholar]
- 22.da Silva AR, Fraga-Silva RA, Stergiopulos N, et al. Update on the role of angiotensin in the pathophysiology of coronary atherothrombosis. Eur J Clin Invest. 2015;45(3):274–287. doi: 10.1111/eci.12401. [DOI] [PubMed] [Google Scholar]
- 23.Parikh V, Singh M. Possible role of adrenergic component and cardiac mast cell degranulation in preconditioning-induced cardioprotection. Pharmacol Res. 1999;40(2):129–137. doi: 10.1006/phrs.1999.0501. [DOI] [PubMed] [Google Scholar]
- 24.Najder A. Sense of coherence, smoking status, biochemical cardiovascular risk factors and body mass in blue collar workers-short report. Am J Mens Health. 2018 doi: 10.1177/1557988317748393. [Epub ahead of print] 1557988317748393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caughey GH. Mast cell proteases as protective and inflammatory mediators. Adv Exp Med Biol. 2011;716:212–234. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virdis A, Giannarelli C, Neves MF, et al. Cigarette smoking and hypertension. Curr Pharm Des. 2010;16(23):2518–2525. doi: 10.2174/138161210792062920. [DOI] [PubMed] [Google Scholar]