Abstract
Purpose
We evaluated the patient-control differences and predictive value of the retina as potential biomarkers for schizophrenia.
Methods
The institutional study included both eyes of 58 schizophrenia spectrum disorder (SSD) patients (age 37.2 ± 12.3 years) and 35 controls (age 41.1 ± 15.2 years). Retinal nerve fiber layer (RNFL), ganglion cell–inner plexiform layer, outer retinal photoreceptor complex, and total macula thicknesses were measured by optical coherence tomography (OCT). Anterior segment parameters including central corneal thickness, anterior chamber depth, and axial length were measured to rule out confounds on the retinal measures.
Results
The peripapillary RNFL was overall significantly thinner in SSD relative to controls (F = 3.97, P = 0.049), most pronounced in the temporal (5.2 µm difference, F = 6.95, P = 0.010) and inferior quadrants (12.1 µm difference, F = 7.32, P = 0.009). There were no significant group differences in thickness for the macular RNFL, ganglion, or photoreceptor cell related measures (P > 0.05). Peripapillary RNFL, central macula, and outer photoreceptor complex thicknesses were together able to classify SSD patients with 80% sensitivity and 71% specificity; area under the curve = 0.82 (95% confidence interval, 0.75–0.88).
Conclusions
SSD patients exhibited significant RNFL thinning relative to controls. Notably, retinal thickness measures including both peripapillary and macular data exhibited improved diagnostic accuracy for SSD as compared to these regions alone.
Translational Relevance
This is the first study to evaluate the predictive value of both the inner and outer retina in SSD. OCT retinal thickness measures including peripapillary data in conjunction with macular data may provide an informative, noninvasive in vivo ocular biomarker for schizophrenia.
Keywords: schizophrenia spectrum disorders, neuro-psychiatric disease, neuroretina, optical coherence tomography, ocular biomarkers
Introduction
Schizophrenia spectrum disorder (SSD) is a severe mental illness, ranking in the top 10 leading causes of disease-related disability in the world.1 Its pathogenesis remains unclear largely because of the difficulty of directly assessing its neuronal level dysfunctions. Aside from psychiatric abnormalities, visual and ophthalmologic impairments from color recognition to motion perception have also been reported.2,3 The retina contains neuronal cell types and neuronal fibers that are direct projections of the central nervous system and can be noninvasively quantified as thickness of the tissue layers using optical coherence tomography (OCT). Therefore OCT can potentially identify the cellular-specific retina layer component deficits in SSD. This may provide a new approach to understand the neuroretina-visual system abnormality in schizophrenia or even biomarkers associated with the pathophysiology of the disease.
Previous studies in schizophrenia reported significantly lower retinal nerve fiber layer (RNFL) thickness in patients as compared to controls.4–15 Two meta analyses showed significant thinning in all RNFL quadrants except the superior quadrant.14,15 Notably, however, results of individual studies have been widely variable, and most prior reports do not control for relevant ocular parameters including intraocular pressure, axial length, and anterior chamber depth, which could be associated with retinal thickness measurements.16,17 Therefore the clinical utility of the retina as a potential biomarker for schizophrenia remains inconclusive. In addition, previous studies have largely evaluated inner retinal changes in schizophrenia with less attention to outer retinal changes as potential markers of disease. The present article aimed to provide a quantitative assessment of both the inner and outer retina in patients with SSD and to investigate the predictive value of retinal thickness changes in diagnosing patients with disease.
Methods
Subjects
This study recruited 58 patients with SSD, including 45 with schizophrenia and 13 with schizoaffective disorder, and 35 controls frequency-matched for age (Table). Patient volunteers were clinically stable outpatients. Diagnoses were determined by Structured Clinical Interview for DSM-5 for all participants. Patients were recruited from the Maryland Psychiatric Research Center outpatient clinics and the neighboring mental health clinics. Controls were recruited by media advertisement, and they had no current Axis I diagnoses and no reported family history of psychotic illness in first-degree relatives. Exclusion criteria for all groups included pre-existing macular pathologies such as age-related macular degeneration, epiretinal membrane or macular hole; other retinopathies such as retinal vascular occlusion or retinal dystrophy; pre-existing ocular diseases such as glaucoma, optic neuropathy, or uveitis; previous intraocular surgery except for uncomplicated cataract surgery; and penetrating ocular trauma. Participants with a history of major neurological or unstable medical illness, substance abuse within the last month, or substance dependence within the last three months (except smoking and marijuana use) were also excluded from the study. Among the SSD patients, 53 were on antipsychotic medications, including eight on first-generation, 40 on second-generation, and five on both first- and second-generation antipsychotics; their current medication dose was recorded as chlorpromazine equivalent dose.17 Data were collected from 2017 to 2020. All participants gave written informed consent as approved by the Institutional Review Board at University of Maryland.
Table.
Cohort Demographics
| Control N = 35 (70 Eyes) | SSD N = 58 (116 Eyes) | |
|---|---|---|
| Age (mean ± SD) | 41.1 ± 15.2 | 37.2 ± 12.3 |
| Male sex* (%) | 16 (46%) | 46 (79%) |
| Race | ||
| African American (%) | 16 (46%) | 29 (50%) |
| Caucasian (%) | 19 (54%) | 29 (50%) |
| logMAR VA (mean ± SD) | 0.0 ± 0.1 | 0.1 ± 0.1 |
| Optic nerve head signal strength (mean ± SD) | 9.3 ± 0.7 | 9.2 ± 1.0 |
| Macula signal strength (mean ± SD) | 9.2 ± 1.0 | 9.2 ± 1.0 |
| Axial length, (mean ± SD) | 24.0 ± 1.9 | 24.1 ± 1.2 |
| Anterior chamber depth, (mean ± SD) | 2.8 ± 0.5 | 2.8 ± 0.7 |
| Intraocular pressure, (mean ± SD) | 15.7 ± 3.5 | 16.0 ± 3.9 |
| Central corneal thickness, (mean ± SD) | 522.4 ± 40.7 | 536.7 ± 37.1 |
| Cup/disc ratio, (mean ± SD) | 0.5 ± 0.2 | 0.5 ± 0.2 |
| Cup volume, (mean ± SD) | 0.2 ± 0.2 | 0.2 ± 0.2 |
SSD, schizophrenia spectrum disorder; VA, visual acuity; SD, standard deviation.
P < 0.05.
Anterior Segment OCT Measurements
The anterior segment parameters were assessed using spectral-domain OCT (Cirrus HD-5000, software v 6.0; Carl Zeiss Meditec). The 200 × 200 cube mode applies a 4 mm square grid by acquiring a series of 128 horizontal scan lines, each comprising 512 A-scans. This mode acquires a pair of high-definition scans through the center of the cube in the vertical and horizontal directions that are composed of 1024 A-scans each. We used these images to measure the central corneal thickness.
The anterior segment five-line raster scans generated high-resolution images of the anterior chamber angle and cornea through 5 parallel lines of equal length at 3 mm. Each line is composed of 4096 A-scans. The lines are horizontal and separated by 250 µm by default, so that the five lines cover a 1 mm width.18 The five-line raster scan was used to measure anterior chamber depth, as well as central corneal thickness.
Axial Length and Intraocular Pressure
Optical biometry was performed to determine axial length using an IOL Master (Zeiss, Dublin, CA, USA). Intraocular pressure was measured using the iCare rebound tonometer (iCare, Helsinki, Finland).
Posterior Segment OCT Imaging
Peripapillary RNFL, ganglion cell-inner plexiform layer (GC-IPL), photoreceptor complex, and total macula thicknesses were measured (Cirrus HD-5000, software v 6.0; Carl Zeiss Meditec, Jena, Germany). Scans were all acquired for Optic Disc Cube 200 × 200 and the Macular Cube 200 × 200 without pupillary dilation.
With respect to the macula, the inner retina was defined as the combined thickness spanning the distance between ganglion cell layer and the inner plexiform layer comprising the GC-IPL. The outer retina was defined as the combined thickness spanning the distance between the outer plexiform layer and the retinal pigment epithelium comprising the photoreceptor complex according to the Zeiss segmentation algorithm. Retinal thickness maps of the macular region were also acquired using the macular cube scan within a 6 × 6 mm2 circular area centered on the fovea. Measurements were averaged over nine retinal subfields, as defined by the Early Treatment Diabetic Retinopathy Study.19
An experienced operator captured all images. Individual scan volumes were reviewed for segmentation errors. Scans with significant motion artifacts, segmentation errors, or signal strength values less than 7 were excluded from analysis. To maximize the reflective signal, polarization was optimized and the scan with best centration of the optic disc was consistently selected. The built-in SD-OCT eye-tracking system provided reproducible measurements with a coefficient of variation of 0.5%.20 An experienced ophthalmologist (O.J.S.) masked to patient diagnoses reviewed each scan individually and excluded any potential abnormality including segmentation errors that could affect the retinal thickness.
Statistical Analysis
The normality assumption for the independent variables was checked with Shapiro-Wilk test, and parametric or nonparametric tests were subsequently used. Group differences in continuous parameters were compared using one-way analysis of variance (ANOVA), and group differences in categorical parameters were compared using χ2 tests. Mixed effect models were used to examine the main effect of diagnosis on retinal thickness where eye laterality and retinal quadrant were two repeated measures and age and sex were covariates, using Greenhouse-Geisser correction. Repeated measure multivariable logistic regression (generalized linear mixed model) was performed on the basis of the forward Wald method. Using a stepwise procedure, the Wald w2-statistic tests the unique contribution of a given predictor while holding the other predictors constant, thus eliminating any overlap between them (avoiding multicollinearity). In the current study, the Wald w2-statistic was used to evaluate the unique contributions of retinal thickness variables on SSD diagnosis. The significant OCT parameters were combined to derive a mathematical model such that that the measurable differences between control and SSD eyes were maximized, as previously described.21,22 Only those with all data points available (retinal thickness for all layers, both eyes, and all regions). Among the 93 subjects, three controls and six SSD subjects were excluded. The final model constituted independently significant predictors. Receiver operating characteristic (ROC) curve analysis was used to assess the potential diagnostic ability of the binary classification system (AUC) and to derive a cutoff for predicted event probability. To test the differences between the ROC curves, the area under the ROC curves were compared using the Hanley–McNeil method.23 Statistical significance was assumed at P < 0.05. Analysis was performed using SPSS V.20 package software.
Results
Of the 102 participants (63 patients and 39 controls), three patients were identified as having retinal diseases including retinopathy and epiretinal membrane. One control was diagnosed as a glaucoma suspect and excluded, and five scans failed quality control (two SSD and three controls). The data from these subjects were excluded.
Anterior Segment
There were no statistically significant differences in axial length and anterior chamber depth between SSD and controls (P > 0.05) (Table).
Intraocular Pressure
As shown in the Table, there was no statistically significant difference in intraocular pressure between SSD and controls (P > 0.05).
Peripapillary RNFL
Figure 1 compares RNFL thickness overall and by quadrant in SSD and controls. Omnibus ANOVA test indicated a significant main effect of diagnosis (F = 3.97, P = 0.049) and a significant quadrant by diagnosis interaction (F = 4.76, P = 0.004). There was no significant effect of eye (P = 0.11) or eye by diagnosis interaction (P = 0.26) or three-way eye by quadrant by diagnosis interaction (P = 0.67). Post-hoc tests were performed to explore the quadrant by diagnosis interaction by averaging the measurements of the left and right eyes. For the temporal quadrant, post-hoc results showed significant effects of diagnosis (F = 6.95, P = 0.009) and age (F = 5.91, P = 0.017).
Figure 1.
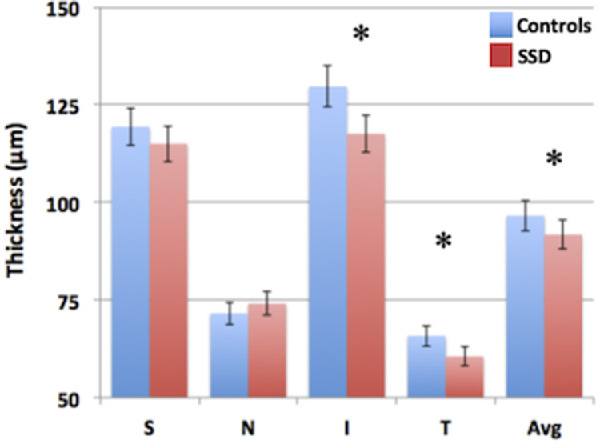
Depicts spectral domain-optical coherence tomography comparison of peripapillary retinal nerve fiber layer (RNFL) thickness between schizophrenia spectrum disorder (SSD) patients and controls. S, superior; N, nasal; I, inferior; T, temporal. Error bars: standard deviation. *P < 0.05.
For the inferior quadrant, post-hoc results showed significant effects of diagnosis (F = 7.32, P = 0.007), sex (F = 4.87, P = 0.030), and age (F = 5.86, P = 0.018).
There were no significant diagnosis effects for superior (F = 1.42, P = 0.24) and nasal (F = 1.01, P = 0.32) quadrants. Further exploration by averaging all quadrants and eyes, peripapillary RNFL was significantly reduced in patients compared with controls (F = 3.97, P = 0.049).
Macular RNFL
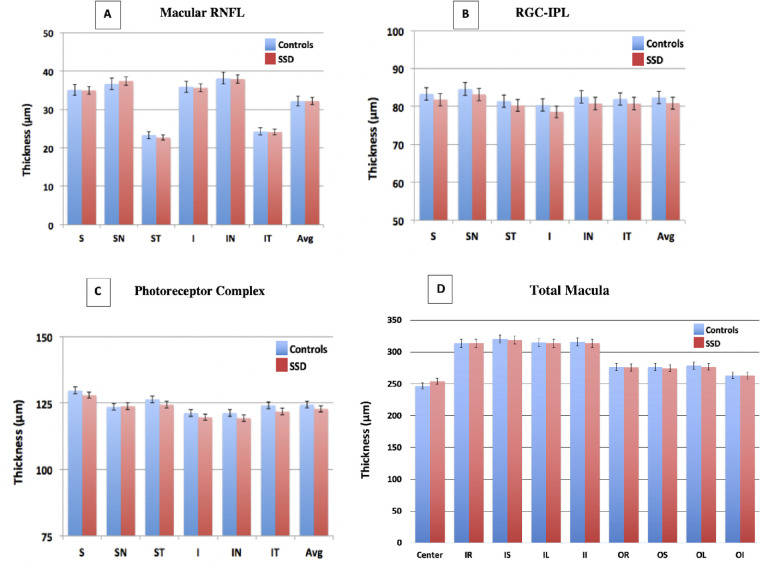
Repeated measure ANOVA showed no significant main effect of diagnosis or any diagnosis related interactions (all main effect and interaction P > 0.05) (Fig. 2A).
Figure 2.
Depicts spectral domain-optical coherence tomography comparison of (A) macular retinal nerve fiber layer, (B) ganglion cell-inner plexiform layer (GC-IPL), (C) outer retinal photoreceptor complex, and (D) total macular thicknesses between schizophrenia spectrum disorder (SSD) patients and controls. S, superior; SN, superior-nasal; ST, superior-temporal; I, inferior; IN, inferior-nasal; IT, inferior-temporal; Avg, average; IR, inner-right; IS, inner-superior; IL, inner-left; II, inner-inferior; OR, outer-right; OS, outer-superior; OL, outer-left; OI, outer-inferior of the Early Treatment Diabetic Retinopathy Study grid. Error bars: standard deviation.
Retinal Ganglion Cell-Inner Plexiform Layer
Repeated measure ANOVA showed no significant main effect of diagnosis or any diagnosis related interactions (all main effect and interaction P > 0.05) (Fig. 2B).
Photoreceptor Complex
Repeated measure ANOVA showed no significant main effect of diagnosis or any diagnosis related interactions (all P > 0.05) (Fig. 2C).
Total Macula
Repeated measure ANOVA showed no significant main effect of diagnosis or any diagnosis related interactions (all P > 0.05) (Fig. 2D). See Supplementary Table S1 for a complete listing of retinal quantitative retinal data.
Correlates With Other Clinical Variables
For measures showing significant effects in SSD, we further explored their potential relationships with other clinical measures. Daily chlorpromazine equivalent dose levels did not significantly correlate with average (P = 0.595), inferior RNFL (P = 0.846), or temporal (P = 0.058) RNFL in the patients. Accounting for age and sex, hypertension was not significantly related to temporal, inferior, or average RNFL (P > 0.05 for all). Accounting for age and sex, diabetes was not significantly related to temporal, inferior, or average RNFL (P > 0.05 for all). Average, inferior, and temporal RNFL quadrants remained significantly different between groups after adjusting for hypertension and diabetes diagnoses (all P < 0.05).
Predicting SSD Diagnosis
Logistic regression was used to predict SSD diagnosis (dependent variable) from retinal OCT indices (independent variables). In the first stage of the analysis, we evaluated the performance of the peripapillary RNFL (temporal, inferior, nasal, average thickness). Inclusion of the temporal, inferior, and nasal RNFL as predictors constituted the strongest model (P < 0.0001); (AUC = 0.76) (95% confidence interval [CI], 0.68–0.83) (Fig. 3). The mathematical model of the binary logistic regression equation was defined as follows: 5.223 − 0.36(RNFL_Temp) − 0.055(RNFL_Inf) + 0.06(RNFL_Nasal).
Figure 3.
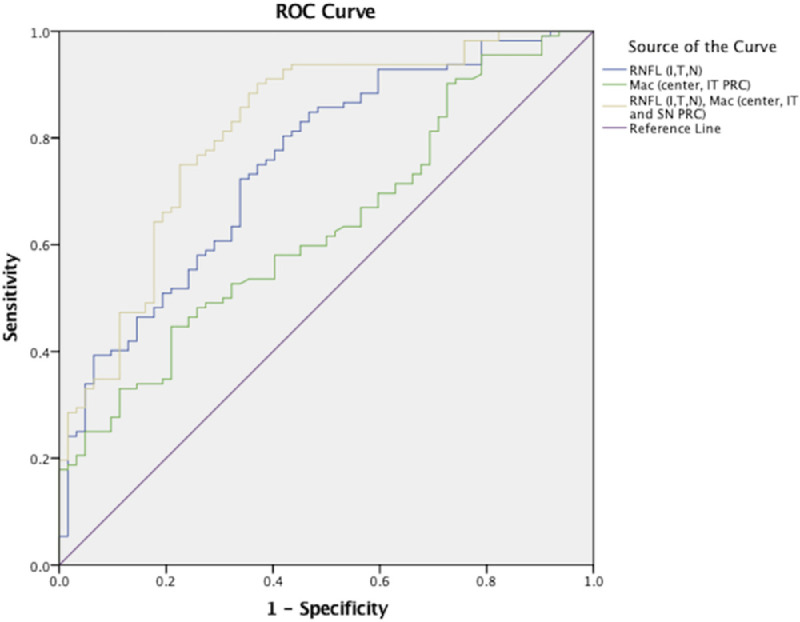
Representation of receiver operator curves (ROCs) of three regression analyses to demonstrate which parameters of optical coherence tomography predict schizophrenia spectrum disorder group classification. The area under the curve (AUC) of the peripapillary retina (inferior, temporal, and nasal retinal nerve fiber layer [RNFL]) was 0.76 (blue curve). The AUC of the macula (center, inferior-temporal, and superior-nasal photoreceptor complex) was 0.63 (green curve). The AUC of the peripapillary retina (inferior, temporal, and nasal RNFL) and macula (center, inferior-temporal, and superior-nasal photoreceptor complex) was 0.82 (gold curve).
We then evaluated the performance of the macula. After running the analysis for all macular layers and quadrants (macular RNFL, GC-IPL, outer retina, total macula), the central macula and temporal-inferior photoreceptor complex constituted the best model (P = 0.001); AUC = 0.63 (95% CI, 0.55–0.72) (Fig. 3). The mathematical model of the binary logistic regression equation was defined as follows: 2.601 − 0.064(Inf_Temp_PRC) + 0.023(Mac_Center).
We further explored a predictive model that could maximize discriminatory potential by combining parameters from both regions. After including all peripapillary and macular measurements, the temporal, inferior, and nasal RNFL in conjunction with the central macula, inferior-temporal and superior-nasal photoreceptor complex comprised the best model (P < 0.0001); AUC = 0.82 (95% CI, 0.75–0.88) (Fig. 3). The mathematical model of the binary logistic regression equation was defined as follows: 3.395 − 0.04(RNFL_Temp) − 0.06(RNFL_Inf) + 0.081(RNFL_Nasal) − 0.105(PRC_Temp_Inf) + 0.071(PRC_Nasal_Sup) + 0.022(Mac_Center). A cutoff for predicted event probability was derived such that sensitivity was at least 80% and specificity was maximized. The selected cutoff of 0.59 yielded 80% sensitivity and 71% specificity. See Supplementary Table S2 for a complete listing of ROC quantitative data.
The AUC of the peripapillary retinal model significantly differed from that of the macular model (P < 0.009). In addition, the AUC of the macular model was statistically significantly different from that of the combined peripapillary and macular retinal model (P < 0.0001). The AUC of the combined peripapillary and macular retinal model was greater than that of the peripapillary retina, although not statistically significant (P = 0.101).
Discussion
We evaluated the patient-control differences in retinal thickness and the sensitivity and specificity of OCT measurements in separating patients with SSD versus controls. This is the first study to evaluate the potential diagnostic accuracy of both inner and outer retinal OCT indexes in SSD. We found significant thinning primarily of the temporal and inferior quadrants of the peripapillary RNFL in SSD in the context of no significant abnormalities in intraocular pressure, anterior segment parameters, and retinal ganglion cell and photoreceptor complexes. Furthermore, we found that the peripapillary and macular retinal regions were together able to predict SSD diagnosis with relatively high sensitivity (80%) and specificity (71%). These findings highlight retinal thinning as a potential neuroretinal biomarker for SSD.
Previous studies have similarly investigated RNFL thickness changes in schizophrenia patients. However, these reports have had conflicting findings. For example, Ascaso et al.4 showed significant RNFL thinning of the nasal quadrant in SSD; Lee et al.7 found RNFL thinning most pronounced in the superior, temporal, and inferior quadrants; and Yilmaz et al.10 reported no significant difference in RNFL thickness in SSD relative to controls. These discrepancies in thickness findings may be attributed to the insufficient control of other potential ophthalmological conditions. Our study deployed a more systematic effort to control for these other potential confounds. Several of the previous studies also relied on self-reported psychiatric disease diagnoses,4,6–8 whereas our study used standard structured interviews in all SSD and control subjects to confirm diagnoses.
The RNFL comprises the axons of the cellular bodies in the ganglion cell layer. Because ganglion cell axons converge onto the optic nerve head, the RNFL is anatomically thinnest in the macular region and thickest in the peripapillary region. These unmyelinated fibers penetrate the sclera to become the myelinated axons comprising the optic nerve. Therefore the RNFL represents the direct neuroretinal contributions to the central nervous system and has been shown to correlate with cerebral white matter atrophy in neurodegenerative disease.24–27 Kochunov et al.28,29 and others recently characterized white matter abnormalities as an underlying neural mechanism in schizophrenia.30–32 Therefore preferential axonal loss in SSD might be related to similar white matter abnormalities evidenced in this disease. Taken together, these findings suggest that cortical white matter changes may be reflected in the neuroretina and, more importantly, can potentially be detected noninvasively by in vivo OCT imaging.
We found no significant differences in the retinal ganglion cell complex or its components between SSD and controls, consistent with a recent metanalysis where the peripapillary RNFL but not the ganglion cell complex was significantly thinner in SSD, relative to controls. It has been speculated that these findings may possibly be explained by retrograde transsynaptic degeneration, which describes ganglion cell loss caused by synaptic dysfunction of optic nerve fibers in the lateral geniculate nucleus.15 A similar mechanism has also been posited in neurodegenerative diseases including Alzheimer's disease (AD) and Parkinson's disease (PD), which are also associated with axonal loss.33,34 Notably, SSD shares some of the AD and PD clinical features including cognitive dysfunction and dopamine dysregulation, respectively.35 Retinal dopaminergic amacrine cells are localized to the inner retina near the ganglion cells.36,37 However, the current study technique does not allow examination on whether RNFL fibers were related to these cells.
Few OCT studies have evaluated the outer retina in SSD. Samani et al.8 reported thinning of the outer nuclear layer and inner segments of the photoreceptors, whereas Bannai et al.13 suggested thickening of the outer plexiform layer. We evaluated the photoreceptor complex, which includes retinal thickness spanning from the outer nuclear layer to the retinal pigment epithelium. Our findings similarly suggest that the outer retina, when considered in total, may be less affected in SSD. Interestingly, these observations are also consistent with our previous report on an epidemiologic association between serious mental illness and glaucoma,38,39 a condition that is characterized by preferential loss of retinal nerve fiber layer early in the disease and sparing of the outer retina. Although the intraocular pressure of schizophrenia patients was normal in the current sample, up to one third of patients with glaucoma have glaucoma at normal intraocular pressure,40 and this is often referred to as normal-tension or normal-pressure glaucoma. Prior studies have shown that glaucoma has numerous contributions that are independent of pressure.41 The reason for this potential overlap between schizophrenia and glaucoma is unclear, but one shared potential cause is microvascular dysfunction such as retinal arterioles that have been observed in both diseases.42,43
We further evaluated the discriminatory potential of the peripapillary and macular retinal parameters for group classification of SSD versus controls. Because higher significance for a given parameter does not automatically imply stronger predictivity and vice versa, we examined all retinal OCT parameters for a given region, as opposed to a priori selection of significant parameters from initial group comparison analysis.44 Notably, we found greater diagnostic ability of the peripapillary RNFL as compared to the macula. Intriguingly, however, our analysis showed that inclusion of outer retinal thickness measures in conjunction with the peripapillary RNFL enhanced the diagnostic ability of the predictive model. Strengths of the current study include rigorous selection of participants after structured interviews in both SSD and control subgroups, accounting for potential ophthalmic (axial length, intraocular pressure) and systemic confounders (hypertension, diabetes, antipsychotic medication) of posterior segment assessment. OCT data correctly predicted group classification with relatively high sensitivity and specificity, supporting our retinal thickness findings in SSD.
The present study is among one of the largest published to date and, more importantly, accounted for anterior segment ocular parameters and intraocular pressure that may influence retinal thickness in healthy and diseased conditions. Our retinal thickness findings may also have been associated with antipsychotic medication use. Electroretinography studies have demonstrated that longer courses of antipsychotic medication treatment are correlated with improved ganglion cell function,45 suggesting a possible role of long-term antipsychotic use in preserving ganglion cells. However, our retinal data found nonsignificant correlations with antipsychotic medication dosage and RNFL. Prospective studies would be needed to confirm the potential medication effect on RNFL. Notably, the superior RNFL quadrant was not a significant predictor of group classification. Future studies are warranted to more precisely elucidate this pattern of retinal involvement. Furthermore, the cross-sectional nature of the study also does not allow causal inference on the cause of the RNFL thinning in SSD, which may require a longitudinal follow-up study.
In conclusion, the study provided a more comprehensive understanding of retinal layer thickness as potential neuroretinal biomarkers for SSD. Because retinal thickness can be more directly visualized as compared to most other neural tissues, it should be further evaluated as potential biomarkers for monitoring the progression of the underlying neuropathology of the disease or for assessing the efficacy of prevention and treatment strategies.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health (R01MH116948 [L.E.H.], K23EY025014 [O.J.S.]).
Disclosure: S. Asanad, None; H. O'Neill, None; H. Addis, None; S. Chen, None; J. Wang, None; E. Goldwaser, None; P. Kochunov, None; L.E. Hong, Mitsubishi (F), Your Energy Systems LLC (F), Neuralstem (F), Taisho (F), Heptares (F), Pfizer (F), Luye Pharma (F), Sound Pharma (F), Takeda (F), Regeneron (F); O.J. Saeedi, NIH Career Development Award K23EY025014 (R), Heidelberg Engineering, Inc. (R), Vasoptic Medical Inc. (R)
References
- 1.World Health Organization. Mental Health Report 2001. Mental Health: New Understanding, New Hope. Geneva: World Health Organization; 2001. [Google Scholar]
- 2. Chen Y, Palafox GP, Nakayama K, et al.. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999; 56(2): 149–154. [DOI] [PubMed] [Google Scholar]
- 3. Bedwell JS, Brown JM, Miller LS. The magnocellular visual system and schizophrenia: what can the color red tell us? Schizophr Res. 2003; 63: 273–284. [DOI] [PubMed] [Google Scholar]
- 4. Ascaso FJ, Cabezon L, Quintanilla MA, et al.. Retinal nerve fiber layer thickness measured by optical coherence tomography in patients with schizophrenia: a short report. Eur J Psychiatry. 2010; 24: 227–35. [Google Scholar]
- 5. Ascaso FJ, Rodriguez-Jimenez R, Cabezón L, et al.. Retinal nerve fiber layer and macular thickness in patients with schizophrenia: Influence of recent illness episodes. Psychiatry Res. 2015; 229(1–2): 230–6. [DOI] [PubMed] [Google Scholar]
- 6. Chu EM-Y, Kolappan M, Barnes TRE, et al.. A window into the brain: an in vivo study of the retina in schizophrenia using optical coherence tomography. Psychiatry Res Neuroimaging. 2012; 203: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee WW, Tajunisah I, Sharmilla K, et al.. Retinal nerve fiber layer structure abnormalities in schizophrenia and its relationship to disease state: evidence from optical coherence tomography. Invest Ophthalmol Vis Sci. 2013; 54: 7785–7792. [DOI] [PubMed] [Google Scholar]
- 8. Samani NN, Proudlock FA, Siram V, et al.. Retinal layer abnormalities as biomarkers of schizophrenia. Schizophr Bull. 2018; 44: 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silverstein SM, Paterno D, Cherneski L, Green S. Optical coherence tomography indices of structural retinal pathology in schizophrenia. Psychol Med. 2018; 48: 2023–33. [DOI] [PubMed] [Google Scholar]
- 10. Topcu-Yilmaz P, Memduha Aydin M, Ilhan BC. Evaluation of retinal nerve fiber layer, macular, and choroidal thickness in schizophrenia: spectral optic coherence tomography findings. Psychiatry Clin Psychopharmacol. 2019; 29(1): 28–33. [Google Scholar]
- 11. Kurtulmus A, Elbay A, Parlakkaya FB, et al.. An investigation of retinal layer thicknesses in unaffected first-degree relatives of schizophrenia patients. Schizophr Res. 2020; 218:255–261. [DOI] [PubMed] [Google Scholar]
- 12. Miller M, Zemon V, Nolan-Kenney R, et al.. Optical coherence tomography of the retina in schizophrenia: Inter-device agreement and relations with perceptual function. Schizophr Res. 2020; 219:13–18. [DOI] [PubMed] [Google Scholar]
- 13. Bannai D, Lizano P, Kasetty M, et al.. Retinal layer abnormalities and their association with clinical and brain measures in psychotic disorders: a preliminary study. Psychiatry Res Neuroimaging. 2020; 299: 111061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan J, Zhou Y, Xiang Y, et al.. Retinal nerve fiber layer thickness changes in Schizophrenia: A meta-analysis of case-control studies. Psychiatry Res. 2018; 270: 786–791. [DOI] [PubMed] [Google Scholar]
- 15. Kazakos CT, Karageorgiou V.. Retinal changes in schizophrenia: a systematic review and meta-analysis based on individual participant data. Schizophr Bull. 2020; 46(1): 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szigeti A, Tátrai E, Varga BE, et al.. The effect of axial length on the thickness of intraretinal layers of the macula. PLoS One. 2015; 10(11): e0142383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003; 64: 663–667. [DOI] [PubMed] [Google Scholar]
- 18. Rodrigues EB, Johanson M, Penha FM. Anterior segment tomography with the cirrus optical coherence tomography. J Ophthalmol. 2012; 2012: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pierro L, Gagliardi M, Iuliano L, et al.. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci. 2012; 53: 5912–5920. [DOI] [PubMed] [Google Scholar]
- 20. Hu J, Gottlieb CB, Barajas DJ, et al.. Improved repeatability of retinal thickness measurements using line-scan ophthalmoscope image-based retinal tracking. Ophthalmic Surg Lasers Imaging Retina. 2015; 46: 310–314. [DOI] [PubMed] [Google Scholar]
- 21. Ghosh A, Martin N, Basu A, Pardo L. A new class of robust two-sample Wald-type tests. Int J Biostats. 2018; 14(2): 20170023. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Martin E, Satue M, Otin S, et al.. Retina measurements for diagnosis of Parkinson disease. Retina. 2014; 34: 971–980. [DOI] [PubMed] [Google Scholar]
- 23. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology . 1982; 143: 29–36. [DOI] [PubMed] [Google Scholar]
- 24. Young KL, Brandt AU, Petzold A, et al.. Loss of retinal nerve fibre layer axons indicates white but not grey matter damage in early multiple sclerosis. Eur J Neurol. 2013; 20: 803–11. [DOI] [PubMed] [Google Scholar]
- 25. Scheel M, Finke C, Oberwahrenbrock T, et al.. Retinal nerve fibre layer thickness correlates with brain white matter damage in multiple sclerosis: a combined optical coherence tomography and diffusion tensor imaging study. Mult Scler. 2014; 20: 1904–1907. [DOI] [PubMed] [Google Scholar]
- 26. Wang R, Tang Z, Sun X, et al.. White matter abnormalities and correlation with severity in normal tension glaucoma: a whole brain atlas-based diffusion tensor study. Invest Ophthalmol Vis Sci. 2018; 59: 1313–1322. [DOI] [PubMed] [Google Scholar]
- 27. Qu M, Kwapong WR, Peng C, et al.. Retinal sublayer defect is independently associated with the severity of hypertensive white matter hyperintensity. Brain Behav. 2020; 10(2): e01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kochunov P, Rowland LM, Fieremans E, et al.. Diffusion-weighted imaging uncovers likely sources of processing-speed deficits in schizophrenia. Proc Natl Acad Sci USA. 2016; 113(47): 13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kochunov P, Coyle TR, Rowland LM, et al.. Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatry. 2017; 74: 958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubicki M, Mccarley RW, Shenton ME. Evidence for white matter abnormalities in schizophrenia. Curr Opin Psychiatry. 2005; 18: 121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cetin-Karayumak S, Di Biase MA, Chunga N, et al.. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. 2020; 25: 3208–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lener MS, Wong E, Tang CY, et al.. White matter abnormalities in schizophrenia and schizotypal personality disorder. Schizophr Bull. 2015; 41: 300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan VTT, Sun Z, Tang S, et al.. Spectral-domain OCT measurements in Alzheimer's disease: a systematic review and meta-analysis. Ophthalmology. 2019; 126: 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. La Morgia C, Barboni P, Rizzo G, et al.. Loss of temporal retinal nerve fibers in Parkinson disease: a mitochondrial pattern? Eur J Neurol. 2013; 20: 198–201. [DOI] [PubMed] [Google Scholar]
- 35. Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophr Res Cogn. 2015; 2: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frederick JM, Rayborn ME, Laties AM, et al.. Dopaminergic neurons in the human retina. J Comp Neurol. 1982; 210: 65–79. [DOI] [PubMed] [Google Scholar]
- 37. Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004; 108: 17–40. [DOI] [PubMed] [Google Scholar]
- 38. Saeedi O, Ashraf H, Malouf M, et al.. Prevalence of diagnosed ocular disease in veterans with serious mental illness. Gen Hosp Psychiatry. 2016; 43: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hood DC . Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog Retin Eye Res. 2017; 57: 46–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sommer A, Tielsch JM, Katz J, et al.. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991; 109: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 41. Mastropasqua R, Fasanella V, Agnifili L, et al.. Advance in the pathogenesis and treatment of normal-tension glaucoma. Prog Brain Res. 2015; 221: 213–232. [DOI] [PubMed] [Google Scholar]
- 42. Adhan I, Bannai D, Lizano P. Commentary: Can retinal imaging biomarkers inform psychosis pathophysiology? Schizophr Res. 2020; 215: 3–5. [DOI] [PubMed] [Google Scholar]
- 43. Mitchell P, Leung H, Wang JJ, et al.. Retinal vessel diameter and open-angle glaucoma: the Blue Mountains Eye Study. Ophthalmology. 2005; 112: 245–250. [DOI] [PubMed] [Google Scholar]
- 44. Lo A, Chernoff H, Zheng T, et al.. Why significant variables aren't automatically good predictors. Proc Natl Acad Sci USA. 2015; 112: 13892–13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Balogh Z, Benedek G, Kéri S. Retinal dysfunctions in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008; 32: 297–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.