Abstract
Purpose
In the field of regenerative medicine, Rho kinase inhibitors (ROCK inhibitors) show a protective effect on the corneal endothelium and promote effective healing in acute surgical wounds. In this study, we investigated the effectiveness of eyedrops containing ripasudil, a ROCK inhibitor approved in Japan for therapeutic use for glaucoma.
Methods
In this retrospective observational study, 13 glaucoma patients (16 eyes) were treated with 0.4% ripasudil eyedrops twice a day after cataract surgery. The control group comprised 13 patients (17 eyes). The averaged corneal endothelial cell density from one central and four paracentral points was <1500/mm2 (range, 527 to 1439/mm2).
Results
The mean rate of increase in the thinnest corneal thickness one week after surgery was 1.25% in the ripasudil group, which was significantly lower than the 5.97% increase observed in the control group (P = 0.0037). The mean endothelial cell density loss 90 to 120 days after surgery, excluding bullous keratopathy patients for whom measurements were not possible was −4.5% in the ripasudil group, which was significantly lower than in control group (14.1%; P = 0.0003).
Conclusions
The results suggest that ripasudil may help maintain corneal endothelial functional integrity and reduce cell loss after cataract surgery in patients with low corneal endothelial cell density, suggesting that it may be more broadly useful for protection of the corneal endothelium after intraocular surgery.
Translational Relevance
The clinically approved ROCK inhibitor ripasudil formulated as an eye drop for glaucoma has a corneal endothelial protective effect in cataract surgery for patients with low corneal endothelial cell density.
Keywords: ripasudil, ROCK inhibitor, corneal endothelium, protection
Introduction
Rho kinase is a protein kinase that participates in the regulation of cell size and shape through the action of the cytoskeleton.1 Rho kinase inhibitors (ROCK inhibitors) have been developed for use in ophthalmology for the treatment of glaucoma2,3 and for corneal endothelium disorders.4,5 The applicability of ROCK inhibitors is also being examined for conditions such as retinal diseases.6 The effectiveness of ROCK inhibitors for corneal endothelium protection has been established in fields such as regenerative medicine, in which human corneal endothelial cell injection with a ROCK inhibitor was able to treat bullous keratopathy (BK) without conventional keratoplasty.7 Its efficacy has also been reported in cases of acute surgical trauma.8
Okumura et al.9 used a rabbit corneal endothelial damage model to examine the transplantation of corneal endothelial cells with ROCK inhibitors. The reconstructed corneal endothelium was shown to be made up of hexagonal monolayer cells with normal expression of function-related markers. Prolonged corneal endothelial cell survival by ROCK inhibitors has also been shown in vitro.10 The protective and pro-proliferative effects of these inhibitors on the corneal endothelium play a critical role in regenerative medicine: one of the milestones in the field was the use of the ROCK inhibitor Y27632 in cultured endothelial cell transplantation.7
In parallel, the efficacy of ROCK inhibitors has been well established in corneal endothelial diseases.4,11–13 Cell loss in the corneal endothelium and decompensation from the endothelium can occur from various causes, such as uveitis, endothelial dystrophy, and surgical trauma during intraocular surgery in humans. This can result in BK, which is characterized by edematous corneal stroma and the development of epithelial and sub-epithelial bullae.14 The ROCK inhibitor Y27632 developed by Koizumi et al.15 is effective for the treatment of several corneal diseases. By contrast, other ROCK inhibitors, such as ripasudil, have not been examined as thoroughly as Y27632 for treating endothelial diseases,16 except for ripasudil use as an adjuvant to corneal surgery.17,18 Furthermore, Okumura et al.16 has reported that ripasudil promotes rabbit corneal endothelial wound healing, suggesting that it may be useful for protection of the corneal endothelium during intraocular surgery. However, there is no consensus for the clinical use of ripasudil for endothelial protection after intraocular surgery in humans.
Ripasudil and Netarsudil are ROCK inhibitors formulated as eye drops that have been approved for clinical use. The safety of ripasudil is well established, and since 2014 it has been available in Japan for the treatment of glaucoma.19,20 In the present study, we sought to assess whether ripasudil may have broader application in the clinic. We assessed this by using the ROCK inhibitor ripasudil in patients with glaucoma with low corneal endothelial cell density who were undergoing cataract surgery. We thereby investigated the protective effect in the clinic of ripasudil administered as an ophthalmic solution for corneal endothelial damage after cataract surgery.
Methods
This retrospective observational study was approved by the institutional review board of Kawasaki Medical School Hospital (approval number 3717). Informed consent was obtained from all study participants according to the Declaration of Helsinki. This study did not have patient or public involvement in its design, participant recruitment or conduct.
For ripasudil group, we included a consecutive series of 13 patients ranging from 64 to 84 yars (mean, 75.1 years), who had undergone cataract surgery (16 eyes) with a corneal endothelial cell density of ≤1500 cells/mm2, presenting with glaucoma because ripasudil is approved only for glaucoma and ocular hypertension. Glaucoma was diagnosed according to the presence of a typical localized glaucomatous RNFL defect in red-free examinations, or glaucomatous optic nerve head damage (cup-to-disc ratio asymmetry of >0.2 between fellow eyes, rim thinning, notching or excavation). Eyes with bullous keratopathy (BK), for which endothelial density measurements were not possible, were additionally included in the study. The causes of endothelial loss were Fuchs endothelial dystrophy (FECD, nine eyes in seven patients), old surgical trauma or corneal surgery (four eyes in three patients), laser iridotomy for closed-angle glaucoma (one eye in one patient), uveitis (one eye in one patient), and cytomegalovirus (CMV)-related corneal endotheliitis (one eye in one patient). Ripasudil hydrochloride hydrate 0.4% (Kowa Company, Ltd., Japan, twice a day) was prescribed from the day after surgery until six months after surgery on the scheduled final examination. In 13 eyes, prostaglandin analogues were used before surgery and stopped after surgery.
For the control group, we included a consecutive series of 13 patients without glaucoma ranging from 68 to 83 years (mean, 76.2 years), who had undergone cataract surgery (17 eyes) with a corneal endothelial cell density of ≤1500 cells/mm2 who were not prescribed 0.4% ripasudil eyedrops. The number of patients was designed to be same as ripasudil group. The age and male/female ratio were not significantly different between ripasudil and control groups (Table). The causes of endothelial loss in the control were FECD (12 eyes in eight patients), old surgical trauma or corneal surgery (two eyes in two patients), laser iridotomy for closed-angle glaucoma (one eye in one patient), and CMV-related corneal endotheliitis (two eyes in two patients).
Table.
Comparison of TCT, CCT, ECD, IOP and the Background Parameters in the Ripasudil and the Control Group
| Ripasudil Group | Control Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Maximum | Minimum | Mean | SD | Maximum | Minimum | P Value Between Ripas-udil and Control Group | |
| ECD loss (%) | -4.5 | 13.0 | 12.6 | -31.4 | 14.1 | 10.3 | 27.8 | -4.6 | 0.0003 |
| TCT change ratio(TCT†/TCT*) | 1.013 | 0.046 | 1.096 | 0.922 | 1.060 | 0.033 | 1.132 | 1.017 | 0.0037 |
| CCT change ratio(CCT†/CCT*) | 1.000 | 0.052 | 1.102 | 0.906 | 1.056 | 0.034 | 1.132 | 1.010 | 0.0038 |
| ECD (cells/mm2)* | 935.6 | 307.1 | 1411 | 527 | 1006.6 | 246.1 | 1439 | 652 | 0.24 |
| ECD (cells/mm2)† | 979.8 | 353.9 | 1686 | 566 | 850.8 | 220.0 | 1381 | 560 | |
| TCT (µm)* | 525.3 | 32.8 | 575 | 475 | 521.1 | 34.7 | 581 | 464 | 0.36 |
| TCT (µm)† | 531.4 | 33.9 | 597 | 486 | 549.7 | 38.4 | 594 | 479 | |
| CCT (µm)* | 549.7 | 37.7 | 628 | 502 | 529.4 | 36.1 | 586 | 470 | 0.06 |
| CCT (µm)† | 553.0 | 39.3 | 622 | 495 | 558.5 | 38.9 | 615 | 484 | |
| IOP (mm Hg)* | 13.2 | 2.0 | 16.1 | 9.7 | 13.2 | 3.4 | 18.3 | 7.6 | 0.35 |
| IOP (mm Hg)† | 13.4 | 5.0 | 23.3 | 5.7 | 13.0 | 2.9 | 16.5 | 6.4 | |
| Age (years) | 75.1 | 5.9 | 84 | 64 | 76.2 | 4.7 | 83 | 68 | 0.28 |
| Male (%)/Females | 3 (18.8%)/13 | 6 (35.3%)/11 | 0.29^ | ||||||
| Duration of surgery (min) | 25.6 | 15.4 | 73 | 10 | 22.1 | 9.5 | 40 | 11 | 0.21 |
| Nuclear hardness graded by the Emery-Little classification | 3.2 | 1.1 | 5 | 2 | 2.8 | 0.8 | 4 | 2 | 0.16 |
P values were calculated using two-sample independent t-test in all instances except for Male (%)/Females where the Pearson chi-squared test was used (^).
TCT, thinnest corneal thickness; CCT, central corneal thickness; ECD, Endothelial cell density; IOP, Intraocular pressure.
Before surgery.
After surgery.
Inclusion criteria were as follows: patients with endothelial cell loss, but without other anterior segment ocular disorders, such as epithelial erosions, infections, conjunctivitis, or nasolacrimal duct obstruction. In contrast, the exclusion criteria involved history of ripasudil use, as well as any active systemic disease, such as cardiovascular disease, or renal disease. Cases of diabetes mellitus and systemic hypertension controlled through the administration of oral medications were allowed in the final cohort.
Phacoemulsification with intraocular lens implantation was performed by the same surgeon in all eyes. During phacoemulsification, 3% sodium hyaluronate with 4% chondroitin sulfate (Viscoat; Alcon Laboratories, Inc., Fort Worth, TX, USA) were used. There were no incidents of surgical complications such as capsule rupture or lens drop. There were no incidents of bullous keratopathy attributable to surgery. All patients were prescribed 1.5% levofloxacin, 0.1% betamethasone, and 0.1% nepafenac eyedrops for three-times daily use from the day after surgery until one month after the surgery. After that, all patients were prescribed 0.1% fluorometholone and 0.1% nepafenac eyedrops for three-times daily use for two months. In the Ripasudil group, ripasudil eyedrops (0.4% twice a day) were prescribed from the day after surgery until six months after surgery.
Outcome measures of interest were the thinnest corneal thickness (TCT), central corneal thickness (CCT), and ECD both before and after surgery. Baseline measurements for TCT and CCT were made one to 30 days before surgery, and seven days after surgery.
Corneal tomographic measurements were obtained using anterior segment optical coherence tomography using CASIA 2 (Tomey Corporation, Inc., Nagoya, Japan) with an infrared light wavelength of 1310 nm. Measurements were obtained along the vertex normal, and the images were centered on the corneal vertex. The analysis software (Tomey Corporation, Inc., Nagoya, Japan) identified and digitized both the anterior and posterior corneal surfaces, as well as aligned the reference axis of the measurement with the vertex normal. Tomographic and pachymetric maps were calculated from 16 radial cross-sectional images through the central 10-mm diameter of the cornea obtained in 0.34 seconds.
Swept-source OCT measurements have been shown to have adequate repeatability in healthy eyes, as well as in eyes with keratoconus.21 TCT and CCT were measured and defined as the pachymetric value at the thinnest location and at the corneal vertex, respectively. Calculations of TCT and CCT were performed inside the central 9-mm diameter of the cornea. Each measurement satisfied the following conditions: more than 96.5% of the data within the central 6-mm diameter had adequate contrast to trace the corneal border; all 16 radial scans within the central 4-mm diameter topographic analysis could be performed for both the anterior and posterior corneal surfaces; the distance from the center of the image to the corneal vertex was less than 0.86 mm; and over 93% of scans inside the central 6-mm diameter topographic analysis could be obtained for both the anterior and posterior surfaces. To avoid the effects of same-day variation on corneal thickness, measurements were performed within one hour of the scheduled measurement at the initial examination.
Measurements for ECD were taken one to 30 days before surgery and 90 to 120 days after surgery.
A non-contact-type specular microscope (Cell Check 16; Konan Co., Ltd, Japan) was used for ECD measurement. The captured images were 0.46 mm high and 0.24 mm wide. We studied the central point, and the four paracentral points for the upper, lower, nasal, and temporal positions from the center. The measured ECD values were averaged (Supplementary Fig. S1). The distance of the paracentral four points from the center was 5°. The number of the cells are counted manually. In two eyes in ripasudil group and two eyes in control group, specular microscopy was completely unavailable because of BK. In some FECD eyes, specular microscopy was not available and discarded in some points so that the measurement location was less than 5 either before or after surgery (Supplementary Fig. S1). For eyes with a K of 42.5 D, 43.5 D, or 44.5 D, 5° corresponds to a distance of 0.65 mm, 0.63 mm, and 0.61 mm, respectively, from the center of the eyes. A total of five corneal points was examined for every eye. The imaging point was controlled by patient fixation; namely, locations were determined on the basis of the patient's primary line of sight and not the vertex normal. The area measured was confirmed by a monitor camera.
Because ripasudil is currently used as an eye drop for glaucoma, intraocular pressure (IOP) was also measured using a non-contact tonometer (NT-4000; NIDEK Co., Ltd, Japan) at every visit. IOP measurements were performed three times, and IOP values were averaged.
Statistical Analysis
Data distributions were assessed for normality using the Shapiro-Wilk test. Following the assumption that all data on outcomes of interest followed a parametric distribution, the two-sample independent t-test was used to compare TCT, CCT, and ECD before and after surgery in both ripasudil and control groups. All analyses were conducted using SPSS version 25.0 (Released 2017. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp., Armonk, NY, USA).
Results
The study included 33 eyes from 26 patients. Of these, 16 eyes from 13 patients were prescribed ripasudil after surgery, whereas 17 eyes from 13 patients in the control group were not. The mean participant age across both groups (ripasudil and control) was 75.5 ± 5.1 years.
In the ripasudil group, the mean TCT before surgery was 525.3 ± 32.8 µm; this slightly increased to 531.4 ± 33.9 µm after surgery. In the control group, the mean TCT before surgery was 521.1 ± 34.7 µm; this increased to 549.7 ± 38.4 µm after surgery. Figure 1 shows the reduction in corneal edema with ripasudil use. The TCT change ratio (TCT after surgery/TCT before surgery) in the control group was 1.060 ± 0.033; this was significantly reduced to 1.013 ± 0.046 in the ripasudil group (P = 0.0037).
Figure 1.
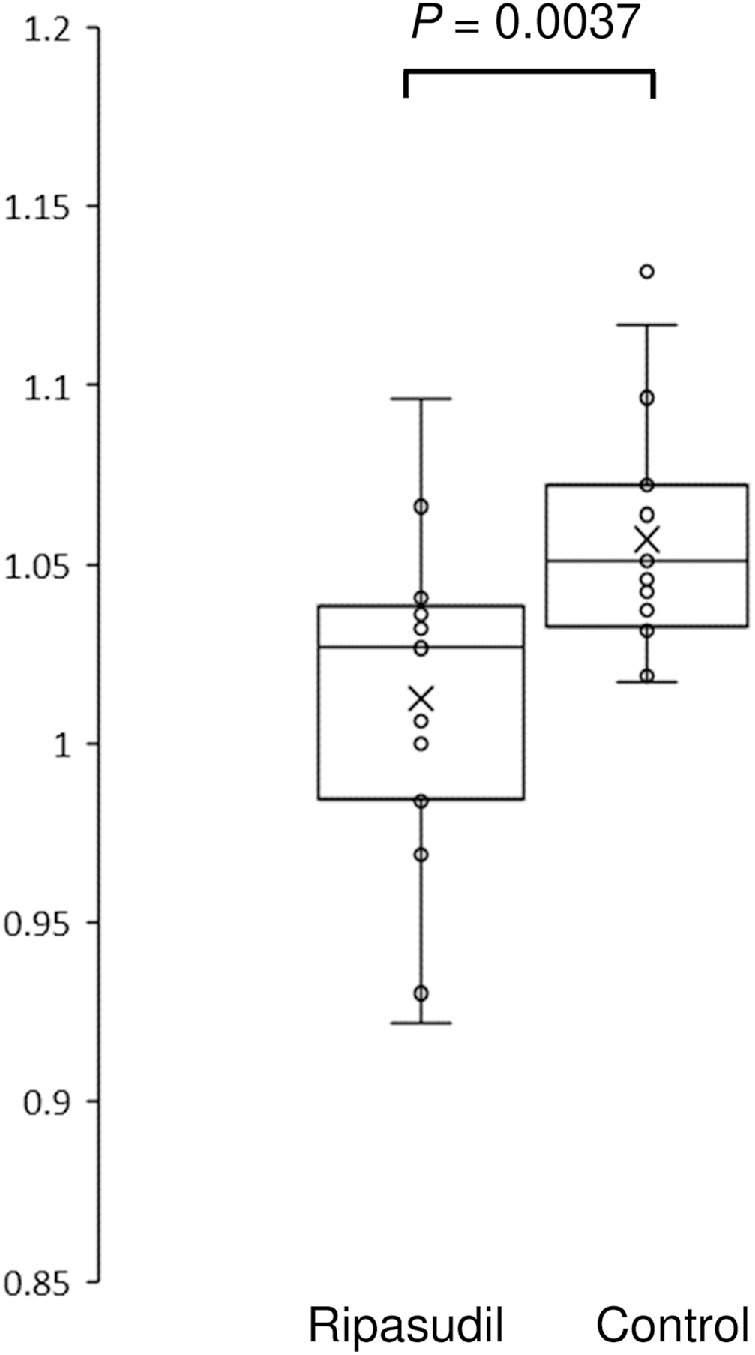
Changes in TCT after surgery in the ripasudil and the control groups. The TCT value one week after surgery divided by the TCT value before surgery is presented. The horizontal lines in the box and whisker plots represent the median values, and the bottom and top of the boxes represent the lower and upper quartiles, respectively. The x represents the mean, and the bars represent the minimum and maximum values within 1.5 times the lower and upper quartiles. TCT, thinnest corneal thickness.
In the ripasudil group, the mean CCT before surgery was 549.7 ± 37.7 µm; this slightly increased to 553.0 ± 39.3 µm after surgery. In the control group, the mean CCT before surgery was 529.4 ± 36.1 µm; this increased to 558.5 ± 38.9 µm after surgery. Figure 2 shows the reduction in corneal edema with ripasudil use. The CCT change ratio (CCT after surgery/CCT before surgery) in the control group was 1.056 ± 0.034; this was significantly reduced to 1.007 ± 0.052 in the ripasudil group (P = 0.0038).
Figure 2.
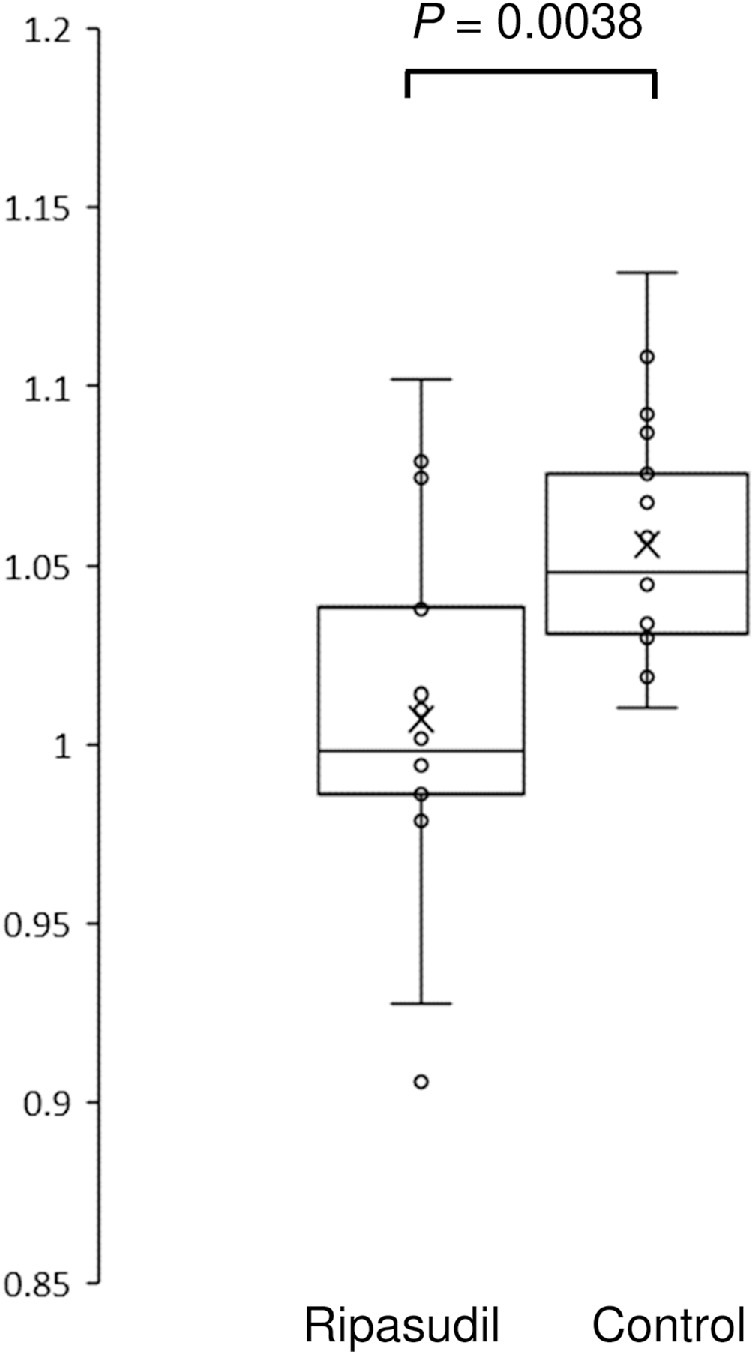
Changes in CCT after surgery in the ripasudil and the control groups. The CCT value one week after surgery divided by the CCT value before surgery is presented. The horizontal lines in the box and whisker plots represent the median values, and the bottom and top of the boxes represent the lower and upper quartiles, respectively. The x represents the mean, and the bars represent the minimum and maximum values within 1.5 times the lower and upper quartiles. CCT, central corneal thickness.
Specular microscopy data was available for 14 eyes from 11 patients in the ripasudil group and 15 eyes from 11 patients in the control group (available for those patients without BK). In the ripasudil group, the mean ECD averaged from one central and four paracentral points before surgery was 935.6 ± 307.1 cells/mm2 compared to 979.8 ± 353.9 cells/mm2 after surgery. In the control group, the mean ECD before surgery was 1006.6 ± 246.1 cells/mm2; this reduced to 850.8 ± 222.0 cells/mm2 after surgery. Figure 3 shows the reduction in endothelial cell loss within paracentral 5 points with ripasudil use. ECD loss [(ECD before surgery – ECD after surgery)/ECD before surgery x 100%] in the control group was 14.1 ± 10.3%; this was significantly reduced to −4.5 ± 13.0% in the ripasudil group (p = 0.0003).
Figure 3.
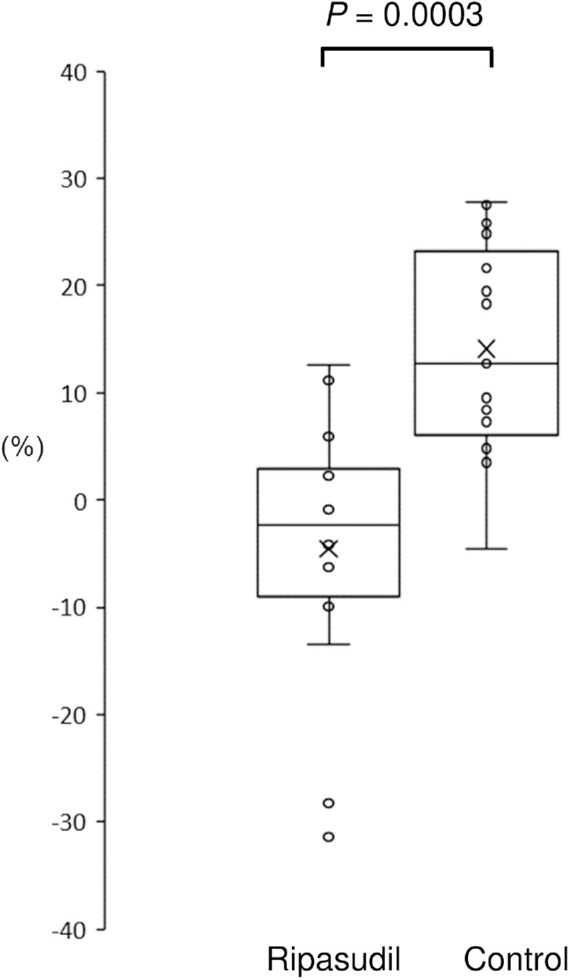
ECD loss after surgery in the ripasudil and the control groups. ECD could be measured for a subset of the cohort (patients without BK). ECD loss is presented as follows: (ECD before surgery − ECD after surgery)/ECD before surgery × 100%. The horizontal lines in the box and whisker plots represent the median values, and the bottom and top of the boxes represent the lower and upper quartiles, respectively. The x represents the mean, and the bars represent the minimum and maximum values within 1.5 times the lower and upper quartiles. ECD, Endothelial cell density.
In the ripasudil group, the mean IOP was 13.2 ± 2.0 mm Hg before surgery and 13.4 ± 5.0 mm Hg after surgery. In the control group, the mean IOP was 13.17 ± 3.41 mm Hg before surgery and 13.0 ± 2.8 mm Hg after surgery. Figure 4 shows the IOP values with ripasudil use. The IOP change ratio (IOP after surgery/IOP before surgery) in the control group was 1.05 ± 0.37; this did not significantly differ from 1.13 ± 0.30 in the ripasudil group (P = 0.398).
Figure 4.
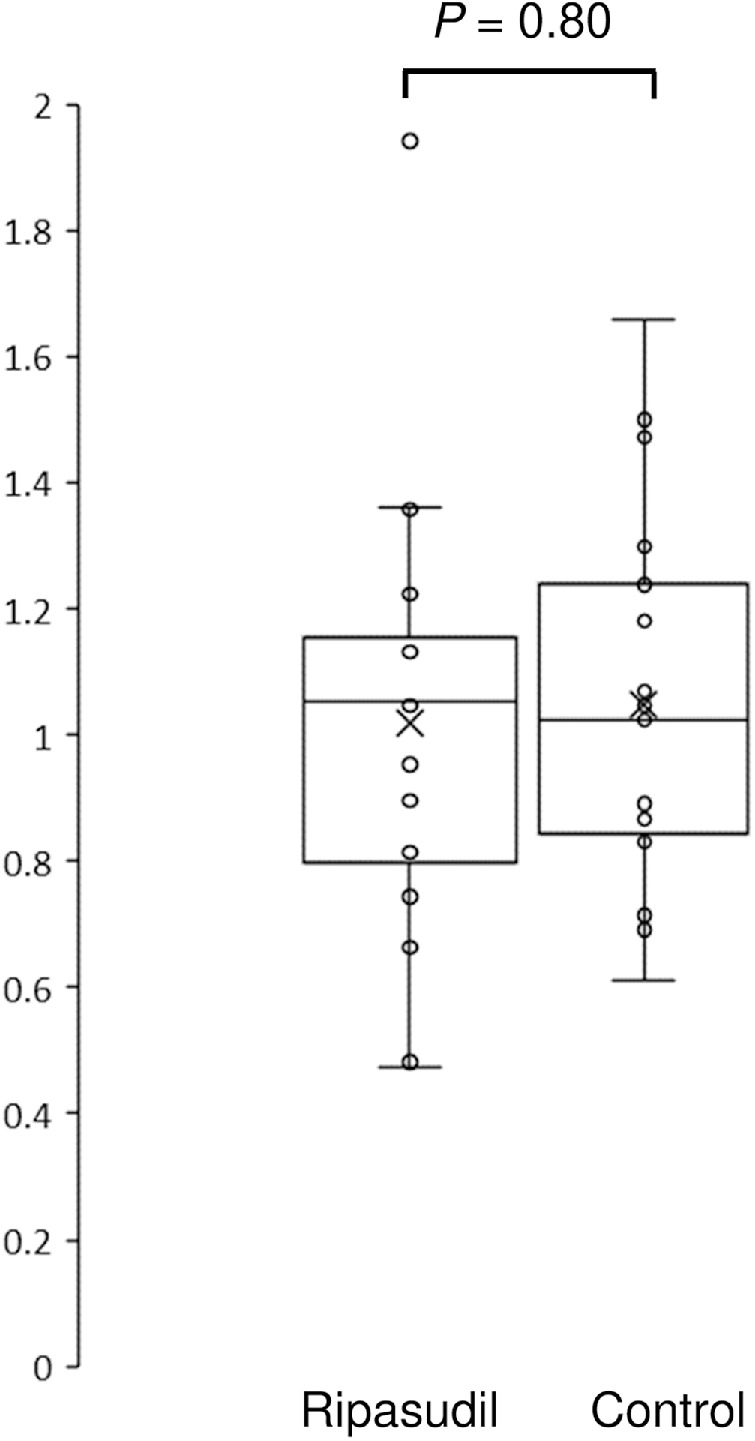
Changes in the IOP after surgery in the ripasudil and the control groups. The IOP value one week after surgery divided by the IOP value before surgery is presented. The horizontal lines in the box and whisker plots represent the median values, and the bottom and top of the boxes represent the lower and upper quartiles, respectively. The x represents the mean and the bars represent the minimum and maximum values within 1.5 times the lower and upper quartiles. IOP, Intraocular pressure.
The mean duration of surgery of 25.6 ± 15.4 and 22.1 ± 9.5 minutes in the ripasudil and control groups, respectively, was not significantly different (Table). The other background measurements examined, age, male/female ratio, TCT and CCT before surgery, and ECD before surgery, nuclear hardness graded by the Emery-Little classification were also not found to be significantly different. The distributions of causes of endothelial loss (FECD, old surgical trauma or corneal surgery, laser iridotomy for closed-angle glaucoma, uveitis, CMV-related corneal endotheliitis) were also not found to be significantly different (P = 0.296, Jonckheere-Terpstra test). In addition, there were no incidents of bullous keratopathy attributable to surgery.
Discussion
Our study revealed that after ripasudil administration, corneal edemas induced by intraocular surgery were significantly reduced in eyes with low corneal endothelial cell density measured by TCT and CCT. Moreover, in cases without BK where specular microscopy data analysis could be performed, ripasudil use suppressed endothelial loss induced after surgery in central and paracentral cornea.
Ripasudil use, which has only been available in Japan for glaucoma treatment, is expected to be extended to other countries, such as the United States, where pseudophakic BK is one of the major causes for corneal transplantation. The administration of the ROCK inhibitor ripasudil in eye drops after intraocular surgery has been shown to be effective in reducing corneal endothelial cell loss and corneal edema, reflective of endothelial inflammation and damage.16 Our study supports the idea that ROCK inhibitors should be formulated as eye drops for the treatment of acute corneal endothelial damage to prevent the progression of BK.8
ROCK inhibitors primarily mediate their effects by promoting endothelial cell migration.8,22,23 The corneal endothelium of the relatively intact region can migrate and compensate for injuries, the surgically damaged region tends to undergo more severe degeneration. An alternative hypothesis is that the peripheral corneal endothelium is resident to migrative, rather than proliferative, stem-like cells.24 In our results, nine of 14 eyes in ripasudil group exhibited negative ECD loss values in the central and para-central cornea, as compared with only one of 15 eyes in the control group. Thus it is possible that the results observed in the present study may be attributed to the migration-promoting effects of ROCK inhibitors on the relatively healthy residual corneal endothelium to the central and paracentral cornea.
In patients with FECD, there is a marked endothelial reduction in the central and paracentral region of the cornea, whereas the endothelium in the surrounding region (periphery) remains comparatively intact.25–28 The treatment of FECD by intentionally removing the Descemet's membrane and endothelium from the progressive loci in the central region and administering ripasudil eye drops has been reported.17 In our results, five of nine eyes in the ripasudil group that had negative ECD loss values also presented with FECD, thus the relatively healthy residual corneal endothelium in the peripheral cornea may migrate to the surgically damaged central and paracentral cornea as a result of ripasudil treatment in these FECD patients.
Okumura et al.16 reported that ripasudil promotes rabbit corneal endothelial wound healing and suggest that it may be useful for protection of the corneal endothelium during intraocular surgery. Rabbit corneal endothelial cells proliferate in physiological conditions, whereas human endothelial cells do not.29 Evidence for the clinical efficacy of ripasudil in humans is required. Our study is the first clinical retrospective examination of the single use of ripasudil in protecting the corneal endothelium after cataract surgery.18
The main limitation of the present study is the retrospective design and the small sample size. Our results need to be verified with a prospective, randomized, masked study in future. The small sample size is reflective of the fact that our patient groups had to also present with glaucoma, because ripasudil has only been approved for clinical use in patients with glaucoma. The data here suggest that examining a large cohort of patients, with and without glaucoma, will be important to confirm that the use of ripasudil has a broad endothelial protective effect.
In conclusion, our results suggest that eyedrops containing ripasudil may help maintain the functional integrity of the corneal endothelium in patients with low endothelial-cell density who undergo cataract surgery. The safety of ripasudil has been proven in glaucoma patients. In addition, these findings suggest that this drug might not only be beneficial for the treatment of cataracts in patients with low corneal endothelial cell density but also be broadly useful for protection of the corneal endothelium after intraocular surgery.
Supplementary Material
Acknowledgments
The authors thank A. Masuda and Y. Mito for discussions, advice, and criticisms that greatly benefited this project.
Supported by Charitable Trust Fund for Ophthalmic Research in Commemoration of Santen Pharmaceutical's Founder 2019 (to H.F.), Research Project Grant 29B-009 from Kawasaki Medical School (to H.F.), and JSPS KAKENHI Grants 25861630 and 16K18378 (to H.F.)
Disclosure: H. Fujimoto, Alcon (F), Otsuka (F), Kowa (F, P), Santen (F), Novartis (F); Y. Setoguchi, Alcon (F); J. Kiryu, Kowa (F), Alcon (F), Novartis (F), Otsuka (F)
References
- 1. Moshirfar M, Parker L, Birdsong OC, et al.. Use of Rho kinase inhibitors in ophthalmology: a review of the literature. Med Hypothesis Discov Innov Ophthalmol J. 2018; 7: 101–111. [PMC free article] [PubMed] [Google Scholar]
- 2. Novack GD. Rho kinase inhibitors for the treatment of glaucoma. Drugs Future. 2013; 38: 107–113. [Google Scholar]
- 3. Inoue T, Tanihara H.. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res. 2013; 37: 1–12. [DOI] [PubMed] [Google Scholar]
- 4. Okumura N, Kinoshita S, Koizumi N.. Application of rho kinase inhibitors for the treatment of corneal endothelial diseases. J Ophthalmol. 2017; 2017: 2646904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jafri M, Colby K.. New insights into corneal endothelial regeneration. Curr Ophthalmol Rep. 2019; 7: 37–44. [Google Scholar]
- 6. Nourinia R, Nakao S, Zandi S, et al.. ROCK inhibitors for the treatment of ocular diseases. Br J Ophthalmol. 2017; 102: 1–5. [DOI] [PubMed] [Google Scholar]
- 7. Kinoshita S, Koizumi N, Ueno M, et al.. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018; 378: 995–1003. [DOI] [PubMed] [Google Scholar]
- 8. Okumura N, Inoue R, Okazaki Y, et al.. Effect of the Rho kinase inhibitor Y-27632 on corneal endothelial wound healing. Invest Ophthalmol Vis Sci. 2015; 56: 6067–6074. [DOI] [PubMed] [Google Scholar]
- 9. Okumura N, Koizumi N, Ueno M, et al.. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am J Pathol. 2012; 181: 268–277. [DOI] [PubMed] [Google Scholar]
- 10. Okumura N, Ueno M, Koizumi N, et al.. Enhancement on primate corneal endothelial cell survival in vitro by a rock inhibitor. Invest Ophthalmol Vis Sci. 2009; 50: 3680–3687. [DOI] [PubMed] [Google Scholar]
- 11. Okumura N, Koizumi N.. Regeneration of the corneal endothelium. Curr Eye Res. 2020; 45: 303–312. [DOI] [PubMed] [Google Scholar]
- 12. Okumura N, Sakamoto Y, Fujii K, et al.. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci Rep . 2016; 6: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mimura T, Yamagami S, Yokoo S, et al.. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci. 2004; 45: 2992–2997. [DOI] [PubMed] [Google Scholar]
- 14. Pricopie S, Istrate S, Voinea L, et al.. Pseudophakic bullous keratopathy. Rom J Ophthalmol. 2017; 61(2): 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koizumi N, Okumura N, Ueno M, et al.. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea. 2014; 33(Suppl 11): S25–S31. [DOI] [PubMed] [Google Scholar]
- 16. Okumura N, Okazaki Y, Inoue R, et al.. Effect of the rho-associated kinase inhibitor eye drop (Ripasudil) on corneal endothelial wound healing. Invest Ophthalmol Vis Sci. 2016; 57: 1284–1292. [DOI] [PubMed] [Google Scholar]
- 17. Moloney G, Petsoglou C, Ball M, et al.. Descemetorhexis without grafting for Fuchs endothelial dystrophy-supplementation with topical ripasudil. Cornea. 2017; 36: 642–648. [DOI] [PubMed] [Google Scholar]
- 18. Moloney G, Congote D, Hirnschall N, et al.. Descemet stripping only supplemented with topical ripasudil for Fuchs endothelial dystrophy 12-month outcomes of the Sydney Eye Hospital Study. Cornea . 2021; 40(3): 320–326. [DOI] [PubMed] [Google Scholar]
- 19. Tanihara H, Kakuda T, Sano T, et al.. Safety and efficacy of ripasudil in Japanese patients with glaucoma or ocular hypertension: 12-month interim analysis of ROCK-J, a post-marketing surveillance study. BMC Ophthalmol. 2020; 20: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kusuhara S, Nakamura M.. Ripasudil hydrochloride hydrate in the treatment of glaucoma: safety, efficacy, and patient selection. Clin Ophthalmol. 2020; 14: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szalai E, Berta A, Hassan Z, et al.. Reliability and repeatability of swept-source Fourier-domain optical coherence tomography and Scheimpflug imaging in keratoconus . J Cataract Refract Surg. 2012; 38: 485–494. [DOI] [PubMed] [Google Scholar]
- 22. Okumura N, Koizumi N, Ueno M, et al.. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 2011; 95: 1006–1009. [DOI] [PubMed] [Google Scholar]
- 23. Koizumi N, Okumura N, Ueno M, et al.. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea . 2013; 32(8): 1167–1170. [DOI] [PubMed] [Google Scholar]
- 24. He Z, Campolmi N, Gain P, et al.. Revisited microanatomy of the corneal endothelial periphery: New evidence for continuous centripetal migration of endothelial cells in humans. Stem Cells. 2012; 30: 2523–2534. [DOI] [PubMed] [Google Scholar]
- 25. Fujimoto H, Maeda N, Soma T, et al.. Quantitative regional differences in corneal endothelial abnormalities in the central and peripheral zones in Fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2014; 55: 5090–5098. [DOI] [PubMed] [Google Scholar]
- 26. Jun AS. One hundred years of Fuchs’ dystrophy. Ophthalmology . 2010; 117: 859–860.e14. [DOI] [PubMed] [Google Scholar]
- 27. Maurice DM. Cellular membrane activity in the corneal endothelium of the intact eye. Experientia. 1968; 24: 1094–1095. [DOI] [PubMed] [Google Scholar]
- 28. Naumann GOH, Schlötzer-Schrehardt U.. Keratopathy in pseudoexfoliation syndrome as a cause of corneal endothelial decompensation: a clinicopathologic study. Ophthalmology. 2000; 107: 1111–1124. [DOI] [PubMed] [Google Scholar]
- 29. Matsubara M, Tanishima T.. Wound-healing of corneal endothelium in monkey: an autoradiographic study. Jpn J Ophthalmol. 1983; 27: 444–450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


