Abstract
The global burden of myopia is growing. Myopia affected nearly 30% of the world population in 2020 and this number is expected to rise to 50% by 2050. This review aims to analyze the impact of myopia on individuals and society; summarizing the evidence for recent research on the prevalence of myopia and high myopia, lifetime pathological manifestations of myopia, direct health expenditure, and indirect costs such as lost productivity and reduced quality of life (QOL). The principal trends are a rising prevalence of myopia and high myopia, with a disproportionately greater increase in the prevalence of high myopia. This forecasts a future increase in vision loss due to uncorrected myopia as well as high myopia-related complications such as myopic macular degeneration. QOL is affected for those with uncorrected myopia, high myopia, or complications of high myopia. Overall the current global cost estimates related to direct health expenditure and lost productivity are in the billions. Health expenditure is greater in adults, reflecting the added costs due to myopia-related complications. Unless the current trajectory for the rising prevalence of myopia and high myopia change, the costs will continue to grow. The past few decades have seen the emergence of several novel approaches to prevent and slow myopia. Further work is needed to understand the life-long impact of myopia on an individual and the cost-effectiveness of the various novel approaches in reducing the burden.
Keywords: myopia, high myopia, direct costs, lost productivity, quality of life, economic impact, disability, utility
Worldwide, one-fifth of blindness is due to refractive error, predominantly myopia.1 Myopia (defined as a spherical equivalent refraction ≤ −0.50 diopter [D]) is an inadequately acknowledged global public health problem and chronic condition that affects almost 30% of the world's population.2 Myopia impacts an individual's early life, imposes disability by way of poor vision, and is life-long. Depending on the age of the individual, magnitude of myopia, and geographical setting (e.g. urban versus remote/rural), it can have severe socio-economic consequences on the individual and, consequently, on society as a whole. It appears inevitable that the proportion of people affected by myopia will increase in coming decades. Projections estimate 50% of the global population will be affected by myopia in the year 2050, of whom 10% will have high myopia.2 As myopia, particularly high myopia, is associated with a significant risk of complications leading to blindness and vision impairment,3 the global burden of myopia is likely to increase.
Understanding the burden of myopia provides a framework to assess and address the condition appropriately. At the societal and individual levels, detection and interventions aimed at delaying the onset and/or slowing myopia progression would reduce the risk of sight-threatening complications as well as the economic burden related to managing the condition. This would result in better visual outcomes that would in turn translate to economic gains as well as improvements in quality of life (QOL). For example, a streamlined approach may improve integrated health care provision at various levels so that the more complex cases are more efficiently channeled or co-managed. This would reduce costs, reduce waiting times, and facilitate higher and earlier rates of detection and more effective intervention for those at risk of myopia development or progression.
In this nonsystematic review, we report recent evidence on the prevalence of myopia and high myopia and identify the direct costs, patient-reported outcomes, and lost productivity associated with the disorder. This review will identify gaps in our understanding of the condition as well as provide evidence to support and advocate for developing appropriate approaches and policies to manage myopia.
Understanding the Burden
Standardized Definition of Myopia
The way myopia is defined or classified is an important consideration when attempting to quantify its burden. In descriptive terms, myopia is “a refractive error in which rays of light entering the eye parallel to the optical axis are brought into focus in front of the retina when ocular accommodation is relaxed. This usually results from the eyeball being too long from front to back, but can be caused by an overly curved cornea and/or a lens with increased optical power.”4 In 2019, the taskforce established by the International Myopia Institute (IMI) reviewed the existing published terminology, definitions, and thresholds for myopia, including the existing World Health Organization (WHO) definitions of myopia, and defined myopia as a condition in which the spherical equivalent is ≤ −0.50 D when ocular accommodation is relaxed, and high myopia as a spherical equivalent of ≤ −6.00 D.4 The WHO expert committee on myopia defined high myopia as ≤ −5.00 D on the basis that uncorrected myopia of this degree impacts visual acuity (VA) to a level that meets the threshold for blindness (<3/60). This criterion may be more relevant for estimating the prevalence and impact of myopia in population-based surveys. Additionally, this definition facilitates comparisons with other causes of blindness and low vision. On the other hand, the IMI definition takes into account the threshold commonly used in published studies and clinical relevance (risk of uncorrectable vision loss in an individual increases with myopia greater than −6.0D). Therefore, a cutoff criterion may be chosen depending on the reason for the inquiry (i.e. whether it is for evaluating the impact of uncorrected myopia in the community versus vision loss in an individual) but the reason for the chosen definition needs to be identified.
Myopia Incidence and Prevalence
Incidence and prevalence estimates of disease are widely used to appreciate the impact and the need for services to manage the burden. Given that data on myopia incidence are sparse, prevalence data are commonly utilized. It was estimated that from the years 2010 to 2020, myopia prevalence increased worldwide from 28.3% to 34%, an increase of about 20% from the baseline prevalence.2
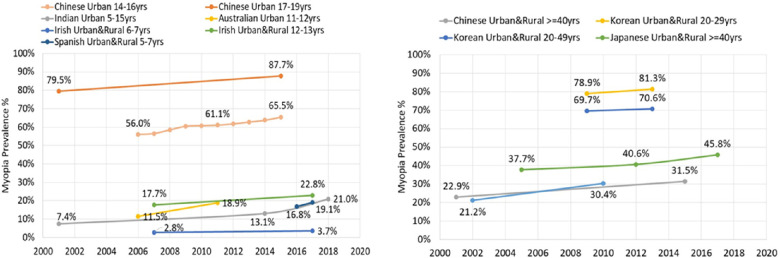
When summarizing data from the years 2000 to 2019 that allowed for comparisons across related ages, it is observed that while the prevalence varies across countries/regions, myopia is rising in children, and young and older adults (Table 1, Fig. 1).
Table 1.
Age and Region-Specific Myopia Prevalence in Children and Adults From 2000 to 2019
| Region | City/Country | Urban/Rural | Age Group (Yrs.) | Year | Myopia % | Reference |
|---|---|---|---|---|---|---|
| Children | ||||||
| East Asia | Beijing, China | Urban | 14–16 | 2006 | 56.0% | Li et al. 20175 |
| 14–16 | 2007 | 56.5% | ||||
| 14–16 | 2008 | 58.5% | ||||
| 14–16 | 2009 | 60.5% | ||||
| 14–16 | 2010 | 60.8% | ||||
| 14–16 | 2011 | 61.1% | ||||
| 14–16 | 2012 | 61.8% | ||||
| 14–16 | 2013 | 62.8% | ||||
| 14–16 | 2014 | 63.8% | ||||
| 14–16 | 2015 | 65.5% | ||||
| Fenghua city, China | Urban | 17–19 | 2001 | 79.5% | Chen et al. 201819 | |
| 17–19 | 2015 | 87.7% | ||||
| South Korea | KHANES IV-V | 5–18 | 2008 | 64.6% | Lim et al. 2018111 | |
| KHANES VII | 5–18 | 2016 | 65.4% | Kim et al. 2020112 | ||
| South Asia | New Delhi, India | Urban | 5–15 | 2001 | 7.4% | Murthy et al. 200210 |
| New Delhi, India | Urban | 5–15 | 2014 | 13.1% | Saxena et al. 201511 | |
| Gurugram, India | Urban | 5–15 | 2018 | 21.0% | Singh et al. 201912 | |
| High Income Asia-Pacific | Hong Kong | Urban | 6–8 | 2000 | 28.4% | Fan et al. 200113 |
| Hong Kong | Urban | 6–8 | 2015 | 25.0% | Yam et al. 201914 | |
| Australasia | Sydney, Australia | Urban | 11–12 | 2006 | 11.5% | French et al. 20137 |
| Sydney, Australia | Urban | 11–12 | 2011 | 18.9% | ||
| Western Europe | Spain | Urban & Rural | 5–7 | 2016 | 16.8% | Alvarez-Peregrina et al 2019137 |
| Urban & Rural | 5–7 | 2017 | 19.1% | |||
| Northern Ireland | Urban & Rural | 6–7 | 2007 | 2.8% | O'Donoghue, et al. 20108 | |
| Urban & Rural | 6–7 | 2017 | 3.7% | Harrington et al 20199 | ||
| Northern Ireland | Urban & Rural | 12–13 | 2007 | 17.7% | O'Donoghue et al. 20108 | |
| Urban & Rural | 12–13 | 2017 | 22.8% | Harrington et al. 20199 | ||
| Adults | ||||||
| East Asia | Beijing, China | Urban & Rural | >= 40 | 2001 | 22.9% | Xu et al. 200515 |
| Yunnan (Han), China | Urban & Rural | >= 40 | 2015 | 31.5% | Wang et al. 201916 | |
| High Income Asia Pacific | South Korea | Urban & Rural | 20–29 | 2009 | 78.9% | Kim et al. 2013115 |
| South Korea | Urban & Rural | 20–29 | 2013 | 81.3% | Han et al. 2019116 | |
| South Korea | Urban & Rural | 20–49 | 2009 | 69.7% | Kim et al. 2013115 | |
| South Korea | Urban & Rural | 20–49 | 2013 | 70.6% | Han et al. 2019116 | |
| Hisayama, Japan | Urban & Rural | >= 40 | 2005 | 37.7% | Ueda et al. 201917 | |
| North Africa and Middle East | Tehran, Iran | Urban | 16–25 | 2002 | 22.5% | Hashemi et al. 2004138 |
| Aligoudarz city, Iran | Urban | 14–21 | 2012 | 33.5% | Hashemi et al. 2014139 | |
| Tehran, Iran | Urban | >= 46 | 2002 | 21.2% | Hashemi et al. 2004138 | |
| Shahroud city, Iran | Urban | >= 45 | 2010 | 30.4% | Hashemi et al. 2012140 | |
Figure 1.
Myopia prevalence from 2000 to 2019 (age and region).
In East Asia, where the prevalence is already high, the prevalence in urban-dwelling children aged 14 to 16 years increased steadily from approximately 56.0% to 65.5% between 2006 and 2015.5 A systematic analysis of 22 studies of myopia prevalence in Chinese children and adolescents found the prevalence had increased steadily between 2000 to 2015 from 25.7% (before 2001), to about 39% (2001–2010) and then to 46.1% (2011–2015).6 Although East Asian countries have the highest prevalence, an increasing myopic shift is also observed in other regions of the world. In Australia, cross-sectional studies of 12-year-old children reported a prevalence that increased from 11.5% in 2006 to 18.9% in 2011.7 In Northern Ireland, the prevalence in 12-year-old children increased from 17.7% in 2007 to 22.8% in 2017.8,9 Similarly, prevalence increased from 7.4% to 13.1% between 2001 and 2014 in New Delhi in children aged 5 to 15 years,10,11 and was 21.1% 4 years later, a steep increase in myopia.12 In contrast to these reports of rising myopia prevalence, prevalence in children aged 6 to 8 years in Hong Kong was high, but decreased slightly over 15 years (28.4% in 2001 to 25.0% in 2019).13,14 Although the authors speculate the role of the academic system, it is possible that differences in administration of cycloplegic drops in this young cohort is responsible for the variation in prevalence.
Data from adult populations also indicates an increasing myopic shift. Between 2001 and 2015, myopia prevalence in adults ≥ 40 years in urban China rose from 22.9% to 31.5%.15,16 In Korea, between 2008 and 2011, myopia prevalence in 20 to 29-year-olds was 78.9% and increased in 2013 to 2014 to 81.3%, and in Japan, myopia in adults ≥ 40 years increased from 37.7% in 2005 to 45.8% in 2017.17 Meta-analysis of studies in Europe show that the age standardized prevalence of myopia increased in older adults, where myopia in the 50 to 79 year age group was observed to be higher by 5.7% in those born later (23.5%) in a decade compared to those born earlier (17.8%).18
As a result of the rising prevalence of myopia, it follows that there could be an increase in the prevalence of high myopia. Table 2 outlines the reported prevalence of high myopia in young adults from recent years. In alignment with the overall prevalence data, high myopia prevalence is much higher in East Asian and Asia-Pacific countries. Significantly, the rate of change in prevalence of high myopia appears to be disproportionately greater compared to the rate of change in the prevalence of myopia. For example, in Fenghua City, China, the prevalence of myopia from 2001 to 2015 rose from 79.5% to 87.7% in 18-year-olds (a 10% increase).19 In comparison, the prevalence of high myopia nearly doubled from 7.9% to 16.6%. Similarly, in Taiwan, the prevalence of myopia and high myopia in a sample of approximately 4000 freshmen (first year students) at university was 91.3% and 23.5% in 1988 and 95.9% and 38.9% in 2005.20 A combination of factors may be responsible for this disproportionate increase in the prevalence of high myopia, including children in Asian countries increasingly developing myopia at earlier ages than before,21 a faster rate of progression of myopia in children of Asian ethnicity compared to Caucasian counterparts,22 and a faster rate of myopia progression in younger compared to older children.23,24 This change in the prevalence pattern toward an increased risk of high myopia creates significant challenges in managing the future burden: increased risk of comorbidities, increased direct costs, and negative effect on QOL and productivity.
Table 2.
Prevalence of Myopia and High Myopia in Young Adults
| High | |||||||
|---|---|---|---|---|---|---|---|
| Myopia % | |||||||
| Region | City/Country | Urban/Rural | Age Group | Year | Myopia % | (≤−6.00 D) | Reference |
| Young Adults | |||||||
| East Asia | Fenghua City, China | Urban | 17–19 y | 2001 | 79.5% | 7.9% | Chen (2018)19 |
| 17–19 y | 2015 | 87.7% | 16.6% | ||||
| South Korea | Urban | 19 | 2010 | 96.5% | 21.6% | Jung et al (2012)113 | |
| South Korea | Rural | 19 | 83.3% | 6.8% | Lee et al. (2013)114 | ||
| South Korea | Urban and Rural | 20–29 y | 2009 | 78.9% | 10.9% | Kim et al. (2013)115 | |
| South Korea | Urban and Rural | 20–29 y | 2013 | 81.3% | 11.1% | Han et al. (2019)116 | |
| Taiwan | Urban | 18–24 | 2010 | 86.1% | 21.2% | Lee et al. (2015)117 | |
| Taiwan | Urban | Freshman (first year university students) | 1988 | 91.3% | 23.5% | Wang et al. (2009)20 | |
| Taiwan | Urban | Freshman (first year university students) | 2005 | 95.9% | 38.4% | Wang et al. (2009)20 | |
| Singapore | Urban | 17–29 | 2009 | 81.6% | 14.7% | Koh et al. 2014118 | |
| Middle East | Israel | 16–22 | 2002 | 28.3% | 2.0% M 2.3% F | Dayan et al. 2005119 | |
Life-Course of Myopia
Although the clinical course of myopia is reasonably well-delineated, certain aspects related to the onset, progression, stability, and associated morbidity are not fully understood. Current evidence shows that myopia is generally detected in children before 10 years of age, but the onset may vary from as young as 3 to 4 years to late teenage or early adulthood depending on ethnic, familial, environmental, and geographical factors.25–27 Usually, the condition is progressive in the early years of life. Two studies found that the annual progression rate was higher in the year before detection and in the year following when myopia was first detected, but declined thereafter.28,29 Annual progression data from spectacle wearers of Asian ethnicity found that the younger the age, the greater the risk of progression, with 7-year-old children progressing approximately 0.9 D/year whereas progression in 12-year-old children was approximately 0.58 D/year.23,30 In a school-based cohort study conducted in Shanghai, the average 2-year progression of cycloplegic spherical equivalent refractive error in myopic children aged 7, 8, and 9 years was 2.0 D, 1.6 D, and 1.8 D, respectively.31 Younger age at baseline predicted a greater risk of high myopia,32 possibly due to the faster progression rate at a younger age.23,29 Although the condition is said to stabilize in teenage years to adulthood, there are no clear data on when exactly this occurs and, additionally, there are reports of onset and progression in adults.33,34 In younger age groups, visual disability by way of impaired distance vision is the characteristic feature of myopia, although in a smaller number of cases, especially in individuals with high myopia, complications, such as retinal breaks, posterior staphylomas, and retinal detachments, may occur.35 Additionally, a small percent of the population may also suffer complications related to corrective modalities, such as Laser-Assisted In-Situ Keratomileusis (LASIK) and contact lenses.36,37
Although there is no threshold or cutoff criterion, increasing age is a risk factor for myopia-related complications. In later years, myopia is associated with an increased risk of cataract, glaucoma, and various conditions affecting the posterior segment of the eye, such as posterior staphyloma, myopic retinopathy, also known as myopic macular degeneration (MMD), and myopic traction retinopathy (Table 3).38,39 Of these, myopic retinopathy or MMD is fast emerging as one of the leading causes of blindness in East Asia and elsewhere.15,17,40 A recent meta-analysis found a pooled prevalence of MMD in the world population of 2.1% (increasing from 1.3% in the 40–49 age group to 4.5% in the 70+ age group)41 and found a higher frequency of MMD from data reported from 2007 to 2019 compared to 1993 to 2006. Vision impairment from MMD was estimated to have affected 10 million people (0.13% of world's population) in 2015 and, if the trajectory for the rising prevalence of myopia and high myopia continues at the same pace, it is set to grow to nearly 56 million (0.6%) by the year 2050.42
Table 3.
Complications Observed in Myopic Eyes
| Ocular Sign or Condition | Myopia/Age of Myope/Prevalence or OR |
|---|---|
| Cataract | High myopia/≥45 yrs/OR-2.79117; 2.55118 |
| Glaucoma | Any myopia/elderly/pooled OR −1.92119 |
| Posterior vitreous detachment | High myopia/20-29 yrs/12.5%120 |
| Myopic maculopathy/Myopic Macular | High myopia/>40 yrs/pooled-47.4%38 |
| Degeneration | |
| - Tessellations of fundus | |
| - Diffuse chorioretinal atrophy | |
| - Patchy chorioretinal atrophy | |
| - Lacquer cracks | |
| - Fuchs spot | |
| - Choroidal neovascularization* | |
| - Macular Atrophy | |
| Posterior Staphyloma | High myopia/6–19 yrs/12.7%121 |
| - Chorioretinal atrophy | High myopia/12–67 yrs/12.0%122 |
| - Macular retinoschisis | |
| - Dome-shaped macula | |
| - Bruch's membrane defects | |
| Myopic traction maculopathy116 | Highly myopic eyes with posterior staphyloma/>40 yrs/Not available |
| - Retinoschisis | |
| - Foveal detachment | |
| - Macular holes | |
| Retinal detachment | High myopia/15–75 yrs/6.3%123 |
| - Epiretinal membranes | |
| - Tractional internal limiting membrane detachment | |
| Peripheral retinal degeneration | |
| - Lattice degeneration | High myopia/15–75 yrs/11.8–37.8%123 |
| - White without Pressure | High myopia/19–25 yrs/46.5–14.6%124 |
Impact of Myopia on Affected Individuals
Impact of Myopia on Education
Numerous studies have shown an association between increased near work and myopia, with educational pressures cited for the increasing prevalence of myopia.43,44 Although it is accepted that these factors may be involved in the onset and progression of myopia, the impact of undercorrected or uncorrected myopia on school performance is of interest as there is evidence linking educational outcomes to adult health.45 The scholastic performance of students in whom myopia is uncorrected relative to those with corrected myopia is a complex area to investigate; however, data from randomized studies from China indicate that providing spectacles for children with myopia who do not have correction can lead to improved academic performance, with demonstrated improvement in mathematics test scores.46,47
A study involving parents, teachers, and students found poor vision or uncorrected visual deficits in children negatively impacted their attention, perseverance, academic performance, and caused psychosocial stress, whereas receiving corrective spectacles improved the students’ academic performance and psychosocial wellbeing.48 Board work remains the mainstay of school education in most parts of the world, although projection or digital technology is becoming increasingly popular, especially in urban areas.49 An assessment of VA demands for classwork in different class grades found that a VA of 0.3 log MAR (6/12 or 20/40 Snellen VA) was required for board work.50 Even low grades of myopia can reduce VA beyond this threshold and hence pose a learning challenge for children with uncorrected or undiagnosed myopia. Additionally, dioptric blur is found to affect reading performance, with speed of reading reduced for large blur.51
While spectacles can help restore vision and enhance academic performance, access to appropriate spectacles and adherence with spectacle wear among children is influenced by many factors. Although myopia, more severe refractive error,52–54 and poorer VA promotes adherence, boys are less likely to be adherent and socio-economic factors, such as cost, accessibility to spectacles, and parental education, are cited to be barriers to spectacle wear and compliance.55 There are also psycho-social barriers, such as fear of discrimination, bullying, and negative societal attitudes.53,55,56 The unmet need for refractive error correction has been reported in many parts of the world, for example, 27% of children in rural China have uncorrected VA worse than 20/40 and 13.1% of children in Philadelphia schools have uncorrected refractive errors. Most of the refractive errors in these cases was myopia.57,58
Estimating Cost of Myopia to Individuals
Direct costs and productivity loss costs are incurred by individuals and their families affected by myopia. Direct costs include expenditure for diagnosis and correction/management, transport costs, and treatment of morbidity. Lost productivity costs may include time spent on eye examinations or returning to clinics to pick up aids, unpaid caregiver time, lost workplace or home productivity, and the value of loss of QOL.
Costs are generally reported as annual costs and, as expected, vary significantly between countries. The most comprehensive data on direct costs to date for myopia have been from Singapore, a country with one of the highest prevalence rates of myopia anywhere in the world. Data gathered from a cross-sectional study in 2006 involving children aged 12 to 17 years, found a mean annual direct cost of myopia of SG $222 (US $148) and a median cost of SG $125 (US $83).59 A more recent paper indicated that costs increase substantially as the individual ages.60 The annual direct costs of myopia per person aged 40 years and above for the year 2011 was reported to be SG $900 (US $709), of which 65% (SG $588 or US $463) was associated with vision products and optometry visits. The higher costs for older individuals relative to costs for children was partly related to complications from myopia.60
In a study in the United States that included participants older than 12 years with distance vision impairment,61 the annual direct costs estimated for refraction and a pair of glasses varied from US $138.60 to $226.48 in the year 2000, depending on whether the fee schedule was based on Centers for Medicare and Medicaid Services or Medical Expenditure Panel Survey. This figure is likely a conservative estimate as it does not take into consideration all the direct health-related costs as well as non-health costs. In providing this figure, the authors suggested that the fee schedule gathered from the Medical Expenditure Panel Survey (US $226.48) was more reflective of the annual figure because it considered lens types, such as contact lenses, as well as any multiple purchases within the year.
Data gathered from optometry and ophthalmology sources in China (multiple sources from Zhongshan Ophthalmic Centre, AIER hospitals as well as data from Shanghai Eye Disease Prevention and Treatment Center for Anhui, Shanghai, and Yunnan region) suggest that the direct annual cost of vision products (assuming 100% of people with myopia purchase spectacles, 10% contact lenses, and 1.5% orthokeratology) and an eye examination is CN ¥ 809 or US $113. If refractive surgery is factored in for 1% of those with myopia, this cost increases to between US $125 to $136. Additionally, data from India (Courtesy: Optometry Council of India, L V Prasad Eye Institute, and private practitioners) suggest that the direct cost of vision products and an eye examination in urban India approximates to Rs3460 or US $48. Additionally, if 1% of those with myopia opt for refractive surgery, this cost increases to between US $54 to $60. When comparing costs between countries, one must be mindful of the relative purchasing power parity to place the cost in the context of society.
Except for cost estimates from Singapore, costs from other countries do not consider expenditures related to pathological myopia. Additionally, the cost estimates for China and India do not consider multiple purchases in a year and do not consider the more specialized lens requirements of those with high myopia. Considering that a progressing and/or a high myope would require more frequent changes, may need specialized lenses and frames, and may attend more frequent or specialist examinations, their costs are likely to be higher than the reported averages. If the proportional increase due to myopia in an older population from the Singapore data can be applied to China and India, then the cost associated with myopia in the older population would approximate to US $510 and $218 in China and India, respectively. Additionally, these figures exclude costs associated with newer myopia control management options, such as novel spectacles and contact lenses, which are likely to be greater than those of a standard lens. In addition to the direct medical costs, affected households and society incur additional productivity costs relating to caregiver time, absenteeism from educational activities, reduced productivity, and reduced QOL. Such costs are also likely to vary from country to country.
Although comprehensive cost data are limited, it is evident that there is a significant financial burden associated with myopia, particularly for poorer communities in countries with higher myopia prevalence and for individuals with high myopia and this is likely to rise in the future. Furthermore, unlike other conditions or diseases that may result in a one-off or a short-term cost, the chronic nature of myopia translates to a life-long burden. The Singapore study for adult myopia estimated a lifetime cost of SG $21,616 (US $17,020) for those with 80 years’ duration of myopia.60
It is therefore important that cost data are evaluated in the context of (a) relationship between incurred costs to health and productivity gains, and (b) ways to reduce burden (i.e. are the costs justified in terms of planned or achieved benefits or outcomes?). For the former issue, considering the Singapore data, a value of $709 per person for myopia care for adults in Singapore was considered to equate to < 2% of Gross Domestic Product (GDP) per person at that time in comparison to blindness and moderate distance vision impairment representing potential lost productivity of 18.7% and 3.1% of GDP, respectively.62 This, therefore, represented a positive trade-off when investing in myopia. However, the cost-benefit of using GDP or any other suitable metric may vary across countries and may or may not result in a positive trade-off. For example, the direct vision costs reported for China and India without the additional costs related to age approximate to 1.5% to 3% of their GDP per capita, but if age-related changes are factored in, these figures will significantly rise to 5% to 10% of GDP per capita. There is a need for further research, data, and modeling on lifetime costs associated with myopia. Several myopia control strategies have been shown to significantly slow myopia,63 and models demonstrate that such myopia control strategies, when applied early and consistently, can significantly reduce the risk of individuals reaching high myopia.23 It is reported that each diopter increase in myopia increases the risk of MMD by 67% or, alternatively, slowing myopia by about 1 D can reduce the likelihood of developing MMD by 40%.64 More data are needed to quantify these impacts from an economic perspective.
Patient-Reported Outcomes – Quality of Life and Myopia
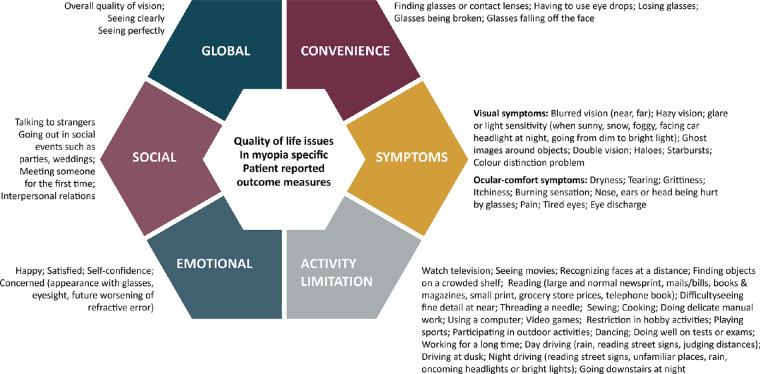
Myopia, either uncorrected or corrected, may impact a person's QOL and the effect on QOL can be evaluated using qualitative (interviews/focus-group discussions)65 or quantitative patient-reported outcome measures (PROMs), such as questionnaires and item-banks.66 PROMs used for myopia (Table 4) contain items on symptoms,67–72 activity limitation,67,69–72 emotional impact,67,68,71,72 social impact,67,69,72 and inconvenience69,72 (Fig. 2). These surveys were intended to evaluate QOL with myopia correction strategies, largely refractive surgery outcomes, and thus lack sufficient content on QOL issues for individuals with myopia in low-resource settings and uncorrected myopes. For example, people in low-resource settings may experience greater inconvenience having to travel far to have their myopia corrected, whereas having vision sufficient to drive may be of greater concern for dwellers from high income countries.65,73
Table 4.
Patient Reported Outcome Measures Used in Myopia
| Types | Sub-Types | Examples of Patient-Reported Outcome Measures |
|---|---|---|
| Generic (non-disease specific) | Domain | Emotional well-being: - Hospital Anxiety and Depression Scale (HADS),82 - General Well-Being Schedule (GWBS)83 - General Health Questionnaire (GHQ),129 - Hudson Index of Self-Esteem (HISE),129 - Adjective check list130 Pain: McGill Pain Questionnaire131 |
| Ophthalmic (non-myopia-specific) | Vision | - National Eye Institute Visual Function Questionnaire (NEI-VFQ),82,90 - Vision Related effect on Quality of Life (VQOL, aka Vision Core Measure-1 [VCM1])76 - Visual Function Index–14 (VF–14)76,86 |
| Refractive error | - Quality of Life Impact of Refractive Correction (QIRC)87 - National Eye Institute Refractive Quality of Life (NEI-RQL)132,133 - Refractive Status and Vision Profile (RSVP)85,134 | |
| Myopia-specific | Myopia correction | Institute for Eye Research Multidimensional Quality of Life for Myopia (MQLM)67 |
| Intervention | RK: Prospective Evaluation of Radial Keratotomy (PERK) Study questionnaire68 PRK: Canadian Refractive Surgery Research Group Quality of Vision Questionnaire (QVQ)69 LASIK: Subjective Vision Questionnaire (SVQ)70 and Myopia-specific Quality of Life Questionnaire (MQLQ)71 | |
| Population | Children: - Pediatric Refractive Error Profile (PREP)72,88,93,94,135 - Modified QIRC91 | |
| Health economic (utility) | Generic | - Time-Trade-off100,103 - Standard Gamble103 |
| Ophthalmic | - Vision Quality of Life index (VISQoL)136 |
LASIK, Laser-assisted In Situ Keratomileusis; PRK, Photorefractive Keratectomy; RK, Radial Keratotomy.
Figure 2.
Quality of life issues explored in myopia specific patient reported outcome measures.
Many existing myopia-specific PROMs are either first generation or second-generation questionnaires (classified based on the development or validation theory used74) that suffer from limitations of being static and inflexible, as every item is administered to every individual irrespective of their QOL issues. Additionally, clinical, demographic, and socio-economic characteristics influence QOL and therefore results vary between populations and groups.73,75 Despite their limitations, existing PROMs have proven valuable in evaluating QOL impacts of myopia and demonstrate that the impact of myopia on QOL is significant. The detrimental impacts of myopia have been observed in diverse aspects of daily living, including activity limitation, economic well-being, emotional well-being, symptoms, and social well-being.66,73,75–77 Generally, poorer QOL has been demonstrated for uncorrected refractive error/poor vision and high myopia as well as with complications associated with myopia. In a group of 16-year-old patients, myopia was an independent risk factor for poorer QOL for both distance and near vision, whereas hyperopia was not associated with any difficulty.78 Although data on the impact of uncorrected myopia on QOL and the benefits of spectacle wear are scarce, in a study of 2346 adolescents from southwestern China,79 where spectacle utilization was low, adolescents not using spectacles had lower psychosocial, emotional, and social functioning health-related QOL scores. Similarly, healthy adolescents with reduced VA reported lower health-related QOL, including social functioning and school functioning in two separate studies conducted in Singapore and China, although the study from Singapore did not find differences between refractive error types.80,81
In general, people with a higher magnitude of myopia are likely to have poorer QOL,76,82 and the impact of high myopia (−10.00 D) has been found to be similar to that of keratoconus.76 Individuals with high myopia have higher QOL concerns regarding cosmetic appearance, especially if they have to wear thick lenses, and they spend more money on spectacles than those with low or moderate myopia, as the cost of thinner and lighter (high index) spectacle lenses is higher. However, the expenditure for contact lenses was found to be similar between high and low myopia groups.76,83 In adults with high myopia (worse than −8.00 D), functional status in daily life was reduced in those with myopia compared to controls and was represented by poor scores on disability (e.g. reading signs), handicap (e.g. unable to perform studies and jobs), and support (understanding from the family structure).83 More significantly, Yokoi et al. reported that about 25% of patients with high myopia were likely to have depression and anxiety disorders, which could substantially lower QOL.82 In older individuals, those with advanced MMD had poorer vision-related QOL than those without.84
Additionally, the impact of myopia on QOL differs by type of myopia. In presbyopic populations, individuals with myopic astigmatism have worse QOL than spherical myopia.85
Correction improves QOL, particularly bringing improvements to visual functioning and symptoms.71,77,86–89 However, it should be noted that refractive correction may not restore QOL to an emmetropic level.88,90,91 For example, spectacle wearers may have concerns about cosmetic appearance and the inconveniences of having to look after their spectacles,73 contact lens wearers may have concerns about possible complications, and those who have undergone refractive surgery may have to live with glare and dry eye-related symptoms.73,92
In children and teenagers with myopia, a better vision-related QOL has been reported with contact lenses than with spectacles, including with contact lenses designed for slowing myopia.93–96 Areas that showed improvement were increased satisfaction with correction, activities, and appearance. Orthokeratology lenses were also well-accepted and brought significant improvement in QOL in children with myopia.88
Although the above data have heterogeneity in factors, including study populations and choice of PROMs, it is clear that uncorrected myopia and high myopia are associated with poorer or reduced outcomes for vision and health-related QOL measures. It is also clear that QOL can be improved with appropriate correction for those with uncorrected myopia. However, for those with high myopia, there remain gaps in the existing literature on interventions that provide improvements in QOL.
For economic evaluations, such as cost-effectiveness analysis, QOL data can be used as quantifiable inputs for assessing the value of health interventions. Here, QOL data are input on a 0 to 1 scale for utility (0 = death and 1 = perfect health) or a disability weight (0 = perfect health and 1 = death or complete disability). In such evaluations, disparate health conditions can be compared, for example, the utility for severe angina has been reported as 0.5,97 whereas utility of complete blindness was reported as 0.26.98 Utilities associated with ocular conditions, including refractive error, were related to the level of VA loss (Fig. 3).99,100 For uncorrected myopia in adults,100 decrement in utility significantly correlated with higher levels of myopia (see Fig. 3).100 Interestingly, utilities reported for uncorrected myopia (causing distance vision impairment) are reportedly similar to those for uncorrected presbyopia (near vision impairment; Table 5) and, generally, utility values for refractive error were reportedly higher than utility values for ocular disease (see Table 5, Fig. 3). Utility studies evaluating corrected myopia (one with teenagers,101 one with medical students,102 and one with adults103) found utility to be close to perfect health and ranged from 0.93 to 0.97. A Chinese study involving 442 patients with myopia who were scheduled to undergo refractive surgery found contact lens users had a significantly better QOL (higher utility) compared to spectacle wearers.103 Although, research has made utility data available for input in economic evaluations of myopia, no such studies are available in the current literature.
Figure 3.
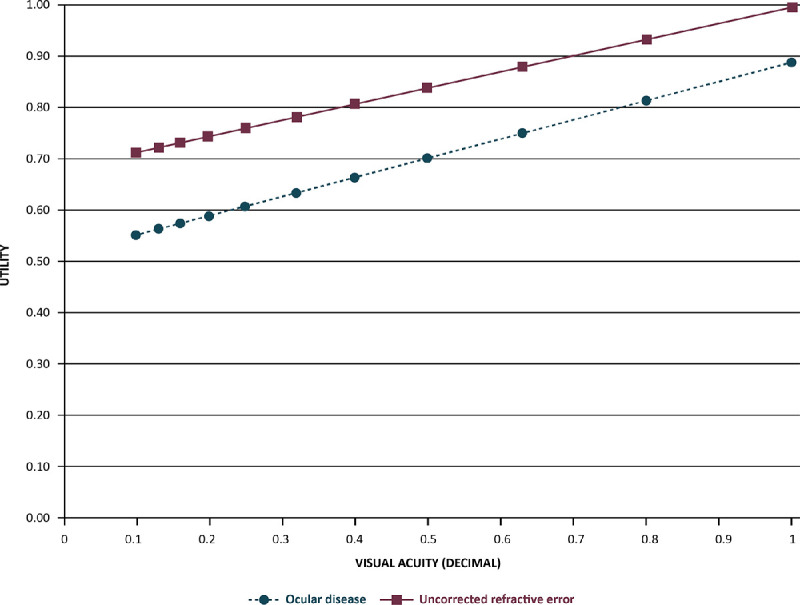
Relationship between utility and visual acuity.100
Table 5.
Utility Findings for Uncorrected Refractive Error and Ocular Disease100
| Uncorrected | Ocular Disease Ranges | ||||
|---|---|---|---|---|---|
| Utility (0-Death; 1-Perfect Health) | Presbyopia: Only Near* Impaired (Mean ± SD) | Myopia: Only Distance† Impaired (Mean ± SD) | Refractive Error: Both Distance and Near Impaired (Mean ± SD) | Moderate VI | Severe VI |
| LogMAR VA | 0.43 ± 0.17 | 0.50 ± 0.24 | 0.64 ± 0.27 | 0.2–0.7 | 0.61-NLP |
| Snellen VA | 6/15–6/19 | 6/19 | 6/24 | 6/9.5–6/30 | 6/24-NLP |
| Decimal VA | 0.37 | 0.32 | 0.23 | 0.6–0.2 | 0.25-NLP |
| Time-trade-off utility | 0.81 ± 0.17 | 0.82 ± 0.16 | 0.68 ± 0.25 | 0.67 | 0.64–0.47 (0.57) |
VA, visual acuity; VI, visual impairment;
VA measure at 40 cm.
VA measure at 6 m.
Disability weight estimates are led by a consortium of research collaborators through the Global Burden of Disease (GBD) program. Table 6 provides a list of disability weights for various degrees of vision impairment and other comparable health disorders calculated since 2004.104 Significant debate in the literature about the validity of methods used to derive recent figures has ensued, with researchers ascribing poor face validity of assessment methods as a factor for unreasonably low estimates for vision impairment and blindness compared to previous values in 2004.105–107 Global Health Estimates (GHEs) have been provided by the WHO in recent years to counter these low values, but in spite of revisions, current disability weights for vision impairment and blindness are still substantially lower than those from 2004.104 Disability weights have been used as inputs in economic evaluations of myopia for quantifying indirect costs to society, including lost productivity.108,109 Using low disability weights in economic evaluations would under-represent the true impact and economic consequences of visually impairing and blinding conditions, such as high myopia. This could lead to lower government prioritization for health spending relative to other medical conditions.
Table 6.
Disability Weights for Vision Loss and Various Health States104
| Disability Weights (0-Perfect Health; 1-Death or Complete Disability) | |||||
|---|---|---|---|---|---|
| Health State | GHE 2015 | GHE 2012 | GDB 2015 | GDB 2010 | GDB 2004 |
| Distance vision: mild impairment | 0.005 | 0.005 | 0.003 | 0.004 | |
| Distance vision: moderate impairment | 0.089 | 0.089 | 0.031 | 0.033 | 0.17 |
| Distance vision: severe impairment | 0.314 | 0.314 | 0.184 | 0.191 | 0.43 |
| Distance vision blindness | 0.338 | 0.338 | 0.187 | 0.195 | 0.6 |
| Near vision impairment | 0.013 | 0.02 | |||
| Hearing loss: mild | 0.01 | 0.005 | 0.01 | 0.005 | 0.04 |
| Hearing loss: moderate | 0.05 | 0.05 | 0.027 | 0.023 | 0.12 |
| Hearing loss: severe | 0.167 | 0.167 | 0.158 | 0.031 | 0.333 |
| Hearing loss: profound | 0.281 | 0.281 | 0.204 | 0.032 | 0.333 |
| Hearing loss: complete | 0.281 | 0.281 | 0.215 | 0.033 | |
| Infertility: primary | 0.056 | 0.056 | 0.008 | 0.011 | 0.18 |
| Dementia: mild | 0.165 | 0.165 | 0.069 | 0.082 | |
| Dementia: moderate | 0.388 | 0.388 | 0.377 | 0.346 | 0.666 |
| Dementia: severe | 0.545 | 0.545 | 0.449 | 0.438 | 0.94 |
GHE, Global Health Estimates; GBD, Global Burden of Disease.
Economic Impact of Myopia on Society
Although limited economic evaluations of myopia are available in the literature, the existing data provide some significant insights on the current and future burden. With respect to direct health expenditure, data from Singapore suggested an annual SG $959 million (US $755 million) in 2011 for direct costs associated with myopia in terms of eye examinations, vision correction, and transport. When placed in context, these are significantly greater than those reported for other chronic conditions in Singapore, such as Parkinson's disease (US $23–41 million), chronic obstructive pulmonary disease (US $9 million), and acute primary angle-closure glaucoma (US $0.2–0.3 million). Even though individual costs of myopia are relatively low in the early years, the higher societal costs are driven by the fact that myopia is more prevalent and complications associated with higher magnitudes of myopia are more common later in life. As Singapore has a high prevalence of myopia and high myopia, it may be inferred from this valuation that countries, particularly those in East Asia with similar prevalence rates, carry a similar burden profile whereby the costs of myopia are significantly greater compared to other diseases or medical conditions.
In a more recent conference proceeding,109 the global costs of myopia and directs costs (including examinations, cost of spectacles and lenses, LASIK, care for complications such as cataract, retinopathy, and glaucoma) were estimated to be US $358.7 billion in 2019 and projected to rise to US $870 billion in 2050. Importantly, these data indicate that whereas costs related to spectacles and lenses are set to double, costs related to cataract care and myopic retinopathy are estimated to quadruple.
In addition to the economic burden associated with direct costs, the burden of productivity costs associated with myopia is also significant. Potential lost productivity due to vision impairment was estimated at US $244 billion from uncorrected myopia and US $6 billion from MMD in 2015.108 These estimates do not include children < 15 years of age. Another study reported a productivity loss of about US $94.5 billion in 2019 from severe vision impairment and blindness projected to rise to US $229.3 billion in 2050.109
Key points that arise from these findings are: first, global costs of myopia and high myopia are set to rise substantially in the future due the increasing prevalence of myopia. The costs attributable to high myopia and related complications, such as MMD, as a percent of overall costs will be rising due to the disproportionately higher increase in the prevalence of high myopia relative to myopia overall. Second, significant benefits in productivity can be gained by managing uncorrected myopia. Even though myopia correction may increase direct health expenditure, the benefits due to improved productivity are significantly greater than the costs associated with managing the burden. Indeed, it has been reported that the global productivity losses far exceed estimated costs of providing the world's population with refractive error correction, including establishing, maintaining, and operating refractive care facilities.110 Third, myopia control strategies that prevent the onset of myopia and/or slow the progression of myopia may result in long term savings in direct and indirect health spending for both the individual and society. However, these interventions do often come at a higher price in the early years and cost-effectiveness evaluations, which weigh the initial outlay of costs against the long-term benefits for these strategies remain to be determined.
Summary
The prevalence of myopia is high and rising worldwide with consequences spanning from childhood to late adult life. Recent evidence reveals that the prevalence of high myopia is growing at a faster rate than the prevalence of overall myopia in conjunction with rising rates of serious blinding complications associated with high myopia, notably MMD. East Asian countries have a higher prevalence of myopia and high myopia and therefore carry a major share of the global burden.
For the individual, particularly a young individual, when vision is impaired either due to uncorrected myopia or due to complications associated with myopia, academic performance and psychosocial well-being are likely to be affected. In adults, myopia results in significant losses in productivity and negative impact on QOL. QOL is adversely affected by uncorrected myopia, high myopia, and complications of high myopia. Although certain corrective modalities appear to improve QOL in certain domains, further information is needed on interventions that provide QOL benefits for those with high myopia. Conventional correction of myopia restores vision but does not slow progression, and direct health expenditure related to myopia is much higher in older individuals, partly due to costs associated with myopia-related complications. Additionally, there is the burden related to lost productivity; although the majority of the current burden is due to uncorrected myopia, data indicate that the proportion resulting from vision impairment due to complications of high myopia will rise in the future.
Currently, the global costs related to direct health expenditure and lost productivity as a result of myopia are in the range of several hundred billion dollars annually. Unless the current trajectory for the rising prevalence of myopia and high myopia is lowered, the costs will continue to grow.
Shifting the trajectory requires a coordinated global effort and it is encouraging that there have been some successes with optical, environmental, and pharmaceutical strategies to prevent the onset and/or effectively slow the progression of myopia. An early and appropriate intervention mitigates the risks and consequences related to uncorrected vision. More importantly, it can reduce the risk of the eye progressing to higher levels of myopia and thus have a positive impact on reducing the burden. Assessment of the costs and cost-effectiveness of these various interventions is in the early stages. Such research will provide individuals, governments, and other decision makers with quantifiable information that will facilitate optimal health resource allocation decisions.
Acknowledgments
The authors thank Monica Jong for facilitation of the process.
Supported by the International Myopia Institute. The publication costs of the International Myopia Institute reports were supported by donations from the Brien Holden Vision Institute, Carl Zeiss Vision, Cooper Vision, Essilor, and Alcon.
Disclosure: P. Sankaridurg, BHVI (E), co-inventor on multiple patents related to myopia (P), Alcon (R), SEED (R), Mark Ennovy (R), Carl Zeiss Vision (R); N. Tahhan, BHVI (E); H. Kandel, None; T. Naduvilath, BHVI (E), co-inventor on patent related to myopia (P); H. Zou, None; K.D. Frick, None; S. Marmamula, None; D.S. Friedman, None; E. Lamarouex, None; J. Keeffe, None; J.J. Walline, Bausch + Lomb (F); T.R. Fricke, BHVI (E); V. Kovai, None; S. Resnikoff, None
References
- 1. Bourne RRA, Flaxman SR, Braithwaite T, et al.. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017; 5(9): e888–e897. [DOI] [PubMed] [Google Scholar]
- 2. Holden BA, Fricke TR, Wilson DA, et al.. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123(5): 1036–1042. [DOI] [PubMed] [Google Scholar]
- 3. Fricke TR, Jong M, Naidoo KS, et al.. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: Systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018; 102(7): 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flitcroft DI, He M, Jonas JB, et al.. IMI – Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Investig Ophthalmol Vis Sci. 2019; 60(3): M20–M30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Liu J, Qi P.. The increasing prevalence of myopia in junior high school students in the Haidian District of Beijing, China: A 10-year population-based survey. BMC Ophthalmol. 2017; 17(1): 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong L, Kang YK, Li Y, Wei W, Bin Jonas J.. Prevalence and time trends of myopia in children and adolescents in CHINA: a systemic review and meta-analysis. Retina. 2019; 40(3): 399–411. [DOI] [PubMed] [Google Scholar]
- 7. French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA.. Prevalence and 5- to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. 2013; 120(7): 1482–1491. [DOI] [PubMed] [Google Scholar]
- 8. O'Donoghue L, McClelland JF, Logan NS, Rudnicka AR, Owen CG, Saunders KJ.. Refractive error and visual impairment in school children in Northern Ireland. Br J Ophthalmol. 2010; 94(9): 1155–1159. [DOI] [PubMed] [Google Scholar]
- 9. Harrington SC, Stack J, Saunders K, O'Dwyer V. Refractive error and visual impairment in Ireland schoolchildren. Br J Ophthalmol. 2019; 103(8): 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murthy GVS, Gupta SK, Ellwein LB, et al.. Refractive error in children in an urban population in New Delhi. Investig Ophthalmol Vis Sci. 2002; 43(3): 623–631. [PubMed] [Google Scholar]
- 11. Saxena R, Vashist P, Tandon R, et al.. Prevalence of myopia and its risk factors in urban school children in Delhi: The North India myopia study (NIM study). PLoS One. 2015; 10(2): e0117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh NK, James RM, Yadav A, Kumar R, Asthana S, Labani S.. Prevalence of myopia and associated risk factors in schoolchildren in North India. Optom Vis Sci. 2019; 96(3): 200–205. [DOI] [PubMed] [Google Scholar]
- 13. Fan DSP, Lam DSC, Lam RF, et al.. Prevalence, incidence, and progression of myopia of school children in Hong Kong. Investig Ophthalmol Vis Sci. 2004; 45(4): 1071–1075. [DOI] [PubMed] [Google Scholar]
- 14. Yam JC, Tang SM, Kam KW, et al.. High prevalence of myopia in children and their parents in Hong Kong Chinese Population: the Hong Kong Children Eye Study [published online ahead of print Jan, 24, 2020]. Acta Ophthalmol, 10.1111/aos.14350. [DOI] [PubMed] [Google Scholar]
- 15. Xu L, Li J, Cui T, et al.. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology. 2005; 112(10): 1676–1683. [DOI] [PubMed] [Google Scholar]
- 16. Wang M, Cui J, Shan G, et al.. Prevalence and risk factors of refractive error: a cross-sectional study in Han and Yi adults in Yunnan, China. BMC Ophthalmol. 2019; 19(1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ueda E, Yasuda M, Fujiwara K, et al.. Trends in the prevalence of myopia and myopic maculopathy in a Japanese population: the Hisayama study. Investig Ophthalmol Vis Sci. 2019; 60(8): 2781–2786. [DOI] [PubMed] [Google Scholar]
- 18. Williams KM, Bertelsen G, Cumberland P, et al.. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology. 2015; 122(7): 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen M, Wu A, Zhang L, et al.. The increasing prevalence of myopia and high myopia among high school students in Fenghua City, Eastern China: a 15-year population-based survey. BMC Ophthalmol. 2018; 18(1): 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang TJ, Chiang TH, Wang TH, Lin LLK, Shih YF.. Changes of the ocular refraction among freshmen in National Taiwan University between 1988 and 2005. Eye. 2009; 23(5): 1168–1169. [DOI] [PubMed] [Google Scholar]
- 21. Ma Y, Qu X, Zhu X, et al.. Age-specific prevalence of visual impairment and refractive error in children aged 3–10 years in Shanghai, China. Investig Ophthalmol Vis Sci. 2016; 57(14): 6188–6196. [DOI] [PubMed] [Google Scholar]
- 22. Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL III, Holden BA. Myopia progression rates in urban children wearing single-vision spectacles. Optom Vis Sci. 2012; 89(1): 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sankaridurg PR, Holden BA.. Practical applications to modify and control the development of ametropia. Eye. 2014; 28(2): 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chua SYL, Sabanayagam C, Cheung YB, et al.. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016; 36(4): 388–394. [DOI] [PubMed] [Google Scholar]
- 25. Williams KM, Hysi PG, Nag A, Yonova-Doing E, Venturini C, Hammond CJ.. Age of myopia onset in a British population-based twin cohort. Ophthalmic Physiol Opt. 2013; 33(3): 336–345. [DOI] [PubMed] [Google Scholar]
- 26. Dirani M, Shekar SN, Baird PN.. Adult-onset myopia: The genes in myopia (GEM) twin study. Investig Ophthalmol Vis Sci. 2008; 49(8): 3324–3327. [DOI] [PubMed] [Google Scholar]
- 27. Ma Y, Qu X, Zhu X, et al.. Age-specific prevalence of visual impairment and refractive error in children aged 3–10 years in Shanghai, China. Investig Ophthalmol Vis Sci. 2016; 57(14): 6188–6196. [DOI] [PubMed] [Google Scholar]
- 28. Mutti DO, Hayes JR, Mitchell GL, et al.. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Investig Ophthalmol Vis Sci. 2007; 48(6): 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiang F, He M, Morgan IG.. Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology. 2012; 119(7): 1478–1484. [DOI] [PubMed] [Google Scholar]
- 30. Liao C, Ding X, Han X, et al.. Role of parental refractive status in myopia progression: 12-year annual observation from the Guangzhou Twin Eye Study. Invest Ophthalmol Vis Sci. 2019; 60(10): 3499–3506. [DOI] [PubMed] [Google Scholar]
- 31. Ma Y, Zou H, Lin S, et al.. Cohort study with 4-year follow-up of myopia and refractive parameters in primary schoolchildren in Baoshan District, Shanghai. Clin Exp Ophthalmol. 2018; 46(8): 861–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parssinen O, Kauppinen M.. Risk factors for high myopia: a 22-year follow-up study from childhood to adulthood. Acta Ophthalmol. 2019; 97(5): 510–518. [DOI] [PubMed] [Google Scholar]
- 33. Iribarren R, Cortinez MF, Chiappe JP.. Age of first distance prescription and final myopic refractive error. Ophthalmic Epidemiol. 2009; 16(2): 84–89. [DOI] [PubMed] [Google Scholar]
- 34. McBrien NA, Adams DW.. A longitudinal investigation of adult-onset and adult-progression of myopia in an occupational group: refractive and biometric findings. Investig Ophthalmol Vis Sci. 1997; 38(2): 321–333. [PubMed] [Google Scholar]
- 35. Tsai ASH, Wong CW, Lim L, et al.. Pediatric retinal detachment in an Asian population with high prevalence of myopia: clinical characteristics, surgical outcomes, and prognostic factors. Retina. 2019; 39(9): 1751–1760. [DOI] [PubMed] [Google Scholar]
- 36. Lim CHL, Stapleton F, Mehta JS.. Review of contact lens–related complications. Eye Contact Lens. 2018; 44(Suppl 2): S1–S10. [DOI] [PubMed] [Google Scholar]
- 37. Sakimoto T, Rosenblatt MI, Azar DT.. Laser eye surgery for refractive errors. Lancet. 2006; 367(9529): 1432–1447. [DOI] [PubMed] [Google Scholar]
- 38. Yokoi T, Ohno-Matsui K.. Diagnosis and treatment of myopic maculopathy. Asia Pac J Ophthalmol (Phila). 2018; 7(6): 415–421. [DOI] [PubMed] [Google Scholar]
- 39. Chang L, Pan CW, Ohno-Matsui K, et al.. Myopia-related fundus changes in Singapore adults with high myopia. Am J Ophthalmol. 2013; 155(6): 991–999. [DOI] [PubMed] [Google Scholar]
- 40. Hsu WM, Cheng CY, Liu JH, Tsai SY, Chou P.. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2004; 111(1): 62–69. [DOI] [PubMed] [Google Scholar]
- 41. Zou M, Wang S, Chen A, et al.. Prevalence of myopic macular degeneration worldwide: a systematic review and meta-analysis. Br J Ophthalmol. 2020; 104(12): 1748–1754. [DOI] [PubMed] [Google Scholar]
- 42. Fricke TR, Jong M, Naidoo KS, et al.. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018; 102(7): 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morgan I, Rose K.. How genetic is school myopia? Prog Retin Eye Res. 2005; 24(1): 1–38. [DOI] [PubMed] [Google Scholar]
- 44. Morgan IG, French AN, Ashby RS, et al.. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018; 62: 134–149. [DOI] [PubMed] [Google Scholar]
- 45. Case A, Fertig A, Paxson C.. The lasting impact of childhood health and circumstance. J Health Econ. 2005; 24(2): 365–389. [DOI] [PubMed] [Google Scholar]
- 46. Ma X, Zhou Z, Yi H, et al.. Effect of providing free glasses on children's educational outcomes in China: cluster randomized controlled trial. BMJ. 2014; 349: g5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ma Y, Congdon N, Shi Y, et al.. Effect of a local vision care center on eyeglasses use and school performance in rural China a cluster randomized clinical trial. JAMA Ophthalmol. 2018; 136(7): 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dudovitz RN, Izadpanah N, Chung PJ, Slusser W.. Parent, teacher, and student perspectives on how corrective lenses improve child wellbeing and school function. Matern Child Health J. 2016; 20(5): 974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hinterlong JE, Holton VL, Chiang CC, Tsai CY, Liou YM.. Association of multimedia teaching with myopia: A national study of school children. J Adv Nurs. 2019; 75(2): 3643–3653. [DOI] [PubMed] [Google Scholar]
- 50. Negiloni K, Ramani KK, Sudhir RR.. Do school classrooms meet the Visual requirements of children and recommended vision standards? PLoS One. 2017; 12(4): e0174983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chung STL, Jarvis SH, Cheung SH.. The effect of dioptric blur on reading performance. Vision Res. 2007; 47(12): 1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wedner S, Masanja H, Bowman R, Todd J, Bowman R, Gilbert C.. Two strategies for correcting refractive errors in school students in Tanzania: randomised comparison, with implications for screening programmes. Br J Ophthalmol. 2008; 92(1): 19–24. [DOI] [PubMed] [Google Scholar]
- 53. Gogate P, Mukhopadhyaya D, Mahadik A, et al.. Spectacle compliance amongst rural secondary school children in Pune district, India. Indian J Ophthalmol. 2013; 61(1): 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khandekar R, Mohammed AJ, Al Raisi A. Compliance of spectacle wear and its determinants among schoolchildren of Dhakhiliya region of Oman: a descriptive study. J Sci Res Med Sci. 2002; 4(1-2): 36–43. [PMC free article] [PubMed] [Google Scholar]
- 55. Morjaria P, McCormick I, Gilbert C.. Compliance and predictors of spectacle wear in schoolchildren and reasons for non-wear: a review of the literature. Ophthalmic Epidemiol. 2019; 26(6): 367–377. [DOI] [PubMed] [Google Scholar]
- 56. Narayanan A, Kumar S, Ramani KK.. Spectacle compliance among adolescents: A qualitative study from Southern India. Optom Vis Sci. 2017; 94(5): 582–587. [DOI] [PubMed] [Google Scholar]
- 57. He M, Huang W, Zheng Y, Huang L, Ellwein LB.. Refractive error and visual impairment in school children in rural Southern China. Ophthalmology. 2007; 114(2): 374–382. [DOI] [PubMed] [Google Scholar]
- 58. Mayro EL, Hark LA, Shiuey E, et al.. Prevalence of uncorrected refractive errors among school-age children in the school district of Philadelphia. J AAPOS. 2018; 22(3): 214–217. [DOI] [PubMed] [Google Scholar]
- 59. Lim MCC, Gazzard G, Sim EL, Tong L, Saw SM.. Direct costs of myopia in Singapore. Eye. 2009; 23(5): 1086–1089. [DOI] [PubMed] [Google Scholar]
- 60. Zheng YF, Pan CW, Chay J, Wong TY, Finkelstein E, Saw SM.. The economic cost of myopia in adults aged over 40 years in Singapore. Investig Ophthalmol Vis Sci. 2013; 54(12): 7532–7537. [DOI] [PubMed] [Google Scholar]
- 61. Vitale S, Cotch MF, Sperduto R, Ellwein L.. Costs of refractive correction of distance vision impairment in the United States, 1999-2002. Ophthalmology. 2006; 113(12): 2163–2170. [DOI] [PubMed] [Google Scholar]
- 62. Congdon N, Burnett A, Frick K.. The impact of uncorrected myopia on individuals and society. Community Eye Health. 2019; 32(105): 7–8. [PMC free article] [PubMed] [Google Scholar]
- 63. Wildsoet CF, Chia A, Cho P, et al.. IMI–Interventions myopia institute: Interventions for controlling myopia onset and progression report. Investig Ophthalmol Vis Sci. 2019; 60(3): M106–M131. [DOI] [PubMed] [Google Scholar]
- 64. Bullimore MA, Brennan NA.. Myopia control: why each diopter matters. Optom Vis Sci. 2019; 96(6): 463–465. [DOI] [PubMed] [Google Scholar]
- 65. Kandel H, Khadka J, Shrestha MK, et al.. Uncorrected and corrected refractive error experiences of Nepalese adults: a qualitative study. Ophthalmic Epidemiol. 2018; 25(2): 147–161. [DOI] [PubMed] [Google Scholar]
- 66. Kandel H, Khadka J, Goggin M, Pesudovs K.. Patient-reported outcomes for assessment of quality of life in refractive error: a systematic review. Optom Vis Sci. 2017; 94(12): 1099–1107. [DOI] [PubMed] [Google Scholar]
- 67. Erickson DB, Stapleton F, Erickson P, Du Toit R, Giannakopoulos E, Holden B. Development and validation of a multidimensional quality-of-life scale for myopia. Optom Vis Sci. 2004; 81(2): 70–81. [DOI] [PubMed] [Google Scholar]
- 68. Bourque LB, Cosand BB, Drews C, Waring GO, Lynn M, Cartwright C.. Reported satisfaction, fluctuation of vision, and glare among patients one year after surgery in the prospective evaluation of radial keratotomy (PERK) study. Arch Ophthalmol. 1986; 104(3): 356–363. [DOI] [PubMed] [Google Scholar]
- 69. Brunette I, Gresset J, Boivin JF, Boisjoly H, Makni H.. Functional outcome and satisfaction after photorefractive keratectomy: Part 1: Development and validation of a survey questionnaire. Ophthalmology. 2000; 107(9): 1783–1789. [DOI] [PubMed] [Google Scholar]
- 70. Fraenkel G, Comaish I, Lawless MA, et al.. Development of a questionnaire to assess subjective vision score in myopes seeking refractive surgery. J Refract Surg. 2004; 20(1): 10–19. [DOI] [PubMed] [Google Scholar]
- 71. Lee J, Lee J, Park K, Cho W, Kim JY, Kang HY.. Assessing the value of laser in situ keratomileusis by patient-reported outcomes using quality of life assessment. J Refract Surg. 2005; 21(1): 59–71. [DOI] [PubMed] [Google Scholar]
- 72. Walline JJ, Jones LA, Chitkara M, et al.. The adolescent and child health initiative to encourage vision empowerment (ACHIEVE) study design and baseline data. Optom Vis Sci. 2006; 83(1): 37–45. [DOI] [PubMed] [Google Scholar]
- 73. Kandel H, Khadka J, Goggin M, Pesudovs K.. Impact of refractive error on quality of life: a qualitative study. Clin Exp Ophthalmol. 2017; 45(7): 677–688. [DOI] [PubMed] [Google Scholar]
- 74. Pesudovs K. Item banking: a generational change in patient-reported outcome measurement. Optom Vis Sci. 2010; 87(4): 285–293. [DOI] [PubMed] [Google Scholar]
- 75. Kandel H, Khadka J, Fenwick EK, et al.. Constructing item banks for measuring quality of life in refractive error. Optom Vis Sci. 2018; 95(7): 575–587. [DOI] [PubMed] [Google Scholar]
- 76. Rose K, Harper R, Tromans C, et al.. Quality of life in myopia. Br J Ophthalmol. 2000; 84: 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kandel H, Khadka J, Shrestha MK, et al.. Uncorrected and corrected refractive error experiences of Nepalese adults: a qualitative study. Ophthalmic Epidemiol. 2018; 25(2): 147–161. [DOI] [PubMed] [Google Scholar]
- 78. Hsieh MH, Lin JC.. Association of refractive error with vision-related quality of life in junior high school students. Taiwan J Ophthalmol. 2016; 6(1): 32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Qian DJ, Zhong H, Li J, Liu H, Pan CW.. Spectacles utilization and its impact on health-related quality of life among rural Chinese adolescents. Eye. 2018; 32(12): 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pan CW, Wu RK, Wang P, Li J, Zhong H.. Reduced vision, refractive errors and health-related quality of life among adolescents in rural China. Clin Exp Optom. 2018; 101(6): 758–763. [DOI] [PubMed] [Google Scholar]
- 81. Wong HB, Machin D, Tan SB, Wong TY, Saw SM.. Visual impairment and its impact on health-related quality of life in adolescents. Am J Ophthalmol. 2009; 147(3): 505–511. [DOI] [PubMed] [Google Scholar]
- 82. Yokoi T, Moriyama M, Hayashi K, et al.. Predictive factors for comorbid psychiatric disorders and their impact on vision-related quality of life in patients with high myopia. Int Ophthalmol. 2014; 34(2): 171–183. [DOI] [PubMed] [Google Scholar]
- 83. Takashima T, Yokoyama T, Futagami S, et al.. The quality of life in patients with pathologic myopia. Nihon Ganka Gakkai Zasshi. 2002; 45(1): 84–92. [PubMed] [Google Scholar]
- 84. Wong Y-L, Sabanayagam C, Wong C-W, et al.. Six-year changes in myopic macular degeneration in adults of the Singapore epidemiology of eye diseases study. Invest Ophthalmol Vis Sci. 2020; 61(4): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Savage H, Rothstein M, Davuluri G, El Ghormli L, Zaetta DM.. Myopic astigmatism and presbyopia trial. Am J Ophthalmol. 2003; 135(5): 628–632. [DOI] [PubMed] [Google Scholar]
- 86. Kanonidou E, Chatziralli IP, Konidaris V, Kanonidou C, Papazisis L.. A comparative study of visual function of young myopic adults wearing contact lenses vs. spectacles. Contact Lens Anterior Eye. 2012; 35(5): 196–198. [DOI] [PubMed] [Google Scholar]
- 87. Meidani A, Tzavara C, Dimitrakaki C, Pesudovs K, Tountas Y.. Femtosecond laser-assisted LASIK improves quality of life. J Refract Surg. 2012; 28(5): 319–326. [DOI] [PubMed] [Google Scholar]
- 88. Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R.. Myopia control with orthokeratology contact lenses in Spain: A comparison of vision-related quality-of-life measures between orthokeratology contact lenses and single-vision spectacles. Eye Contact Lens. 2013; 39(2): 153–157. [DOI] [PubMed] [Google Scholar]
- 89. Esteso P, Castanon A, Toledo S, et al.. Correction of moderate myopia is associated with improvement in self-reported visual functioning among Mexican school-aged children. Investig Ophthalmol Vis Sci. 2007; 48(11): 4949–4954. [DOI] [PubMed] [Google Scholar]
- 90. Sandhu RK, Munoz BE, Swenor BK, West SK.. Refractive error and visual function difficulty in a Latino population. Ophthalmology. 2012; 119(9): 1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhao F, Zhao G, Zhao Z.. Investigation of the effect of orthokeratology lenses on quality of life and behaviors of children. Eye Contact Lens Sci Clin Pract. 2018; 44(5): 335–338. [DOI] [PubMed] [Google Scholar]
- 92. Kandel H, Khadka J, Lundström M, Goggin M, Pesudovs K.. Questionnaires for measuring refractive surgery outcomes. J Refract Surg. 2017; 33(6): 416–424. [DOI] [PubMed] [Google Scholar]
- 93. Rah MJ, Walline JJ, Jones-Jordan LA, et al.. Vision specific quality of life of pediatric contact lens wearers. Optom Vis Sci. 2010; 87(8): 560–566. [DOI] [PubMed] [Google Scholar]
- 94. Walline JJ, Gaume A, Jones LA, et al.. Benefits of contact lens wear for children and teens. Eye Contact Lens. 2007; 33(6 Pt 1): 317–321. [DOI] [PubMed] [Google Scholar]
- 95. Pomeda AR, Pérez-Sánchez B, Cañadas Suárez MDP, Prieto Garrido FL, Gutiérrez-Ortega R, Villa-Collar C. MiSight Assessment Study Spain: a comparison of vision-related quality-of-life measures between MiSight contact lenses and single-vision spectacles. Eye Contact Lens. 2018; 44(Suppl 2): S99–S104. [DOI] [PubMed] [Google Scholar]
- 96. Plowright AJ, Maldonado-Codina C, Howarth GF, Kern J, Morgan PB.. Daily disposable contact lenses versus spectacles in teenagers. Optom Vis Sci. 2015; 92(1): 44–52. [DOI] [PubMed] [Google Scholar]
- 97. Phillips KA, Shlipak MG, Coxson P, et al.. Health and economic benefits of increased β-blocker use following myocardial infarction. J Am Med Assoc. 2000; 284(21): 2748–2754. [DOI] [PubMed] [Google Scholar]
- 98. Brown MM, Brown GC, Sharma S, Kistler J, Brown H.. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001; 85(3): 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sharma S, Brown GC, Brown MM, et al.. Converting visual acuity to utilities. Can J Ophthalmol. 2000; 35(5): 267–272. [DOI] [PubMed] [Google Scholar]
- 100. Tahhan N, Papas E, Fricke TR, Frick KD, Holden BA.. Utility and uncorrected refractive error. Ophthalmology. 2013; 120(9): 1736–1744. [DOI] [PubMed] [Google Scholar]
- 101. Saw SM, Gazzard G, Au Eong KG, Koh D. Utility values and myopia in teenage school students. Br J Ophthalmol. 2003; 87(3): 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lim WY, Saw SM, Singh MK, Au Eong KG. Utility values and myopia in medical students in Singapore. Clin Exp Ophthalmol. 2005; 33(6): 598–603. [DOI] [PubMed] [Google Scholar]
- 103. Li S, Wang G, Xu Y, Gray A, Chen G.. Utility values among myopic patients in mainland China. Optom Vis Sci. 2014; 91(7): 723–729. [DOI] [PubMed] [Google Scholar]
- 104. World Health Organisation. WHO methods and data sources for global burden of disease estimates 2000-2015, https://www.who.int/healthinfo/global_burden_disease/data_sources_methods/en/. Accessed March 26, 2020.
- 105. Taylor HR, Jonas JB, Keeffe J, et al.. Disability weights for vision disorders in Global Burden of Disease study. Lancet. 2013; 381(9860): 23. [DOI] [PubMed] [Google Scholar]
- 106. Nord E. Disability weights in the Global Burden of Disease 2010: Unclear meaning and overstatement of international agreement. Health Policy. 2013; 111(1): 99–104. [DOI] [PubMed] [Google Scholar]
- 107. Braithwaite T, Taylor H, Bourne R, Keeffe J, Pesudovs K.. Does blindness count? Disability weights for vision loss. Clin Exp Ophthalmol. 2017; 45(3): 217–220. [DOI] [PubMed] [Google Scholar]
- 108. Naidoo KS, Fricke TR, Frick KD, et al.. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. 2019; 126(3): 338–346. [DOI] [PubMed] [Google Scholar]
- 109. Holy C, Kulkarni K, Brennan NA.. Predicting costs and disability from the myopia epidemic–a worldwide economic and social model. Invest Ophthalmol Vis Sci. 2019; 60(9): 5466. [Google Scholar]
- 110. Fricke T, Holden B, Wilson D, et al.. Global cost of correcting vision impairment from uncorrected refractive error. Bull World Health Organ. 2012; 90(10): 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lim DH, Han J, Chung T-Y, Kang S, Yim HW.. The high prevalence of myopia in Korean children with influence of parental refractive errors: The 2008-2012 Korean National Health and Nutrition Examination Survey. PLoS One. 2018; 13(11): e0207690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim H, Seo JS, Yoo WS, et al.. Factors associated with myopia in Korean children: Korea National Health and nutrition examination survey 2016-2017 (KNHANES VII). BMC Ophthalmol. 2020; 20(1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jung SK, Lee JH, Kakizaki H, Jee D.. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Investig Ophthalmol Vis Sci. 2012; 53(9): 5579–5583. [DOI] [PubMed] [Google Scholar]
- 114. Lee JH, Jee D, Kwon JW, Lee WK.. Prevalence and risk factors for myopia in a rural Korean population. Investig Ophthalmol Vis Sci. 2013; 54(8): 5466–5471. [DOI] [PubMed] [Google Scholar]
- 115. Kim EC, Morgan IG, Kakizaki H, Kang S, Jee D.. Prevalence and risk factors for refractive errors: Korean National Health and Nutrition Examination Survey 2008-2011. PLoS One. 2013; 8(11): e80361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Han SB, Jang J, Yang HK, Hwang JM, Park SK.. Prevalence and risk factors of myopia in adult Korean population: Korea national health and nutrition examination survey 2013-2014 (KNHANES VI). PLoS One. 2019; 14(1): e0211204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lee YY, Lo CT, Sheu SJ, Yin L-T.. Risk factors for and progression of myopia in young Taiwanese men. Ophthalmic Epidemiol. 2015; 22(1): 66–73. [DOI] [PubMed] [Google Scholar]
- 118. Koh V, Yang A, Saw SM, et al.. Differences in prevalence of refractive errors in young Asian males in Singapore between 1996-1997 and 2009-2010. Ophthalmic Epidemiol. 2014; 21(4): 247–255. [DOI] [PubMed] [Google Scholar]
- 119. Dayan YB, Levin A, Morad Y, et al.. The changing prevalence of myopia in young adults: A 13-year series of population-based prevalence surveys. Investig Ophthalmol Vis Sci. 2005; 46(8): 2760–2765. [DOI] [PubMed] [Google Scholar]
- 120. Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K.. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013; 156(5): 948–957. [DOI] [PubMed] [Google Scholar]
- 121. Tang Y, Wang X, Wang J, et al.. Prevalence of age-related cataract and cataract surgery in a Chinese adult population: The Taizhou eye study. Investig Ophthalmol Vis Sci. 2016; 57(3): 193–1200. [DOI] [PubMed] [Google Scholar]
- 122. Kanthan GL, Mitchell P, Rochtchina E, Cumming RG, Wang JJ.. Myopia and the long-term incidence of cataract and cataract surgery: The Blue Mountains Eye Study. Clin Exp Ophthalmol. 2014; 42(4): 347–353. [DOI] [PubMed] [Google Scholar]
- 123. Marcus MW, De Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: A systematic review and meta-analysis. Ophthalmology. 2011; 118(10): 1989–1994. [DOI] [PubMed] [Google Scholar]
- 124. Morita H, Funata M, Tokoro T.. A clinical study of the development of posterior vitreous detachment in high myopia. Retina. 1995; 15(2): 117–124. [DOI] [PubMed] [Google Scholar]
- 125. Tanaka N, Shinohara K, Yokoi T, et al.. Posterior staphylomas and scleral curvature in highly myopic children and adolescents investigated by ultra-widefield optical coherence tomography. PLoS One. 2019; 14(6): e0218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Guo X, Xiao O, Chen Y, He M.. Fundus changes in highly myopic eyes with different shapes identified by high-resolution three-dimensional magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2015; 56(7): 4081. [Google Scholar]
- 127. Elnahry AG, Khafagy MM, Esmat SM, Mortada HA.. Prevalence and associations of posterior segment manifestations in a cohort of Egyptian patients with pathological myopia. Curr Eye Res. 2019; 44(9): 955–962. [DOI] [PubMed] [Google Scholar]
- 128. Chen DZ, Koh V, Tan M, et al.. Peripheral retinal changes in highly myopic young Asian eyes. Acta Ophthalmol. 2018; 96(7): e846–e851. [DOI] [PubMed] [Google Scholar]
- 129. Kidd B, Stark C, McGhee CNJ.. Screening for psychiatric distress and low self-esteem in patients presenting for excimer laser surgery for myopia. J Refract Surg. 1997; 13(1): 40–44. [DOI] [PubMed] [Google Scholar]
- 130. Toczolowski J, Oles P, Zagórski Z, Szymona KU, Baltaziak L, Rymgayllo-Jankowska B.. The sense of self-concept change in patients after radial keratotomy. J Refract Surg. 2001; 17(2): 134–137. [DOI] [PubMed] [Google Scholar]
- 131. Vetrugno M, Maino A, Quaranta GM, Cardia L.. A randomized, double-masked, clinical study of the efficacy of four nonsteroidal anti-inflammatory drugs in pain control after excimer laser photorefractive keratectomy. Clin Ther. 2000; 22(6): 719–731. [DOI] [PubMed] [Google Scholar]
- 132. Nichols JJ, Twa MD, Mitchell GL.. Sensitivity of the National Eye Institute Refractive Error Quality of Life instrument to refractive surgery outcomes. J Cataract Refract Surg. 2005; 31(12): 2313–2318. [DOI] [PubMed] [Google Scholar]
- 133. McDonnell PJ, Mangione C, Lee P, et al.. Responsiveness of the National Eye Institute refractive error quality of life instrument to surgical correction of refractive error. Ophthalmology. 2003; 110(12): 2302–2309. [DOI] [PubMed] [Google Scholar]
- 134. Lane SS, Waycaster C.. Correction of high myopia with a phakic intraocular lens: interim analysis of clinical and patient-reported outcomes. J Cataract Refract Surg. 2011; 37(8): 1426–1433. [DOI] [PubMed] [Google Scholar]
- 135. Li L, Moody K, Tan DTH, Yew KC, Ming PY, Long QB.. Contact lenses in pediatrics study in Singapore. Eye Contact Lens. 2009; 35(4): 188–195. [DOI] [PubMed] [Google Scholar]
- 136. Chen CY, Keeffe JE, Garoufalis P, et al.. Vision-related quality of life comparison for emmetropes, myopes after refractive surgery, and myopes wearing spectacles or contact lenses. J Refract Surg. 2007; 23(8): 752–759. [DOI] [PubMed] [Google Scholar]
- 137. Alvarez-Peregrina CC, Angel M, Sanchez-Tena MA, Martinez-Perez CC, Villa-Collar CC. Prevalence and risk factors of myopia in Spain. J. Ophthalmol. 2019, doi: 10.1155/2019/3419576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hashemi H, Fotouhi A, Mohammad K.. The age- and gender-specific prevalences of refractive errors in Tehran: The Tehran eye study. Ophthalmic Epidemiol. 2004; 11(3): 213–225. [DOI] [PubMed] [Google Scholar]
- 139. Hashemi H, Rezvan F, Beiranvand A, et al.. Prevalence of refractive errors among high school Students in Western Iran. J Ophthalmic Vis Res. 2014; 9(2): 232–239. [PMC free article] [PubMed] [Google Scholar]
- 140. Hashemi H, Khabazkhoob M, Jafarzadehpur E, et al.. High prevalence of myopia in an adult population, Shahroud, Iran. Optom Vis Sci. 2012; 89(7): 993–999. [DOI] [PubMed] [Google Scholar]