Abstract
Purpose
To assess alterations in quantitative dynamic pupil responses to light in relation to neurologic disability and retinal axonal loss in patients with multiple sclerosis (MS).
Methods
Twenty-five patients with relapsing-remitting MS and 25 healthy subjects were included in this cross-sectional study. Pupillary responses were measured with an infrared dynamic pupillometry unit, and peripapillary retinal nerve fiber layer (RNFL) thickness was measured with spectral-domain optical coherence tomography. Neurologic disability was assessed by the Expanded Disability Status Scale (EDSS). Patients with a history of optic neuritis (ON) within 6 months were excluded. Only the right eyes were assessed, except in 11 patients with a history of unilateral ON in whom both eyes were further analyzed to evaluate the effect of previous ON.
Results
The initial pupil diameter (P = 0.003) and pupil contraction amplitude (P = 0.027) were lower in patients with MS compared with healthy controls. Initial pupil diameter correlated with EDSS score (ρ = −0.458; P = 0.021), and RNFL correlated with contraction latency (ρ = −0.524; P = 0.007). There were no significant differences in any of the pupil parameters between eyes with and without a history of ON, and between the ON and fellow eyes of the 11 patients with previous unilateral ON.
Conclusions
Dynamic pupillometry reveals significant alterations in pupillary light reflex responses associated with neurologic disability and retinal axonal loss, independent of previous ON.
Translational Relevance
Dynamic pupillometry is a simple, noninvasive tool that may be useful in detecting autonomic dysfunction in patients with MS.
Keywords: dynamic pupillometry, multiple sclerosis, optic neuritis, pupillary light reflex, retinal nerve fiber layer
Introduction
Autonomic dysfunction occurs in patients with multiple sclerosis (MS) and can manifest with a range of symptoms including incontinence, urinary retention, gastric and intestinal dysmotility, sexual dysfunction, orthostatic intolerance, vasomotor dysfunction, and sweating and thermoregulatory disorders.1–5 Demyelination and axonal degeneration affecting the autonomic pathway in the brain stem, hypothalamus, and spinal cord, as well as peripheral autonomic nerve fibers are presumably responsible for such disturbances. Autonomic function tests including urodynamic tests, heart rate responses to deep breathing, Valsalva ratio, sympathetic skin responses, and electrochemical skin conductance are abnormal in patients with MS5–9 and have been related to the severity of neurologic disability7–9 and lesions detected on brain magnetic resonance imaging (MRI).9
Dynamic pupillometry allows quantitative measurement of pupil responses to light and has been used to evaluate autonomic dysfunction in diabetes mellitus, Alzheimer disease, overactive bladder, and MS.10–14 Although recent clinical and neuroanatomic studies have demonstrated the additional role of the striate and extra-striate cortex on sensory input to the pupillary system,15–17 the sphincter and dilator muscles of the iris are mainly innervated by the autonomic nervous system. Previous studies have described abnormalities in the pupillary light response in patients with MS, which were associated with spinal cord atrophy but not demyelinating lesions on MRI.14,18 In another study utilizing multifocal pupil perimetry, patients with MS had reduced pupil contraction amplitude and delayed time-to-peak contraction that correlated with disease severity, whereas subjects with two or more contrast enhancing lesions on MRI paradoxically demonstrated an increase in amplitude and a decrease in time-to-peak, suggesting that pupillary abnormalities were related to neuronal degeneration rather than inflammation.19 We have previously demonstrated corneal nerve fiber loss using corneal confocal microscopy and reduced retinal nerve fiber layer (RNFL) thickness using optical coherence tomography (OCT) in patients with MS.20 In this study, we aimed to evaluate pupillary light reflex measures in relation to neurologic disability, RNFL thickness, and previous history of optic neuritis (ON).
Methods
Twenty-five consecutive patients with relapsing-remitting MS and 25 healthy control subjects were enrolled in this cross-sectional study conducted at a tertiary referral university hospital. The diagnosis of MS was based on both clinical and radiologic findings, according to the revised McDonald criteria.21 Neurologic disability and disease severity were assessed using the Kurtzke Expanded Disability Status Scale (EDSS), and the Multiple Sclerosis Severity Score (MSSS), which was calculated from the EDSS score and disease duration. A history of previous episodes of ON and the medications used by the patients were recorded. Exclusion criteria were previous ocular trauma or surgery, use of anticholinergic agents or beta blockers and any other medication that might influence the autonomic nervous system, a history of ON within 6 months of enrollment, diabetes, or any other neurologic disorders that might cause autonomic neuropathy. Patients with a visual acuity of lower than 20/25 were also excluded. The study was approved by the Research Ethics Committee of the Necmettin Erbakan University and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants after they were informed in detail about the course and possible results of the study.
Each participant underwent detailed ophthalmologic examination including visual acuity assessment, slit-lamp anterior segment biomicroscopy, and fundus examination. The peripapillary RNFL thickness was measured using a spectral-domain OCT device (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany). Scans were acquired at 3.4-mm-diameter circle positioned at the center of the optic nerve head and the average peripapillary RNFL thickness was recorded.
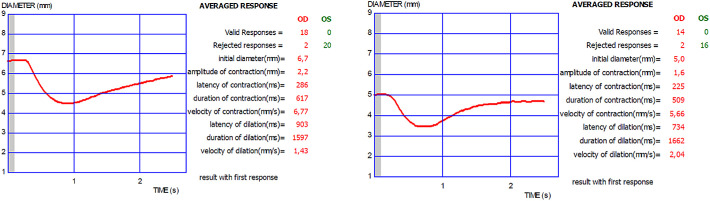
Pupillary light reflex responses were recorded by using an infrared dynamic pupillometry unit (MonPack One; Metrovision, Pérenchies, France), which utilizes near-infrared illumination (880 nm) and a high-resolution infrared imaging sensor that allows the measurement of pupil parameters in complete darkness. Dynamic pupillary responses were elicited with white-light flashes (total luminance 100 cd/m2, stimulation on time 200 ms, stimulation off time 3300 ms) and recorded with an infrared camera. Automated real-time image processing was performed (30 images per second) and pupillary contours were outlined by the proprietary software provided in the device with a measurement sensitivity of 0.1 mm. After 5 minutes of dark adaptation, one eye was occluded and at least 10 measurements were performed monocularly. The following eight parameters were automatically quantified: initial pupil diameter (mm), contraction amplitude (mm), contraction latency (ms), contraction duration (ms), contraction velocity (mm/s), dilation latency (ms), dilation duration (ms), and dilation velocity (mm/s) (Fig. 1). To reduce the potential influence of diurnal variations on the results, all examinations were performed between 9 AM and 12 AM.
Figure 1.
Pupillary light reflex responses measured with dynamic pupillometry in the right eyes of a healthy subject (left) and a patient with MS (right). OD, oculus dextrus; OS, oculus sinister.
Only the data obtained from the right eyes of participants were included in comparisons between patients with MS and healthy control subjects. A separate comparison including both eyes of the 11 patients with unilateral ON was performed to evaluate the intereye differences in pupillary responses in relation to a history of ON.
Statistical analyses of the data were performed with SAS software (SAS Institute, Cary, NC). Power analysis based on a previously published study14 revealed that a minimum sample size of 10 was needed to detect a significant difference with a power (1-β) of 0.80 and a significance level (α) of 0.05. Basic descriptive statistics were calculated and reported as the mean ± SD or median (interquartile range [IQR]), as appropriate. The Kolmogorov-Smirnov test was used to evaluate the normality distribution of continuous numeric data. The Pearson χ2 test was used to compare the categorical parameters. Binomial logistic regression models were used to assess the individual effects of multiple study variables between patients with MS and control participants, and between MS patients with and without a history of ON. The associations between pupillary light reflex parameters, RNFL thickness, and disease severity scores were measured using Pearson correlation coefficient for normally distributed data and Spearman correlation coefficient for non-normally distributed data. For all evaluations, a P value of less than 0.05 was considered statistically significant.
Results
The mean ages of the patients with MS and healthy control subjects were 34.8 ± 8.0 years and 34.2 ± 7.0 years, respectively. There were no significant differences between patients with MS and control group for age (P = 0.779) and sex (P = 0.382). The mean duration of MS was 9.5 ± 3.9 years, the median (IQR) value of the EDSS score was 3.0 (2.3–4.0), and the mean value of MSSS was 4.21 ± 1.59. Of the 25 patients with MS, 6 (24%) had a history of bilateral ON, 11 (44%) had a history of unilateral ON, and 8 (32%) had not experienced ON. Twenty-two patients (88%) were receiving disease-modifying agents; 11 (44%) patients were on fingolimod, 9 (36%) were on interferon-beta, 2 (8%) were on azathioprine; and 3 (12%) patients were not receiving any disease-modifying therapy. Only two eyes had a decimal visual acuity of 0.9, whereas all remaining eyes in patients with MS and control subjects had a best-corrected visual acuity of 1.0 or better.
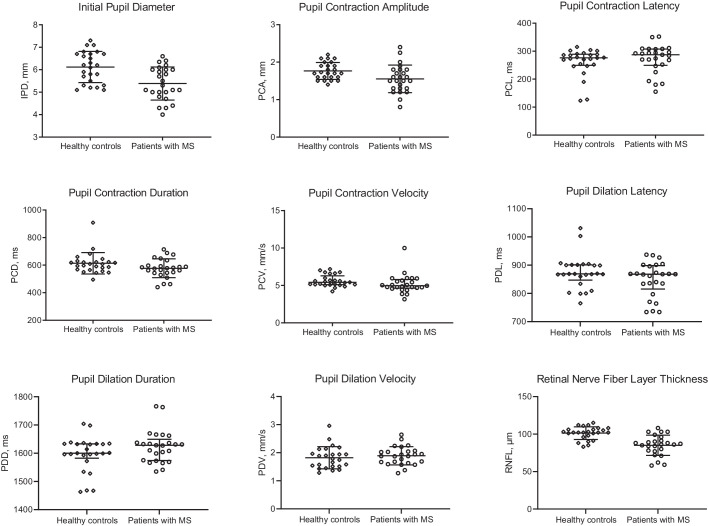
A logistic regression analysis showed that patients with MS had a significantly lower initial pupil diameter (5.39 ± 0.74 vs. 6.12 ± 0.69 mm, P = 0.003), pupil contraction amplitude (1.55 ± 0.37 vs. 1.76 ± 0.23 mm, P = 0.027), and RNFL thickness (85.1 ± 13.5 vs. 101.3 ± 8.5 µm, P < 0.001) compared with control subjects. Other pupillary light reflex parameters did not differ significantly between patients with MS and controls (Table 1, Fig. 2). Table 1 also gives the effect-size as Cohen's d for the parameters. There were no significant differences in any of the study parameters between male and female subjects with MS or healthy controls.
Table 1.
Pupillary Light Reflex Responses and Peripapillary RNFL Thickness in Patients With MS and Healthy Control Subjects
| Healthy | Patients With | Cohen's d | ||
|---|---|---|---|---|
| Controls (n = 25) | MS (n = 25) | P Valuea | Effect Size | |
| Initial pupil diameter (mm) | 6.12 ± 0.69 | 5.39 ± 0.74 | 0.003 | 1.0243 |
| Contraction amplitude (mm) | 1.76 ± 0.23 | 1.55 ± 0.37 | 0.027 | 0.6884 |
| Percentage change in size (%) | 29.1 ± 4.5 | 28.8 ± 5.2 | 0.818 | 0.0641 |
| Contraction latency (ms) | 276.0 (249.5–289.0) | 287.0 (249.5–307.5) | 0.435 | −0.2199 |
| Contraction duration (ms) | 613.4 ± 77.5 | 577.2 ± 68.4 | 0.102 | 0.4963 |
| Contraction velocity (mm/s) | 5.42 (5.10–6.28) | 4.95 (4.63–5.80) | 0.185 | 0.3917 |
| Dilation latency (ms) | 869.0 (847.0–901.0) | 868.0 (815.5–898.5) | 0.148 | 0.4221 |
| Dilation duration (ms) | 1600.0 (1582.5–1633.0) | 1628.0 (1574.0–1649.5) | 0.119 | −0.4828 |
| Dilation velocity (mm/s) | 1.85 ± 0.40 | 1.89 ± 0.32 | 0.660 | −0.1224 |
| RNFL thickness (µm) | 101.3 ± 8.5 | 85.1 ± 13.5 | <0.001 | 1.4400 |
Data are expressed as mean ± SD for parametric variables and median (IQR) for nonparametric variables.
Binomial logistic regression analysis.
Figure 2.
Dynamic pupillometry parameters and RNFL thickness in healthy control subjects and patients with MS, showing a significant reduction in IPD (P = 0.003), PCA (P = 0.027), and RNFL thickness (P < 0.001), and no change in PCL (P = 0.435), PCD (P = 0.102), PCV (P = 0.185), PDL (P = 0.148), PDD (P = 0.119), and PDV (P = 0.660) in patients with MS.
Among the subjects with MS, 12 had previous ON in their right eyes. There were no significant differences in any of the pupil parameters and RNFL thickness between eyes with and without previous ON (Table 2), as well as between ON eyes and fellow eyes of the patients with a history of unilateral ON (n = 11, Table 3).
Table 2.
Comparison of the Study Parameters According to a History of Previous ON in the Right Eyes of Patients With MS
| Eyes With | Eyes Without | Cohen's d | ||
|---|---|---|---|---|
| Previous ON (n = 12) | Previous ON (n = 13) | P Valuea | Effect Size | |
| Initial pupil diameter (mm) | 5.40 ± 0.79 | 5.37 ± 0.72 | 0.904 | 0.0464 |
| Contraction amplitude (mm) | 1.60 ± 0.32 | 1.52 ± 0.41 | 0.579 | 0.2160 |
| Percentage change in size (%) | 29.5 ± 3.5 | 28.2 ± 6.4 | 0.534 | 0.2426 |
| Contraction latency (ms) | 279.0 (248.3–303.3) | 293.0 (231.5–308.5) | 0.818 | −0.0886 |
| Contraction duration (ms) | 573.1 ± 74.2 | 580.9 ± 65.4 | 0.771 | −0.1124 |
| Contraction velocity (mm/s) | 5.05 (4.80–5.86) | 4.77 (4.47–5.70) | 0.937 | 0.0304 |
| Dilation latency (ms) | 865.0 (776.8–896.3) | 868.0 (834.5–898.5) | 0.608 | −0.1986 |
| Dilation duration (ms) | 1618.5 (1567.8–1654.3) | 1632.0 (1587.0–1651.0) | 0.758 | −0.1188 |
| Dilation velocity (mm/s) | 1.87 ± 0.30 | 1.91 ± 0.36 | 0.749 | −0.1235 |
| RNFL thickness (µm) | 81.9 ± 13.6 | 88.0 ± 13.2 | 0.262 | −0.4541 |
Data are expressed as mean ± SD for parametric variables and median (IQR) for nonparametric variables.
Binomial logistic regression analysis.
Table 3.
Comparison of the Study Parameters Between Eyes With Previous ON and Fellow Eyes of the 11 Patients With MS With a History of Unilateral ON
| ON Eye (n = 11) | Non-ON Eye (n = 11) | P Valuea | Cohen's d Effect Size | |
|---|---|---|---|---|
| Initial pupil diameter (mm) | 5.12 ± 0.79 | 5.15 ± 0.87 | 0.936 | −0.0870 |
| Contraction amplitude (mm) | 1.56 ± 0.49 | 1.57 ± 0.42 | 0.961 | −0.0963 |
| Percentage change in size (%) | 30.1 ± 6.7 | 30.4 ± 5.3 | 0.926 | −0.0948 |
| Contraction latency (ms) | 268.0 (238.0–307.0) | 269.0 (243.0–293.0) | 0.889 | −0.0911 |
| Contraction duration (ms) | 561.8 ± 85.2 | 558.9 ± 71.4 | 0.928 | 0.0543 |
| Contraction velocity (mm/s) | 5.47 (4.34–5.97) | 5.06 (4.73–5.84) | 0.920 | −0.1999 |
| Dilation latency (ms) | 834.0 (737.0–869.0) | 834.0 (767.0–869.0) | 0.983 | −0.0099 |
| Dilation duration (ms) | 1631.0 (1601.0–1670.0) | 1637.0 (1630.0–1703.0) | 0.700 | −0.1749 |
| Dilation velocity (mm/s) | 2.02 ± 0.32 | 1.95 ± 0.43 | 0.623 | 0.2115 |
| RNFL thickness (µm) | 83.9 ± 15.7 | 91.8 ± 13.4 | 0.223 | −0.7297 |
Data are expressed as mean ± SD for parametric variables and median (IQR) for nonparametric variables.
Binomial logistic regression analysis.
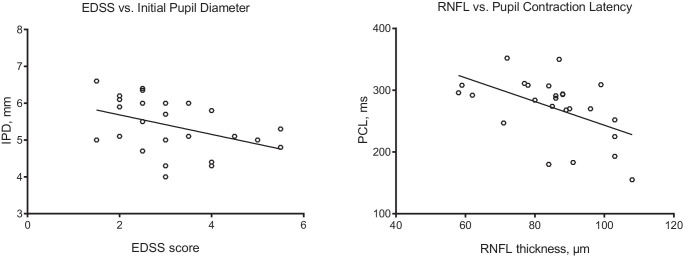
The initial pupil diameter showed an inverse correlation with the EDSS score (ρ = −0.458; P = 0.021, Fig. 3), and pupil contraction latency showed an inverse correlation with RNFL (ρ = −0.524; P = 0.007). Regression analysis using a standard linear model identified significant associations between EDSS and initial pupil diameter (β = −0.64; P = 0.040), and MSSS and pupil contraction velocity (β = −0.93; P = 0.014). The units of the two fitted β were mm/EDSS-step and mm/s/MSSS-step, respectively. There was no significant correlation between age and pupil parameters or RNFL thickness.
Figure 3.
Scatter-plot graphs showing a significant inverse correlation between EDSS score and initial pupil diameter (ρ = −0.458; P = 0.021), and RNFL thickness and pupil contraction latency (ρ = −0.524; P = 0.007).
Discussion
The current study shows that patients with MS had smaller initial pupil diameter and reduced amplitude of pupil contraction but no abnormality in dilation compared with healthy subjects. Furthermore, alterations in pupillary light response were related with the severity of neurologic disability and RNFL thickness, regardless of a history of ON. The pupillary light reflex is a useful indicator of autonomic nervous system function reflecting both sympathetic and parasympathetic activity. The initial pupil diameter is determined primarily by sympathetic innervation, whereas the amplitude and velocity of pupil contraction are indicators of parasympathetic activity.22 Therefore the reduction in initial pupil diameter observed in this study may indicate a diminished sympathetic tone, and the reduction in amplitude of pupil contraction may reflect a reduced parasympathetic activity in patients with MS. Although the smaller initial pupil size could contribute to the reduction in amplitude of pupil contraction, a study in diabetic patients with a small resting pupil size showed that reduced amplitude was associated with parasympathetic dysfunction.23 Indeed, a reduced initial pupil size and decreased pupil contraction amplitude has been found in diabetic patients without cardiac autonomic neuropathy, suggesting that pupillary alterations may be the earliest signs of diabetic autonomic neuropathy.10,24
In patients with MS, Surakka et al.13 showed a significant reduction in initial pupil diameter and time to 75% redilation, but no correlation to neurologic disability. In another study by Pozzessere et al.,18 although pupil contraction amplitude and velocity were reduced in patients with MS, they did not correlate with latency of the visual evoked response or demyelinating lesions on MRI. Similarly, de Seze et al.14 demonstrated reduced pupil contraction amplitude and increased contraction latency in different subtypes of MS but found no relation to EDSS score or previous history of ON. The current study demonstrates reduced initial pupil size and decreased amplitude of pupil contraction consistent with these studies, but additionally, we show significant associations between the initial pupil diameter and EDSS score, and pupil contraction velocity and MSSS. Ali et al.19 have utilized multifocal pupil perimetry in patients with MS to show that the amplitude of pupil contraction was reduced, and time-to-peak contraction was increased, and the latter correlated with EDSS. They also demonstrated that patients with two or more contrast enhancing lesions on MRI had a slightly increased contraction amplitude and reduced time-to-peak contraction, suggesting that pupillary responses reflect axonal damage rather than inflammation.
We have found no significant differences in pupil parameters between patients with and without previous ON, as well as between the affected and unaffected eye of patients with unilateral ON, consistent with previous studies.14,18 Van Diemen et al.25 have shown prolonged contraction latency in eyes with previous ON compared with fellow eyes of patients with MS. This discrepancy may be owing to differences in study populations, as the visual acuity of patients in our study ranged between 0.9 and 1.0, whereas it was between 0.016 and 1.0 in their study, suggesting the presence of an anterior pathway defect rather than efferent autonomic denervation. This is further supported by the inverse correlation between RNFL thickness and pupil contraction latency observed in our study.
Peripapillary RNFL thickness is an established surrogate measure of axonal degeneration and is particularly reduced after ON in MS.26–28 In the current study, we also demonstrated a significant reduction in peripapillary RNFL thickness between patients with MS and healthy controls. However, although RNFL thickness was lower in patients with previous ON, this was not statistically significant because of the small sample size. In addition to retinal axonal degeneration, we and others have recently shown axonal damage in corneal nerve fibers of patients with MS.20,29–31 Corneal nerve fiber loss is related to sudomotor dysfunction, reduced heart rate variability, and erectile dysfunction in diabetic patients,32–34 suggesting that corneal confocal microscopy might be useful in detecting autonomic neuropathy. Further studies evaluating both corneal nerve fibers and pupillary light reflex parameters are required to better understand the association between axonal damage and autonomic dysfunction in patients with MS.
Limitations of the current study are the small sample size and cross-sectional study design, which precludes us drawing any conclusions about the natural history of alterations in pupillary light responses in MS.
Conclusions
We show an abnormality in pupillary light reflex parameters, which were associated with neurologic disability and retinal axonal loss but not with a history of ON. Further studies with a larger sample size are required to determine the utility of quantifying dynamic pupillary responses in patients with MS and to evaluate the potential role of axonal degeneration in the pathophysiology of pupillary abnormalities.
Acknowledgments
Disclosure: G. Bitirgen, None; Z. Akpinar, None; H.B. Turk, None; R.A. Malik, None
References
- 1. Kragt JJ, Hoogervorst EL, Uitdehaag BM, Polman CH.. Relation between objective and subjective measures of bladder dysfunction in multiple sclerosis. Neurology. 2004; 63: 1716–1718. [DOI] [PubMed] [Google Scholar]
- 2. el-Maghraby TA, Shalaby NM, Al-Tawdy MH, Salem SS.. Gastric motility dysfunction in patients with multiple sclerosis assessed by gastric emptying scintigraphy. Can J Gastroenterol. 2005; 19: 141–145. [DOI] [PubMed] [Google Scholar]
- 3. Drulovic J, Kisic-Tepavcevic D, Pekmezovic T.. Epidemiology, diagnosis and management of sexual dysfunction in multiple sclerosis. Acta Neurol Belg. 2020; 120(4): 791–797. [DOI] [PubMed] [Google Scholar]
- 4. Allen DR, Huang MU, Morris NB, et al.. Impaired thermoregulatory function during dynamic exercise in multiple sclerosis. Med Sci Sports Exerc. 2019; 51: 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Findling O, Hauer L, Pezawas T, Rommer PS, Struhal W, Sellner J.. Cardiac autonomic dysfunction in multiple sclerosis: a systematic review of current knowledge and impact of immunotherapies. J Clin Med. 2020; 9: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haensch CA, Jörg J.. Autonomic dysfunction in multiple sclerosis. J Neurol. 2006; 253(Suppl. 1): I3–I9. [DOI] [PubMed] [Google Scholar]
- 7. Kale N, Magana S, Agaoglu J, Tanik O.. Assessment of autonomic nervous system dysfunction in multiple sclerosis and association with clinical disability. Neurol Int. 2009; 1: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan A, Kamran S, Ponirakis G, et al.. Peripheral neuropathy in patients with multiple sclerosis. PLoS One. 2018; 13: e0193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saari A, Tolonen U, Pääkkö E, et al.. Cardiovascular autonomic dysfunction correlates with brain MRI lesion load in MS. Clin Neurophysiol. 2004; 115: 1473–1478. [DOI] [PubMed] [Google Scholar]
- 10. Ferrari GL, Marques JL, Gandhi RA, et al.. Using dynamic pupillometry as a simple screening tool to detect autonomic neuropathy in patients with diabetes: a pilot study. Biomed Eng Online. 2010; 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chougule PS, Najjar RP, Finkelstein MT, Kandiah N, Milea D.. Light-induced pupillary responses in Alzheimer's disease. Front Neurol. 2019; 10: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aydogmus Y, Uzun S, Gundogan FC, Ulas UH, Ebiloglu T, Goktas MT.. Is overactive bladder a nervous or bladder disorder? Autonomic imaging in patients with overactive bladder via dynamic pupillometry. World J Urol. 2017; 35: 467–472. [DOI] [PubMed] [Google Scholar]
- 13. Surakka J, Ruutiainen J, Romberg A, Puukka P, Kronholm E, Karanko H.. Pupillary function in early multiple sclerosis. Clin Auton Res. 2008; 18: 150–154. [DOI] [PubMed] [Google Scholar]
- 14. de Seze J, Arndt C, Stojkovic T, et al.. Pupillary disturbances in multiple sclerosis: correlation with MRI findings. J Neurol Sci. 2001; 188: 37–41. [DOI] [PubMed] [Google Scholar]
- 15. Gamlin PD. The pretectum: connections and oculomotor-related roles. Prog Brain Res. 2006; 151: 379–405. [DOI] [PubMed] [Google Scholar]
- 16. Rosli Y, Carle CF, Ho Y, et al.. Retinotopic effects of visual attention revealed by dichoptic multifocal pupillography. Sci Rep. 2018; 8(1): 2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sabeti F, James AC, Carle CF, Essex RW, Bell A, Maddess T.. Comparing multifocal pupillographic objective perimetry (mfPOP) and multifocal visual evoked potentials (mfVEP) in retinal diseases. Sci Rep. 2017; 7: 45847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pozzessere G, Rossi P, Valle E, Froio CP, Petrucci AF, Morocutti C.. Autonomic involvement in multiple sclerosis: a pupillometric study. Clin Auton Res. 1997; 7: 315–319. [DOI] [PubMed] [Google Scholar]
- 19. Ali EN, Maddess T, James AC, Voicu C, Lueck CJ.. Pupillary response to sparse multifocal stimuli in multiple sclerosis patients. Mult Scler. 2014; 20(7): 854–861. [DOI] [PubMed] [Google Scholar]
- 20. Bitirgen G, Akpinar Z, Malik RA, Ozkagnici A.. Use of corneal confocal microscopy to detect corneal nerve loss and increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. 2017; 135: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Polman CH, Reingold SC, Banwell B, et al.. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaji K, Hirata Y, Usui S.. A method for monitoring autonomic nervous activity by pupillary flash response. Systems Computers Japan. 2000; 31: 22–31. [Google Scholar]
- 23. Smith SA, Smith SE.. Reduced pupillary light reflexes in diabetic autonomic neuropathy. Diabetologia. 1983; 24: 330–332. [DOI] [PubMed] [Google Scholar]
- 24. Pittasch D, Lobmann R, Behrens-Baumann W, Lehnert H.. Pupil signs of sympathetic autonomic neuropathy in patients with type 1 diabetes. Diabetes Care. 2002; 25: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 25. Van Diemen HA, Van Dongen MM, Nauta JJ, Lanting P, Polman CH.. Pupillary light reflex latency in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1992; 82: 213–219. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Martin E, Pueyo V, Ara JR, et al.. Effect of optic neuritis on progressive axonal damage in multiple sclerosis patients. Mult Scler. 2011; 17: 830–837. [DOI] [PubMed] [Google Scholar]
- 27. Behbehani R, Al-Hassan AA, Al-Khars A, Sriraman D, Alroughani R. Retinal nerve fiber layer thickness and neurologic disability in relapsing-remitting multiple sclerosis. J Neurol Sci. 2015; 359: 305–308. [DOI] [PubMed] [Google Scholar]
- 28. Abalo-Lojo JM, Treus A, Arias M, Gómez-Ulla F, Gonzalez F.. Longitudinal study of retinal nerve fiber layer thickness changes in a multiple sclerosis patients cohort: a long term 5 year follow-up. Mult Scler Relat Disord. 2018; 19: 124–128. [DOI] [PubMed] [Google Scholar]
- 29. Mikolajczak J, Zimmermann H, Kheirkhah A, et al.. Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density. Mult Scler. 2017; 23: 1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petropoulos IN, Kamran S, Li Y, et al.. Corneal confocal microscopy: an imaging endpoint for axonal degeneration in multiple sclerosis. Invest Ophthalmol Vis Sci. 2017; 58: 3677–3681. [DOI] [PubMed] [Google Scholar]
- 31. Bitirgen G, Akpinar Z, Uca AU, Ozkagnici A, Petropoulos IN, Malik RA.. Progressive loss of corneal and retinal nerve fibers in patients with multiple sclerosis: a 2-year follow-up study. Transl Vis Sci Technol. 2020; 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ponirakis G, Fadavi H, Petropoulos IN, et al.. Automated quantification of neuropad improves its diagnostic ability in patients with diabetic neuropathy. J Diabetes Res. 2015; 2015: 847854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sivaskandarajah GA, Halpern EM, Lovblom LE, et al.. Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes Care. 2013; 36: 2748–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Azmi S, Ferdousi M, Alam U, et al.. Small-fibre neuropathy in men with type 1 diabetes and erectile dysfunction: a cross-sectional study. Diabetologia. 2017; 60: 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]