Abstract
BACKGROUND
Autoimmune pulmonary alveolar proteinosis (aPAP) is a rare disease characterized by progressive surfactant accumulation and hypoxemia caused by disruption of signaling by granulocyte/macrophage-colony stimulating factor (GM-CSF), which pulmonary alveolar macrophages require to clear surfactant. Recently, inhaled GM-CSF was shown to improve arterial oxygen tension in aPAP patients.
METHODS
We conducted a double-blind, placebo-controlled, 3-group study in 138 aPAP patients randomized to receive molgramostim (300 μg/day) continuously (n=46) or intermittently every other week (n=45), or matching placebo (n=47) by once-daily inhalation for 24-weeks, followed by an open-label treatment-extension period.
RESULTS
After excluding invalid A-aDO2 data for four patients who received nasal oxygen therapy during arterial blood gas measurement (1 in each molgramostim group and 2 in placebo), improvement in the primary endpoint – change in A-aDO2 from baseline to 24 weeks – was greater in patients receiving continuous molgramostim than placebo (estimated treatment difference (ETD) −6.2 mmHg, P=0.025, least square mean (LSMean) comparison). Patients receiving continuous molgramostim also had an improvement in secondary endpoints compared to placebo including Saint George’s Respiratory Questionnaire total score (ETD −7.4, P=0.012, LSMean). Improvement in multiple endpoints was greater for continuous than intermittent molgramostim administration. Rates of adverse events and serious adverse events were similar among groups.
CONCLUSION
Daily molgramostim administration improved clinical, physiological, biochemical, and radiologic outcome measures and was safe in patients with aPAP. Inhaled molgramostim may be useful as therapy of aPAP.
Autoimmune pulmonary alveolar proteinosis (aPAP) is an alveolar filling disease characterized by progressive accumulation of surfactant in alveoli, hypoxemia and, in some patients, development of serious infections or pulmonary fibrosis.1-3 Although multiple mechanistically distinct PAP-causing diseases exist, aPAP accounts for 90% of cases4 and has a reported prevalence of 7 – 27 per million.4-6 Pathogenesis is driven by autoantibodies that block GM-CSF signaling,7-10 which alveolar macrophages require to clear surfactant from alveoli.11-13 Disease progression is associated with an increase in the alveolar-arterial difference in oxygen concentration (A-aDO2) due to reduced arterial oxygen tension (PaO2), and reduced diffusing capacity for carbon monoxide (DLCO), restrictive lung function impairment, ground glass opacification (GGO) of the lungs seen by computed tomography (CT), progressive dyspnea, polycythemia (a systemic manifestation of chronic hypoxemia), and increased serum biomarkers.3,14
No drugs are approved as therapy of aPAP in any country; aPAP is treated by whole lung lavage (WLL), a procedure to physically remove excess surfactant sediment. Since alveoli are typically well-preserved in aPAP, an effective therapy – one that removes the excess accumulated surfactant sediment – would be expected to improve multiple disease manifestations simultaneously. Preclinical studies,15-17 case-reports, and small open-label trials all indicate inhaled GM-CSF improves lung function as well as clinical and radiographic manifestations of aPAP.18-23 A meta-analysis found GM-CSF therapy of aPAP was effective and that administration by inhalation was superior to subcutaneous injection.24 A recent controlled trial found administration of inhaled GM-CSF for 24 weeks improved arterial oxygen tension and radiologically-measured lung density.25 However, no controlled trials have reported inhaled GM-CSF improves clinical manifestations of aPAP.
Inhaled molgramostim, an Escherichia coli-produced recombinant GM-CSF formulated as a nebulizer solution, is pharmacologically active after aerosolization and well-tolerated in healthy people.26 Here, we report a controlled study showing inhaled molgramostim is safe, well-tolerated, and improves gas exchange and functional health status in patients with aPAP.
METHODS
PATIENTS
Eligible patients were at least 18 years of age; had a diagnosis of aPAP based on a chest CT, lung biopsy, or bronchoalveolar lavage (BAL) cytology; and a positive serum GM-CSF autoantibody test. Patients were required to have an A-aDO2 of ≥25 mmHg; PaO2 of <75 mmHg at rest or a desaturation of >4% points during a 6-minute walk test (6MWT); and unremitting or progressive aPAP judged by improvement of less than 5 % in the vital capacity (VC) or 10% in DLCO within two months before enrollment. Exclusion criteria included pregnancy, breast-feeding, a PAP-causing disease other than aPAP; and treatment with carbocysteine or ambroxol currently or WLL, GM-CSF, plasmapheresis, or rituximab, respectively, within 1, 3, or 6 months before enrollment. Patients provided written informed consent. Detailed eligibility criteria are provided in the Supplementary Appendix.
OVERSIGHT
The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice and underwent periodic review by an independent data safety monitoring committee. The protocol was designed by the sponsor, Savara Pharmaceuticals, in collaboration with the investigators and approved by the institutional review board/ethics committee at each participating site. The sponsor gathered and analyzed the data. The authors (B.C.T. and G.W.) decided to publish the paper and wrote the first draft; all authors had access to the data, contributed to writing and approval of the final manuscript, and attest to the accuracy and completeness of the data and the fidelity of trial conduct to the protocol, which is available at NEJM.org.
TRIAL DESIGN AND TREATMENTS
This trial was conducted at 34 sites in 18 countries; included a 24-week, double-blind, placebo-controlled intervention period comprising a baseline and monthly follow-up visits; and an open-label treatment extension period of up to 48 weeks. Patients were randomized 1:1:1 into three groups to receive inhaled molgramostim (300 μg/day) every week or every other week, or matched placebo – continuous molgramostim, intermittent molgramostim, and placebo groups, respectively; administered once daily using a vibrating mesh nebulizer (eFlow; PARI Pharma, Germany). During the blinded intervention period, patients receiving intermittent molgramostim received placebo on ‘off’-weeks to maintain blinding. During the open-label period, all patients received molgramostim (300 μg/day) every other week because prior open-label studies had shown positive results for intermittent administration.22,27 Patients were stratified by receipt of WLL within two months before baseline. For ethical reasons, administration of supplemental oxygen therapy (to maintain adequate blood oxygen levels) and WLL (as rescue therapy for PAP progression) were permitted according to the protocol and administered at the discretion of site investigators. Patients receiving such therapy continued receiving study drug as assigned. Details of trial sites, design, and management are included in the Supplemental Appendix.
ENDPOINTS AND ASSESSMENTS
The primary endpoint, the change in the A-aDO2 from baseline to week 24, was chosen based on investigator recommendations and extensive use in prior studies.22,25. Key secondary endpoints informing direct patient benefit included functional health status measured using the St. George’s Respiratory Questionnaire (SGRQ) total score28, distance covered on a 6-minute walk test (6MWT-distance)29 – both evaluated as change from baseline to 24 weeks, and the use of WLL – evaluated as a time-to-event analysis after randomization. Other endpoints included change from baseline to week 24 in DLCO,30 SGRQ component scores (activity, impact, symptoms), number of WLL’s, forced expiratory volume in one second (FEV1), forced vital capacity (FVC), vital capacity (VC),31 GGO score on chest CT,22 and serum biomarkers (Table S1 in the Supplementary Appendix). These endpoints permitted evaluation of a range of PAP-pathogenesis-driven abnormalities. Safety was assessed by monitoring adverse events. Additional pre specified outcome variables and details regarding trial assessments, data collection, analysis, and interpretation are provided in the Supplemental Appendix.
STATISTICAL ANALYSIS
The study was initially powered to detect a difference in mean change in A-aDO2 of 10 mmHg between molgramostim- and placebo-treated patients. After meeting with the US Food and Drug Administration, the trial was expanded to include US sites, several outcome measures (SGRQ total score, 6MWT-distance, and time from baseline to first WLL use) were promoted as key secondary endpoints, and the sample size was recalculated to detect a difference in mean change in 6MWT-distance of 50 m between the continuous molgramostim group and placebo group with a power of 90% at the 5% significance level.
As prespecified in the statistical analysis plan (SAP - available at NEJM.org), the primary endpoint was evaluated using analysis of covariance (ANCOVA) with treatment, WLL within two months before baseline (stratification), and geographic region (Japan vs other countries) as factors, and baseline values as covariates; a least squares mean (LSMean)-based P-value of <5% was considered to indicate statistical significance. To control type I error, key secondary endpoints were analyzed using a testing hierarchy (Fig. S1A in the Supplemental Appendix) wherein the continuous molgramostim and placebo groups were compared first and if statistical significance was reached for comparison of any key secondary endpoints, then evaluation of the intermittent molgramostim and placebo groups would proceed, first by evaluation of the primary endpoint and if statistical significance was reached, then by comparison of the key secondary endpoints. The threshold indicating statistical significance for analyses of key secondary endpoints was adjusted for multiplicity using the truncated Hochberg procedure. Analyses of all other endpoints were considered supportive, not adjusted for multiplicity and P-values are not reported.
The full analysis set (FAS) included results for all patients receiving at least one dose of intervention (Fig. S2 in the Supplemental Appendix) and was used for the initial analysis of all numeric data. After unblinding and prespecified analysis, a non-physiologic (large negative) A-aDO2 value (−42 mmHg) was identified in one patient. A thorough re-examination of the data revealed this patient and three others (one in the continuous molgramostim group, one in the intermittent molgramostim group, and two in the placebo group) had undergone blood gas analysis while breathing supplemental oxygen via nasal canula, which precluded calculation of the true A-aDO2 because the FiO2 was unknown.32 Therefore, a revised FAS was established treating the invalid blood gas results for these four patients as missing data (with replacement by multiple imputation) and used for analysis of the primary endpoint. Although not prespecified, for consistency, missing data for the primary endpoint, key secondary endpoints, and GGO, but not other endpoints, during the double-blind period were replaced using multiple imputation (details provided in the Supplemental Appendix). The FAS was used for analysis of all other endpoints. Numerical results are presented as mean (±SD) and analyses were performed using SAS software (version 9.4; SAS Institute).
RESULTS
PATIENTS
From February 2016 through May 2019, 235 patients were assessed for eligibility and 138 with aPAP were randomized and received continuous molgramostim (n=46), intermittent molgramostim (n=45), or placebo (n=47) (Fig. S2 in the Supplemental Appendix). Of these, 97.8% of patients in each molgramostim group and 93.6% of patients in the placebo group completed the blinded intervention period and 131 were enrolled into an open-label treatment extension period. One third of patients required supplemental oxygen therapy during the study. The baseline characteristics were similar among the groups (Table 1, Table S2 in the Supplemental Appendix).
Table 1.
Selected Clinical Characteristics of the Patients at Baseline. *
| Characteristic | Continuous Molgramostim Group (N=46) |
Intermittent Molgramostim Group (N=45) |
Placebo Group (N=47) |
|---|---|---|---|
| Age – yr | 54.0±13.3 | 49.2±14.1 | 46.1±14.8 |
| Female gender – no. (%) | 18 (39) | 19 (42) | 22 (47) |
| Pulmonary gas exchange | |||
| A-aDO2 – mm Hg ‡ | 40.5±19.6 | 40.9±20.2 | 40.2±14.3 |
| DLCO – percent of predicted value | 51.9±18.5 | 46.1±14.5 | 49.6±14.3 |
| Radiologic evaluation – CT GGO score § | 10.9±3.2 | 10.8±3.0 | 10.9±2.8 |
| Functional health status | |||
| SGRQ Total score¶ | 47.2±20.4 | 44.4±21.4 | 44.1±21.7 |
| Distance walked on 6–min walk test (m) | 411±143 | 447±117 | 447±125 |
| Prior or concomitant therapy of PAP | |||
| WLL therapy** | |||
| Any prior WLL – no. of patients (%) | 23 (50) | 31 (69) | 30 (64) |
| No. of prior WLL procedures | 3.8 | 3.7 | 2.8 |
| Time since last WLL procedure – months | 24.3±52.6 | 18.9±24.0 | 17.7±20.7 |
| GM-CSF therapy †† | |||
| Any prior GM-CSF – no. of patients (%) | 6 (13) | 7 (16) | 6 (13) |
| Time since last administration – months | 35.4±35.9 | 37.8±26.4 | 18.3±22.6 |
Plus-minus values are means ± SD. Results represent data for N=46, 45, or 47 patients in the Continuous Molgramostim, Intermittent Molgramostim, and Placebo groups, respectively, unless indicated otherwise; missing data were replaced by multiple imputation. A-aDO2 denotes alveolar-arterial difference in oxygen concentration, CT computed tomography, DLCO diffusing capacity for carbon monoxide, GGO ground glass opacification score, GM-CSF granulocyte/macrophage-colony stimulating factor, R-FAS revised full analysis set, WLL whole lung lavage. See Table S2 in the Supplemental Appendix for additional clinical characteristics of the patients at Baseline.
In the R-FAS, the A-aDO2 results for four patients were invalid and treated as missing data. These four patients were distributed in the three groups: continuous molgramostim group (n=1), placebo group (n=2), and 1 in the intermittent molgramostim group.
Calculated with the use of the following equation: A-aDO2 = (FiO2 x (PB-PH20) - PaCO2/R) – PaO2, where FiO2 indicates fraction of inspired oxygen (assumed to be 0.21 for patients breathing room air), PaCO2 partial pressure of arterial carbon dioxide, PB barometric pressure measured by validated barometers, PH20 partial pressure of water vapor in inspired air (assumed to be 47 mm Hg), and R the respiratory quotient (assumed to be 0.8).
The GGO scores range from 0 to 15, with higher scores indicating a higher proportion of the area of the lung parenchyma on CT scan images corresponding to regions affected by ground glass opacification, an indication of the abnormal accumulation of surfactant sediment in patients with aPAP. Baseline GGO Score data for one patient in the Intermittent Molgramostim group was unavailable and was not imputed.
Scores on the SGRQ range from 0 to 100, with higher scores indicating more severe effects on a patient’s functional health status.
The lifetime utilization of WLL therapy of PAP before the randomization, including any prior use, the number of single lung WLL treatments performed, and the time elapsed since the most recent WLL treatment.
The lifetime utilization of GM-CSF as therapy of PAP before the randomization.
EFFICACY OF CONTINUOUS MOLGRAMOSTIM DURING THE BLINDED INTERVENTION PERIOD
Effects on Pulmonary Gas Transfer
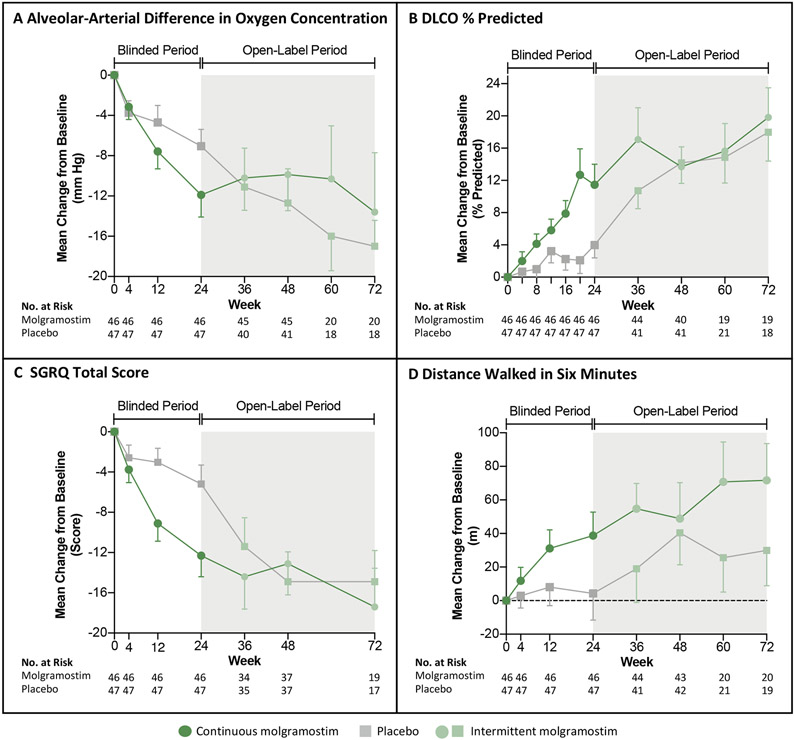
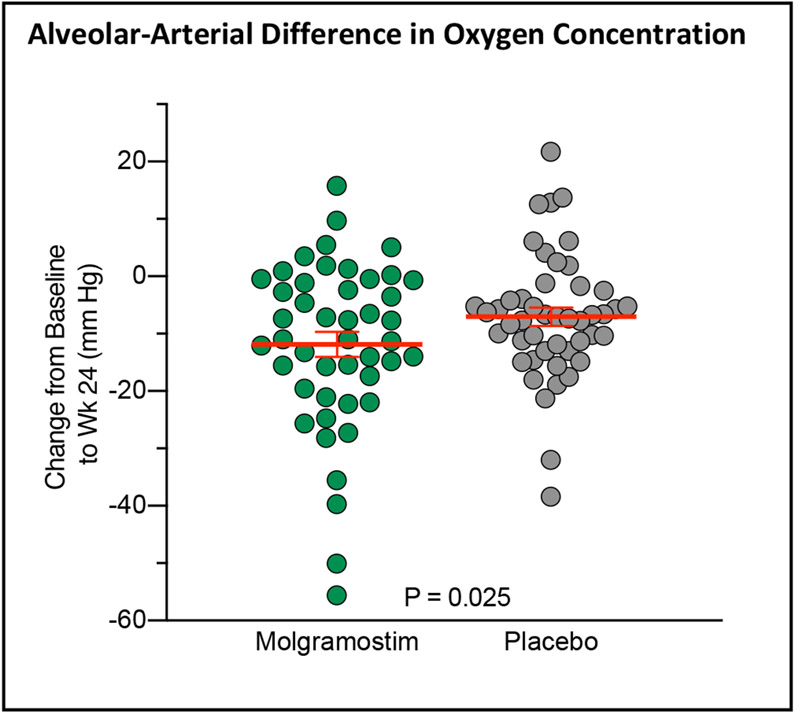
The primary endpoint, the mean change from Baseline at week 24 in A-aDO2, was not significantly different between the continuous molgramostim and placebo groups when analyzed using the FAS (estimated treatment difference (ETD) −5.2 mm Hg; n= 46, 47, respectively; P=0.118). However, after replacing the invalid data (by imputation) for patients breathing supplemental oxygen during blood gas measurement, the change was greater in patients receiving molgramostim than placebo (ETD −6.2 mm Hg; n=46, 47, respectively; P=0.025; Fig. 2, Table 2, see also, Fig. S3 and Table S3-S5 in the Supplemental Appendix). The improvement in A-aDO2 was supported by another pre-specified measure of pulmonary gas transfer, the mean change from Baseline at week 24 in DLCO, which was greater in the molgramostim group than placebo (ETD 7.8 (95% CI 2.3 to 13.3) percent of predicted; n=46, 47, respectively; Table S3 in the Supplemental Appendix) and also by sensitivity analyses (Fig. S4 in the Supplemental Appendix).
Figure 2. Changes in the Primary Endpoint and Selected Secondary Endpoints Over Time.
Panels A-D show the mean change from baseline during the blinded intervention period (white regions) and open-label treatment period (grey regions) for patients who received continuous molgramostim (n=46, green circles) or placebo (n=47, grey squares) from week 0 to week 24 and intermittent molgramostim from week 24 to week 72 for the following end points: alveolar-arterial difference in oxygen concentration (A-aDO2) (Panel A), diffusing capacity for carbon monoxide (DLCO percent of predicted) (Panel B), Saint George Respiratory Questionnaire (SGRQ) Total Score (Panel C), and distance covered in a six-minute walk test (6MWT) (Panel D). The number of patients for whom results were available at each time point is shown; data missing during the double-blind period were replaced using multiple imputation. The difference in numbers of patients included between weeks 24 – 48 and weeks 48 – 72 was due to a protocol amendment permitting use of a longer open-label period for some patients. T bars indicate standard errors.
Table 2.
Effects of Continuous Molgramostim on Primary and Selected Secondary Outcome Variables After 24 Weeks of Treatment. *
| Value at 24 Weeks |
Change from Baseline |
Estimated Difference |
|||
|---|---|---|---|---|---|
| Variable | Molgramostim (N = 47) |
Placebo (N = 47) |
Molgramostim (N = 47) |
Placebo (N = 47) |
Continuous Molgramostim vs. Placebo (95% CI) † |
| Pulmonary gas exchange | |||||
| A-aDO2,mm Hg ‡ (R-FAS) | 26.4±13.7 | 31.6±12.7 | −11.9±14.9 | −7.0±11.4 | −6.2 (−11.7 to −0.8) |
| DLCO, percentage of predicted | 63.3±22.5 | 53.6±15.5 | 11.5±17.4 | 4.0±11.0 | 7.8 (2.3 to 13.3) |
| Radiological evaluation of the lungs | |||||
| GGO score § | 7.5±3.6 | 10.0±3.5 | −3.4±3.8 | −1.2±2.6 | −2.5 (−3.7 to −1.2) |
| Functional health status | |||||
| SGRQ total score ¶ | 35.1±21.3 | 38.9±23.7 | −12.1±14.3 | −5.2±13.0 | −7.4 (−13.1 to −1.6) |
| Distance walked in six minutes, m | 450±135 | 451±145 | 38.7±95.6 | 4.3±109.0 | 24.6 (−15.3 to 64.4) |
Plus-minus values are empirical means ± SD. A-aDO2 denotes alveolar-arterial difference in oxygen concentration, CI confidence interval, DLCO diffusion capacity of the lungs for carbon monoxide, GGO ground glass opacification. See Table S3 in the Supplemental Appendix for the results of other end points.
In the revised full analysis set (R-FAS), A-aDO2 results for four patients (described in Table 1) were invalid and treated as missing data. See Table S3 in the Supplemental Appendix) and text for additional details.
Between-group differences for the change from baseline are expressed as least square means and 95% confidence intervals with use of an analysis of covariates model (all treatment groups included in the same model) with treatment, WLL within 2 months prior to Baseline (stratification) and geographic region (Japan vs other countries) as factors and Baseline values as covariates.
The GGO scores range from 0 to 15, with higher scores indicating a higher proportion of the area of the chest CT scan images corresponding to lung parenchyma affected by ground glass opacification, an indication of the abnormal accumulation of surfactant sediment in patients with aPAP.
Scores on the SGRQ range from 0 to 100, with higher scores indicating more severe effects on a patient’s functional health status.
Effects on Health Status and Functional Exercise Capacity
The mean change from Baseline at week 24 in SGRQ total score was significantly greater in the molgramostim group than placebo (ETD −7.4 (95%CI −13.1 to −1.6); n=46, 47, respectively; P=0.012) (Table 2) as were SGRQ component scores for activity and impact but not symptoms (Table S3 in the Supplemental Appendix). Responder analysis was also greater at a 4-point and 8-point threshold but not a 12-point threshold (Fig S5 in the Supplemental Appendix).
The mean change from baseline at week 24 in 6MWT-distance was not significantly different between the molgramostim and placebo groups (Table 2).
Effects on Use of WLL
The use of WLL therapy in the two years preceding enrollment was not different among the three groups (Fig. S6 in the Supplemental Appendix). Compared to the use of WLL before enrollment, the use rate after randomization was similar in the placebo group but lower in the molgramostim group during the blinded-intervention period and reduced further in the open-label treatment period (Fig. S7A in the Supplemental Appendix). The time from Baseline to the first use (or frequency) of WLL therapy was not statistically different in patients receiving molgramostim or placebo (Fig. S7B in the Supplemental Appendix).
Effects on Radiographic and Biochemical Measures of aPAP Pathology
The reduction in alveolar surfactant burden from baseline to week 24 reflected by the change in GGO was greater in patients receiving molgramostim than placebo (ETD −2.5 [95%CI −3.7 to −1.2]) (Fig. 2B, Table 2).
Serum levels of aPAP biomarkers were improved in patients receiving continuous molgramostim compared to placebo (Fig. S8, S9 in the Supplemental Appendix).
EFFICACY OF INTERMITTENT MOLGRAMOSTIM DURING THE BLINDED INTERVENTION PERIOD
Because prior uncontrolled studies had evaluated intermittent GM-CSF administration in aPAP,22,27 an intermittent molgramostim arm was included in this study. Compared to placebo, the mean change for most outcomes was less in patients receiving intermittent molgramostim than continuous molgramostim (Table S3, Fig. S10-11 in the Supplemental Appendix). The WLL use rate in patients receiving intermittent molgramostim was less than placebo and similar to continuous molgramostim during the double-blinded period (Fig. S7B in the Supplemental Appendix).
EFFICACY OF MOLGRAMOSTIM DURING THE OPEN LABEL INTERVENTION PERIOD
Improvement was observed in A-aDO2, DLCO, SGRQ, and 6MWT-distance during the open-label treatment extension period in patients who received continuous molgramostim, intermittent molgramostim, or placebo during the double-blind period (Fig. 3A-D, Fig. S11 in the Supplemental Appendix). The rate of WLL use during the open-label period was less than in any group during the double-blind period and less than before enrollment (Fig. 2A, Fig. S7B in the Supplemental Appendix).
SAFETY
No deaths occurred during the study. The numbers of serious adverse events and adverse were similar among the three groups during the blinded intervention period except for a slight increase in the number of patient with mild chest discomfort in the continuous molgramostim group and no treatment-related serious adverse events were reported in open-label treatment extension period (Table S7-8 in the Supplemental Appendix). The serum molgramostim/GM-CSF antibody titer was similar among the three groups at baseline and did not change during the double blinded treatment period (Table S3 in the Supplemental Appendix).
DISCUSSION
This study showed treatment with inhaled molgramostim for 24 weeks had beneficial effects in patients with aPAP including improvement in pulmonary gas transfer (A-aDO2, DLCO), health status (SGRQ), and pathology (GGO score, serum biomarkers); however, no significant improvement was observed in 6MWT-distance or need for WLL therapy. Molgramostim was not associated with an increase in adverse effects.
The observation of synchronous improvement across multiple outcomes reflecting physiological, clinical, radiological, and biochemical disease manifestations provides strong support for a treatment effect of molgramostim in aPAP. Further support comes from the consistent trend to greater efficacy when molgramostim was administered continuously rather than intermittently. Our data on SGRQ show an improvement in the health status of aPAP patients in a controlled trial.
Although the minimal clinically important difference (MCID), which is a change that patients can perceive, has not been established in aPAP for any outcome variable, the magnitude of the treatment effects of molgramostim are similar to or larger than those observed in prior studies evaluating GM-CSF as therapy of aPAP.22,25,27 In a recent controlled study in 33 patients with mild to moderate aPAP (the PAGE trial), administration of inhaled GM-CSF for 24 weeks improved the placebo-adjusted change in A-aDO2 by −5.7 (95% CI −10.5 to −1.4) mmHg and DLCO by 6.9 (95% CI 0.62 to 13.1) percent of predicted.25 Our observation of placebo-adjusted changes of −6.4 mm Hg in A-aDO2 and 7.9 percent of predicted in DLCO for continuous molgramostim administered for a similar duration compare favorably. Continuous molgramostim also improved the placebo-adjusted SGRQ total score by 7.6, a value greater than the MCID of 4 previously determined for patients with chronic obstructive pulmonary disease.33 Molgramostim improved the GGO score, similar to results for the PAGE trial in which inhaled GM-CSF improved radiographically-measured lung density.25 Finally, molgramostim improved serum biomarkers of PAP consistent with results for KL-6 and MCP-1 in the PAGE trial.25
Effects on several outcome measures have also been evaluated for WLL therapy, which is known to be efficacious in aPAP patients.3 WLL improved the mean A-aDO2 by 18 mm Hg in 21 patients in one study,34 15.2 mm Hg in 26 patients in another study,35 and 20.7 mm Hg in 80 patients in a third study.36 In these uncontrolled studies, WLL also improved DLCO by 8.0, 11.3, and 10.6, percent of predicted, respectively. These values are higher than we observed during the 24-week blinded intervention period although it is noteworthy that improvement continued during the open-label treatment-extension period. Together, these observations suggest a longer duration of inhaled molgramostim therapy may be needed to achieve the maximal treatment benefit.
The measurement of A-aDO2 while administering supplemental oxygen in patients requiring this therapy continuously was an important limitation of the study. The protocol-specified use of the same oxygen flow rate for each such patient during all study visits was not an adequate remedial measure and it was necessary to replace the A-aDO2 data for several such patients, which was justified because their data were invalid and the occurrences were few (4 of 138 participants) and balanced among the groups. The observed improvement in A-aDO2 was further supported by sensitivity analysis, concurrent improvement in DLCO - an independent measure of pulmonary gas transfer unaffected by supplemental oxygen, and by a number of prior uncontrolled and controlled studies reporting a treatment benefit of inhaled of GM-CSF in aPAP.24, 25 Another study limitation was the short (24-week) duration of the blinded intervention period that was considered to be too short to expect an effect on the use of WLL for which the median time between therapeutic procedures is 35-60 weeks.14,37
In conclusion, our small relatively short study demonstrates that daily administration of inhaled molgramostim improved the physiological, radiological, biochemical, and clinical manifestations of aPAP, and was more beneficial when administered continuously than on alternating weeks. Further studies are needed to define the duration of treatment required for maximal treatment benefit and to evaluate the potential utility of differential dosing for induction and maintenance therapy.
Supplementary Material
Figure 1. Changes in Alveolar-Arterial Difference in Oxygen Concentration (A-aDO2).
The figure shows the change in A-aDO2 from baseline to week 24 in the continuous molgramostim group (46 patients) and placebo group (47 patients) during the 24-week randomized intervention period analyzed using the revised full analysis set. Each symbol represents the results for one patient; data for one patient in the molgramostim and two patients in the placebo group were invalid, treated as missing, and replaced using multiple imputation (see text for details).
ACKNOWLEDGEMENTS
Supported by Savara Pharmaceuticals.
Drs. Ganslandt, Jouhikainen, and Tarnow are employees of Savara Pharmaceuticals. Dr. Inoue reports having served as an advisor to Savara Pharmaceuticals. Drs. Bonella, Jouneau, and Trapnell, report having served as consultants to Savara Pharmaceuticals. Drs. Baba, Campo, Kramer, Kreuter, Morgan, Papiris, Veltkamp, and Yamaguchi report no financial relationships to disclose related to this work.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank our patients for their participation in the study, Dr. Raphael Borie, Paris, France and Dr. Marlies Wijsenbeek-Lourens, Rotterdam, the Netherlands for serving on the data and safety monitoring board, Dr. Ulrich Costabel, Essen, Germany, Dr. Maurizio Luisetti, Pavia, Italy for advice regarding trial design, the Savara Team for help with conduct of the trial, and Frank McCormack (Cincinnati, OH, USA), and Jeffrey A. Whitsett (Cincinnati, OH, USA) for critical reading of the manuscript.
Funding:
A statement has been added to the end of the text of the manuscript that describes all sources of financial support for this study.
Footnotes
Trial Identifiers: Savara Clinical Trial Number - MOL-PAP-002 EudraCT number - 2015-003878-33 ClinicalTrials.gov number - NCT02702180
Disclosures: All authors submitted a disclosure statement through the Convey website.
Confidentiality: The clinical site investigators who conducted the clinical trial all agreed to provide the sponsor 45 days to review any manuscripts proposed for publication before submission.
REFERENCES
- 1.Rosen SG, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med 1958;258:1123–42. [DOI] [PubMed] [Google Scholar]
- 2.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003;349:2527–39. [DOI] [PubMed] [Google Scholar]
- 3.Trapnell BC, Nakata K, Bonella F, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers 2019;5:16. [DOI] [PubMed] [Google Scholar]
- 4.Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008;177:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarthy C, Avetisyan R, Carey BC, Chalk C, Trapnell BC. Prevalence and healthcare burden of pulmonary alveolar proteinosis. Orphanet J Rare Dis 2018;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura N, Ohkouchi S, Tazawa R, et al. Incidence of autoimmune pulmonary alveolar proteinosis estimated using Poisson distribution. ERJ Open Res 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura T, Tanaka N, Watanabe J, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 1999;190:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida K, Nakata K, Trapnell BC, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 2004;103:1089–98. [DOI] [PubMed] [Google Scholar]
- 9.Sakagami T, Uchida K, Suzuki T, et al. Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med 2009;361:2679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakagami T, Beck D, Uchida K, et al. Patient-derived granulocyte/macrophage colony-stimulating factor autoantibodies reproduce pulmonary alveolar proteinosis in nonhuman primates. Am J Respir Crit Care Med 2010;182:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol 2002;64:775–802. [DOI] [PubMed] [Google Scholar]
- 12.Sallese A, suzuki T, Bridges J, et al. Pulmonary Alveolar Proteinosis Is Driven by Reduced GM-CSF-Dependent Cholesterol Clearance by Alveolar Macrophages, Not an Inability to Catabolize Surfactant Phospholipids. American Journal of Respiratory and Critical Care Medicine 2016:A6598. [Google Scholar]
- 13.McCarthy C, Lee E, Bridges JP, et al. Statin as a novel pharmacotherapy of pulmonary alveolar proteinosis. Nat Commun 2018;9:3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. American Joural of Respiratory and Critical Care Medicine 2002;166:215–35. [DOI] [PubMed] [Google Scholar]
- 15.Huffman JA, Hull WM, Dranoff G, Mulligan RC, Whitsett JA. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest 1996;97:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed JA, Ikegami M, Cianciolo ER, et al. Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF- deficient mice. Am J Physiol 1999;276:L556–63. [DOI] [PubMed] [Google Scholar]
- 17.Zsengeller ZK, Reed JA, Bachurski CJ, et al. Adenovirus-mediated granulocyte-macrophage colony-stimulating factor improves lung pathology of pulmonary alveolar proteinosis in granulocyte-macrophage colony-stimulating factor-deficient mice. Hum Gene Ther 1998;9:2101–9. [DOI] [PubMed] [Google Scholar]
- 18.Seymour JF, Dunn AR, Vincent JM, Presneill JJ, Pain MC. Efficacy of granulocyte-macrophage colony-stimulating factor in acquired alveolar proteinosis. N Engl J Med 1996;335:1924–5. [DOI] [PubMed] [Google Scholar]
- 19.Seymour JF, Presneill JJ, Schoch OD, et al. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am J Respir Crit Care Med 2001;163:524–31. [DOI] [PubMed] [Google Scholar]
- 20.Schoch OD, Schanz U, Koller M, et al. BAL findings in a patient with pulmonary alveolar proteinosis successfully treated with GM-CSF. Thorax 2002;57:277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tazawa R, Hamano E, Arai T, et al. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2005;171:1142–9. [DOI] [PubMed] [Google Scholar]
- 22.Tazawa R, Trapnell BC, Inoue Y, et al. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2010;181:1345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tazawa R, Inoue Y, Arai T, et al. Duration of benefit in patients with autoimmune pulmonary alveolar proteinosis after inhaled granulocyte-macrophage colony-stimulating factor therapy. Chest 2014;145:729–37. [DOI] [PubMed] [Google Scholar]
- 24.Sheng G, Chen P, Wei Y, Chu J, Cao X, Zhang HL. Better approach for autoimmune pulmonary alveolar proteinosis treatment: inhaled or subcutaneous granulocyte-macrophage colony-stimulating factor: a meta-analyses. Respir Res 2018;19:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tazawa R, Ueda T, Abe M, et al. Inhaled GM-CSF for Pulmonary Alveolar Proteinosis. N Engl J Med 2019;381:923–32. [DOI] [PubMed] [Google Scholar]
- 26.Tarnow I, Nymark K, Vinge M, Nielsen KA, Ganslandt C. A randomized double-blind, placebo-controlled, single-centre, single ascending dose adn multiple ascending dose study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of molgramostim (rhGM-CSF) administered by inhalation to healthy adult subjects. Am J Resp Crit Care Med 2016;34:553–64. [Google Scholar]
- 27.Wylam ME, Ten R, Prakash UB, Nadrous HF, Clawson ML, Anderson PM. Aerosol granulocyte-macrophage colony-stimulating factor for pulmonary alveolar proteinosis. Eur Respir J 2006;27:585–93. [DOI] [PubMed] [Google Scholar]
- 28.Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respir Med 1991;85 Suppl B:25–31; discussion 3-7. [DOI] [PubMed] [Google Scholar]
- 29.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. [DOI] [PubMed] [Google Scholar]
- 30.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–35. [DOI] [PubMed] [Google Scholar]
- 31.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- 32.Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care 2005;50:604–9. [PubMed] [Google Scholar]
- 33.Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014;189:250–5. [DOI] [PubMed] [Google Scholar]
- 34.Beccaria M, Luisetti M, Rodi G, et al. Long-term durable benefit after whole lung lavage in pulmonary alveolar proteinosis. Eur Respir J 2004;23:526–31. [DOI] [PubMed] [Google Scholar]
- 35.Byun MK, Kim DS, Kim YW, et al. Clinical features and outcomes of idiopathic pulmonary alveolar proteinosis in Korean population. J Korean Med Sci 2010;25:393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao YY, Huang H, Liu YZ, Song XY, Li S, Xu ZJ. Whole Lung Lavage Treatment of Chinese Patients with Autoimmune Pulmonary Alveolar Proteinosis: A Retrospective Long-term Follow-up Study. Chin Med J (Engl) 2015;128:2714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campo I, Luisetti M, Griese M, et al. Whole lung lavage therapy for pulmonary alveolar proteinosis: a global survey of current practices and procedures. Orphanet J Rare Dis 2016;11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.