Abstract
Purpose
Corneal confocal microscopy (CCM) is an ophthalmic imaging technique that has been used to identify increased corneal immune cells in patients with immune-mediated peripheral neuropathy. Given that multiple sclerosis has an immune-mediated etiology, we have compared corneal immune cell (IC) density and near-nerve distance in different subtypes of patients with multiple sclerosis (MS) to controls.
Methods
This is a blinded, cross-sectional study conducted at a tertiary hospital. Patients with clinically isolated syndrome (CIS) (n = 9), relapsing-remitting multiple sclerosis (RRMS) (n = 43), secondary progressive multiple sclerosis (SPMS) (n = 22), and control subjects (n = 20) underwent CCM. The total, mature, and immature corneal IC density and their nearest nerve distance were quantified.
Results
The total IC density was higher in patients with MS (P = 0.02), RRMS (P = 0.01), and SPMS (P = 0.04) but not CIS (P = 0.99) compared to controls. Immature IC density was higher in patients with MS (P = 0.03) and RRMS (P = 0.02) but not SPMS (P = 0.10) or CIS (P = 0.99) compared to controls. Mature IC density (P = 0.15) did not differ between patients with MS and controls. The immature IC near-nerve distance was significantly greater in patients with MS (P = 0.001), RRMS (P = 0.007), and SPMS (P = 0.002) compared to controls. Immature IC density correlated with the Symbol Digit Modalities Test (r = –0.281, P = 0.02) and near-nerve distance correlated with the Expanded Disability Status Scale (r = 0.289, P = 0.005).
Conclusions
In vivo CCM demonstrates an increase in immature IC density and the near-nerve distance in patients with MS. These observations merit further studies to assess the utility of CCM in assessing neuroimmune alterations in MS.
Translational Relevance
Multiple sclerosis is an immune-mediated neurodegenerative disease. Dendritic cells mediate communication between the innate and adaptive immune systems. We have used in vivo CCM to show increased corneal ICs and suggest it may act as an imaging biomarker for disease status in patients with MS.
Keywords: corneal confocal microscopy, corneal nerves, immune cells, multiple sclerosis
Introduction
Multiple sclerosis (MS) is a progressive immune-mediated inflammatory and progressive neurodegenerative disease.1 Disease severity and progression can be assessed from neurologic disability and magnetic resonance imaging of the brain to identify inflammation and brain atrophy.2 In vivo imaging of immune cells could provide insight into the role of immune activation in disease relapse and progression and to evaluate the effect of therapies targeting immune activation in clinical trials of MS.
Corneal immune cells (ICs) are a subtype of bone marrow–derived antigen-presenting cells and a key component of the corneal immune defense system.3,4 They appear as white reflective structures using in vivo corneal confocal microscopy as confirmed by ex vivo tissue studies.5–7 Most ICs located at the center of the cornea are immature cells without dendritic structures while those located in the periphery are mature cells with dendritic structures8 and reside primarily in the basal epithelium or subbasal layer.9
In vivo corneal confocal microscopy (CCM) has been used to quantify ICs in normal and pathologic human corneas.3,9–11 Increased IC density correlates with increased proinflammatory tear cytokines and reduced subbasal nerve density.12 IC density has also been used as an endpoint in clinical trials of anti-inflammatory drugs for inflammatory ocular surface disease.13 We and others have shown an increase in IC density in a number of immune-mediated conditions, including Behcet disease,14 chronic inflammatory demyelinating polyneuropathy,15 diabetic neuropathy,16 Fabry disease17 and fibromyalgia.11 Of relevance to the current study, we and others have also demonstrated subbasal corneal nerve loss with normal18,19 or increased20 corneal ICs in small cohorts of patients with relapsing remitting MS.
This study quantified corneal IC density and distribution relative to corneal nerves in different subtypes of patients with MS.
Patients and Methods
Seventy-four patients (MS, n = 65 and clinically isolated syndrome [CIS], n = 9) attending the neurology outpatient clinic at Hamad General Hospital in Doha, Qatar, and 20 healthy age- and gender-matched controls were studied. Patients with MS were classified as having relapsing-remitting MS (RRMS, n = 43) and secondary progressive MS (SPMS, n = 22), fulfilling the 2010 revised McDonald criteria.21 This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of Weill Cornell Medicine-Qatar (approval no. 15-00064). Informed, written consent was obtained from all participants before participation in the study.
Patients with MS and healthy controls who wore contact lenses or were diagnosed with dry eyes, glaucoma, vitreoretinal or corneal disorders; had active optic neuritis; or had undergone refractive surgery within 1 year of their participation were excluded. Patients with other metabolic, ophthalmologic, rheumatologic, or neurologic disorders that may cause neuropathy were excluded from participation in the study based on HbA1c, antinuclear antibody, serum B12/folate, and immunoglobulins and medical history.
Neurologic Evaluation
The duration of MS was calculated from the date the patient was first diagnosed to the time of assessment. The severity of neurologic disability was evaluated using the Expanded Disability Status Scale (EDSS),22 Timed 25-foot Walk Test (T25FWT) to assess the gait speed, Nine-Hole Peg Test (9-HPT) to assess upper extremity function, and the Symbol Digit Modality Test (SDMT) to assess cognitive and motor performance.23 The T25FWT requires an assessment of the time taken for the patient to walk to and from a marked point on a flat surface over a distance of 25 feet (7.62 m). The 9-HPT is a standardized test in which the time taken for each participant to pick up nine pegs from a shallow container one at a time and place them in each of nine holes and then remove them one at a time back into the shallow container is recorded. Two consecutive trials with the dominant hand are immediately followed by two consecutive trials with the nondominant hand. For the SDMT, the participant is presented with a page headed by a key that pairs the single digits 1 to 9 with nine symbols. The participant's task is to write or orally report the correct pairing of the number to the symbol, and the total score is the number of correct responses within 90 seconds.24
Corneal Confocal Microscopy
Corneal confocal microscopy (Heidelberg Retinal Tomograph III Rostock Cornea Module; Heidelberg Engineering GmbH, Heidelberg, Germany) was undertaken by two experienced examiners and took approximately 10 minutes for both eyes using our established protocol.19 Image analysis was performed by a single trained investigator masked to the participant's clinical status. Based on depth, contrast and focus, six images (three from each eye) of the subbasal nerve plexus per participant were selected and analyzed.
Immune Cell Density
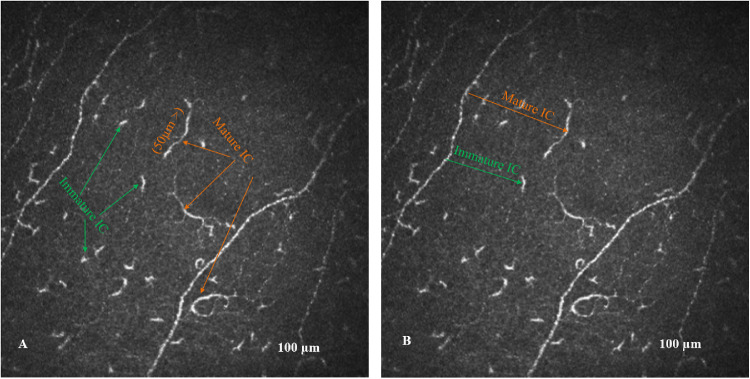
Zhivov et al.3 described three types of ICs—(1) individual cell bodies without processes, (2) cell-bearing dendrites, and (3) cells arranged in a network through long interdigitating dendrites—and proposed that the absence or presence of dendrites could be used to differentiate immature from mature ICs. This was based on a suggestion by Hamrah et al.8 that “cells in the center of the cornea often lacked the dendrites, most likely underlining their immature phenotype,” and studies by Teunissen et al.25 in epidermal ICs that had shown that maturation of ICs is associated with an increase in size and the development of numerous long dendrites. We have adopted the absence or presence of dendrites as the primary criterion to differentiate immature from mature cells, respectively,15 and in addition, we introduced a cell length cutoff of 50 µm as an additional objective measure to help differentiate mature from immature cells.16 ICs with a length <50 µm without dendritic structures were considered immature, and those with a length >50 µm and dendritic structures were considered mature cells (Fig. 1A). The length of each cell was quantified using the nerve fiber length feature and IC number was measured using the nerve branch density feature of the CCMetrics software (University of Manchester, Manchester, UK) and expressed as the density (n/mm2).
Figure 1.
(A) CCM image of the subbasal nerve plexus from the center of the cornea with mature (yellow arrow) and immature (green arrow) ICs. (B) Nearest nerve distance (yellow and green lines) between the IC body and subbasal nerve fiber.
IC Near-Nerve Distance
The near-nerve distance (µm) was the nearest perpendicular distance between the IC cell body and nearest nerve. The same images were used to manually measure the near-nerve distance based on the HRT III image resolution of 0.00104167 mm/pixel, using ImageJ software (National Institutes of Health, Bethesda, MD, USA) (Fig. 1B). The IC near-nerve distance measurement was undertaken to provide an objective measure of the interaction between ICs and corneal nerves. ICs can move closer to the nerves, thereby reducing the near-nerve distance, but this can also increase with the loss of nerves. Studies have shown a reduction in corneal nerves in MS,20 which will reduce the interaction between ICs and nerve fibers and hence increase the near-nerve distance, irrespective of a change in the IC density. To account for this, we have also divided the IC near-nerve distance by corneal nerve fiber length.
Statistical Analysis
All statistical analyses were performed using SPSS (SPSS, Inc., Chicago, IL, USA), and graphic illustrations were generated using Prism 6 (version 6.0g for Windows; GraphPad Software, La Jolla, CA, USA). The Kolmogorov–Smirnov test and visual inspection of histogram and normal Q-Q plot were used to assess for normality of the data. Data are expressed as the mean ± standard deviation. Statistical justification for the number of participants was based on a power analysis using the freeware program G*Power (Heinrich Heine University, Dusseldorf, Germany) version 3.0.10 for α (type I error) of 0.05 and power (1 − type II error) of 0.80 using dendritic cell density mean (17.3 and 27.7) and standard deviation (7.1 and 9.8) comparing healthy controls to patients with MS.20
To assess within- and between-group differences, we used one-way analysis of variance (nonparametric, Kruskal–Wallis test) and Dunnett's T3 for post hoc test corrections. The χ2 test was used for demographic variables and Fisher exact test for proportions. Student t test and Mann–Whitney tests were used to compare healthy controls to patients with MS. Spearman rank test was used and expressed as a correlation coefficient (r) to estimate the strength of the relationship between clinical parameters and CCM measures. P < 0.05 was significant.
Results
The clinical demographic and CCM results of patients with MS and control participants are summarized in Table 1. There was no significant difference in age and gender between patients with MS and control participants. Patients with MS had a disease duration of 7.88 ± 4.38 years with 1.89 ± 2.09 relapses and an EDSS of 2.00 ± 2.38. Patients with MS were on a range of treatments, including interferon-β (48/74; 64.9%), dimethyl fumarate (16/74; 21.6%), fingolimod (15/74; 20.3%), teriflunomide (8/74; 10.8%), natalizumab (4/74; 5.4%), mycophenolate (2/74; 2.7%), azathioprine (2/74; 2.7%), alemtuzumab (1/74; 1.4%), and mitoxantrone (1/74; 1.4%) (Table 2). Patients with MS had a significantly lower SDMT score (39.09 ± 14.15 vs. 53.17 ± 10.27, P = 0.002), longer T25FWT (7.50 ± 4.41 vs. 4.07 ± 1.32, P < 0.001), and longer 9-HPT (27.72 ± 16.23 vs. 20.52 ± 3.28, P = 0.007) compared to control participants.
Table 1.
Clinical Demographic and Neurologic Assessment in Healthy Controls (HC) Compared to Patients with MS, CIS, RRMS, and SPMS
| Parameters | HC (n = 20) | MS (n = 74) | CIS (n = 9) | RRMS (n = 43) | SPMS (n = 22) |
|---|---|---|---|---|---|
| Age, y | 38.27 ± 10.91 | 36.75 ± 9.15 | 36.40 ± 6.62 | 34.77 ± 8.80 | 40.76 ± 9.72 |
| Gender, M/F | 9/11 | 25/49 | 3/6 | 13/30 | 9/13 |
| MS duration, y** | — | 7.88 ± 4.38 | 3.75 ± 2.82 | 7.47 ± 3.43a | 10.44 ± 4.93a |
| Relapses (n)*** | — | 1.89 ± 2.09 | — | 1.26 ± 1.00 | 3.53 ± 2.63b |
| EDSS (score)*** | — | 2.00 ± 2.38 | 0.67 ± 0.66 | 0.93 ± 1.26 | 4.64 ± 2.45a,b |
| Symbol Digit Modality Test (score)** | 53.17 ± 10.27 | 39.09 ± 14.15c | 44.40 ± 9.76 | 42.84 ± 12.37 | 30.71 ± 15.14b,d |
| 25-Foot Walk Test* | 4.07 ± 1.32 | 7.50 ± 4.41c | 6.20 ± 1.18 | 6.27 ± 2.76d | 11.44 ± 6.50 |
| 9-HP Test (s)*** | 20.52 ± 3.28 | 27.72 ± 16.23c | 22.37 ± 1.34 | 23.19 ± 5.35 | 37.51 ± 25.46b,d |
| Mature lC density (n/mm2) | 4.20 ± 3.37 | 7.22 ± 7.27 | 5.56 ± 6.23 | 6.67 ± 6.49 | 8.95 ± 8.90 |
| Immature lC density (n/mm2) | 24.75 ± 18.74 | 48.04 ± 45.10c | 28.22 ± 26.76 | 51.84 ± 49.67d | 48.73 ± 40.83 |
| Total lC density (n/mm2)* | 28.95 ± 19.76 | 55.26 ± 47.99c | 33.78 ± 29.15 | 58.51 ± 53.18d | 57.68 ± 42.21d |
| Mature lC near-nerve distance (µm) | 32.21 ± 10.67 | 37.74 ± 20.45 | 36.00 ± 14.33 | 35.74 ± 18.00 | 42.25 ± 26.33 |
| Immature lC near-nerve distance (µm)** | 20.65 ± 6.10 | 29.32 ± 12.02c | 24.78 ± 8.32 | 28.23 ± 11.56d | 33.32 ± 13.42d |
| Mature lC near-nerve distance/CNFL ratio*** | 1.42 ± 0.52 | 3.58 ± 7.56c | 2.27 ± 1.12 | 2.59 ± 2.35d | 5.90 ± 13.06 |
| Immature lC near-nerve distance/CNFL ratio*** | 0.93 ± 0.35 | 2.61 ± 4.02c | 1.66 ± 0.88 | 2.07 ± 1.76d | 4.00 ± 6.75 |
Results expressed as mean ± SD. Statistically significant differences between different types of MS groups using analysis of variance:
P < 0.05,
P < 0.01,
P < 0.001. CNFL, corneal nerve fiber length; lC, XXX
Post hoc results differ significantly from CIS (P < 0.05).
Post hoc results differ significantly from RRMS (P < 0.05).
Results differ significantly from healthy controls (P < 0.05).
Post hoc results differ significantly from healthy control group (P < 0.05).
Table 2.
lC Parameters in Patients Treated with Interferon-β Compared to Other Disease-Modifying Medications
| Parameters | Interferon-β | Other Medications | P Value |
|---|---|---|---|
| Mature lC density (n/mm2) | 7.08 ± 7.73 | 7.46 ± 6.47 | 0.832 |
| Immature lC density (n/mm2) | 45.27 ± 41.99 | 53.15 ± 50.84 | 0.477 |
| Total lC density (n/mm2) | 52.35 ± 44.29 | 60.62 ± 54.68 | 0.483 |
| Mature lC near-nerve distance (µm) | 36.43 ± 21.89 | 40.04 ± 17.85 | 0.494 |
| Immature lC near-nerve distance (µm) | 30.35 ± 12.84 | 27.42 ± 10.30 | 0.320 |
Results expressed as mean ± SD.
MS Subtypes
There were no significant differences in age and gender between groups, and the duration of disease was significantly shorter in patients with CIS compared to RRMS (P = 0.021) and SPMS (P = 0.001). EDSS was significantly higher in SPMS (P < 0.001) compared to CIS (P < 0.001) and RRMS (P < 0.001), and the number of relapses was higher in SPMS versus RRMS (P = 0.016). The SDMT score was lower and 9-HPT time was higher in SPMS versus RRMS (P = 0.049, P = 0.001) with no difference for the T25FWT between subtypes (Table 1).
Immune Cell Density
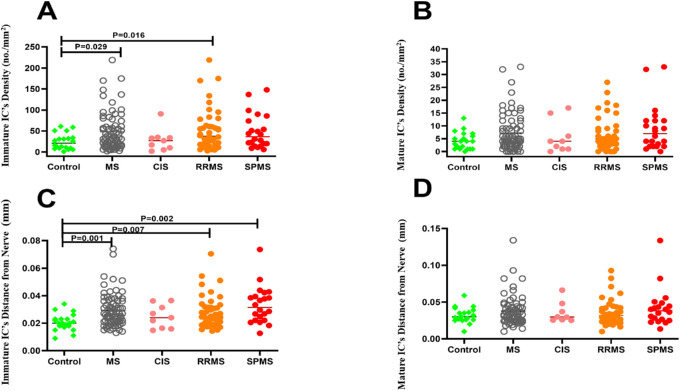
The total IC density (n/mm2) was higher in patients with MS (55.26 ± 47.99 vs. 28.95 ± 19.76, P = 0.02), RRMS (58.51 ± 53.18 vs. 28.95 ± 19.76, P = 0.01), and SPMS (57.68 ± 42.21 vs. 28.95 ± 19.76, P = 0.04) but not CIS (33.78 ± 29.15 vs. 28.95 ± 19.76, P = 0.999) compared to controls. Immature IC density (n/mm2) was significantly higher in patients with MS (48.04 ± 45.10 vs. 24.75 ± 18.74, P = 0.03) and RRMS (P = 0.02) with no difference in SPMS (P = 0.10) and CIS (P = 0.99) compared to controls. Mature IC density (n/mm2) (7.22 ± 7.27 vs. 4.20 ± 3.37, P = 0.15) did not differ between patients with MS and controls (Fig. 2). The proportion of individuals with immature (86.9% vs. 85.5%, P = 0.845) and mature (13.1% vs. 14.5%, P = 0.814) ICs was comparable between patients with MS compared to control participants (Table 1).
Figure 2.
Immature IC density (A), mature IC density (B), immature IC near-nerve distance (C), and mature IC near-nerve distance (D) in healthy controls compared to patients with MS and subtype of MS.
Immune Cell Near-Nerve Distance
The IC near-nerve distance (µm) for immature ICs was significantly greater in patients with MS (29.32 ± 12.02 vs. 20.65 ± 6.10, P = 0.001), RRMS (28.23 ± 11.56 vs. 20.65 ± 6.10, P = 0.007), and SPMS (33.32 ± 13.42 vs. 20.65 ± 6.10, P = 0.002) compared to controls (Figs. 2A, 2C). After correction for corneal nerve fiber length, the IC near-nerve distance was also greater in patients with MS and controls for immature ICs (2.61 ± 4.02 vs. 0.93 ± 0.35, P = 0.00) and mature ICs (3.58 ± 7.56 vs. 1.42 ± 0.52, P = 0.00) (Table 1). Moreover, the near-nerve distance/corneal nerve fiber length ratio remained significantly greater for both immature (P = 0.001) and mature (P = 0.04) ICs in RRMS compared to controls (Table 1).
Effect of Treatment
There was no significant difference in total IC density (P = 0.483), mature IC density (P = 0.832), immature IC density (P = 0.477), mature IC near-nerve distance (P = 0.494), and immature IC near-nerve distance (P = 0.320) for patients with MS on treatment with interferon-β compared to other disease-modifying therapies (Table 2).
Correlation Between IC Density and Near-Nerve Distance with Neurologic Disability
EDSS correlated with MS duration (r = 0.265, P = 0.045), number of relapses (r = 0.561, P < 0.001), SDMT score (r = –0.367, P = 0.003), T25FWT (r = 0.646, P < 0.001), 9-HPT (r = 0.622, P < 0.001), and immature IC near-nerve distance (r = 0.289, P = 0.005) (Table 3). There was a negative correlation between SDMT and immature IC density (r = –0.281, P = 0.024) and total IC density (r = –0.295, P = 0.017).
Table 3.
Correlation between Clinical and Neurologic Disability Measures and lC Parameters in Patients with MS
| MS | Symbol Digit | 25- Foot | ||||
|---|---|---|---|---|---|---|
| Parameter | Duration | Relapses | EDSS | Modality Test | Walk Test | 9-HP Test |
| EDSS | ||||||
| Coefficient (r) | 0.265 | 0.561 | 1.000 | −0.367 | 0.646 | 0.622 |
| P | 0.045 | 0.000 | 0.003 | 0.000 | 0.000 | |
| Mature lC density | ||||||
| Coefficient (r) | 0.053 | 0.145 | 0.147 | −0.128 | −0.005 | −0.128 |
| P | 0.692 | 0.286 | 0.157 | 0.308 | 0.972 | 0.283 |
| Immature lC density | −0.220 | 0.061 | 0.163 | −0.281 | 0.055 | −0.007 |
| Coefficient (r) | ||||||
| P | 0.098 | 0.654 | 0.116 | 0.024 | 0.676 | 0.957 |
| Total lC density | ||||||
| Coefficient (r) | −0.189 | 0.095 | 0.186 | −0.295 | 0.085 | 0.010 |
| P | 0.156 | 0.486 | 0.072 | 0.017 | 0.513 | 0.931 |
| Mature lC near-nerve distance | ||||||
| Coefficient (r) | −0.126 | 0.016 | 0.069 | −0.157 | 0.125 | 0.126 |
| P | 0.372 | 0.910 | 0.530 | 0.234 | 0.356 | 0.317 |
| Immature lC near-nerve distance | ||||||
| Coefficient (r) | 0.015 | 0.229 | 0.289 | −0.099 | 0.154 | 0.091 |
| P | 0.913 | 0.090 | 0.005 | 0.434 | 0.237 | 0.445 |
Statistically significant correlations are in bold.
Discussion
This study has used corneal confocal microscopy to show an increase in immature corneal ICs in patients with RRMS and SPMS but not CIS. Previously, we have shown an increase in IC density in both children16 and adults26 with type 1 diabetes, and Leppin et al.27 showed a significant increase in ICs that was related to reduced corneal nerve fiber length in Streptozotocin (STZ)-diabetic mice. We have also demonstrated an increase in ICs in patients with Bechet disease14 and Fabry disease.17 Moreover, we have shown an increase in IC density and reduction in corneal nerves in chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy15 and related these changes to the severity of motor deficits and pain and level of antineuronal antibodies, suggesting that CCM could be used to assess immune activation and treatment effect in immune-mediated neuropathies.28 In patients with MS, we have also previously shown a reduction in corneal nerves and an increase in ICs.20
Studies have suggested a possible two-way interaction between ICs and nerves in relation to nerve degeneration and repair29 indicating neuroimmune communication.30 Nerves can influence immune cell activity by releasing cytokines and neuropeptides,31 and neuropeptide receptors are expressed by resident immune cells.32 ICs express neurotrophic factors such as ciliary neurotrophic factor, which promotes reinnervation, and corneal denervation has been associated with a reduction in dendritic cell density.33 Despite an increase in IC density in the present study, we observed an overall increase in the distance between ICs and their nearest subbasal nerve in patients with MS. Ongoing treatment for the underlying disease may potentially impact on ICs. Thus, Marsovszky et al.34 found increased IC density in patients with rheumatoid arthritis but not in patients treated with anti–tumor necrosis factor therapy or glucocorticoids. Similarly, IC density was increased in patients with glaucoma but differed depending on the formulation of topical travaprost therapy used for the treatment of glaucoma.35 Despite adjusting for the loss of corneal nerves in patients with MS,18,19 the near-nerve distance was greater in patients with RRMS, suggesting that the reduced interaction between ICs and nerves was only partly due to a loss of nerves. A reduced association between ICs and subbasal nerves has also been reported in patients with fibromyalgia.11
As expected, there were significant associations between EDSS with the duration of MS, number of relapses, and different measures of neurologic disability. There was also a correlation between IC density and the symbol digit modality test and between near-nerve distance and EDSS. However, given the modest correlation, we can only speculate that altered corneal ICs may be associated with disability in MS. We also found no difference in IC density or near-nerve distance between patients with MS on different treatments, including interferon-β and other disease-modifying therapies.
The limitations of this study include the small sample size and cross-sectional design of the study, along with a possible effect of ongoing therapy on ICs. Nevertheless, CCM demonstrates an increase in immature ICs in patients with RRMS and SPMS but not CIS. These findings merit further investigation to assess the potential of quantifying corneal immune cells as an imaging biomarker for disease progression, relapses, and the effect of ongoing therapy in MS.
Acknowledgments
The authors thank all the participants involved in the study.
Supported by a Qatar National Research Fund (grant BMRP 20038654) and a Merck Serono Grant for Multiple Sclerosis Innovation 2016 (grant 201701.10249.POT).
Disclosure: A. Khan, None; Y. Li, None; G. Ponirakis, None; N. Akhtar, None; H. Gad, None; P. George, None; F.M. Ibrahim, None; I.N. Petropoulos, None; B.G. Canibano, None; D. Deleu, None; A. Shuaib, None; S. Kamran, None; R.A. Malik, None
References
- 1. Absinta M, Lassmann H, Trapp BD.. Mechanisms underlying progression in multiple sclerosis. Curr Opin Neurol. 2020; 33: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trapp BD, Nave K-A.. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008; 31: 247–269. [DOI] [PubMed] [Google Scholar]
- 3. Zhivov A, Stave J, Vollmar B, Guthoff R.. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol. 2005; 243: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 4. Knickelbein JE, Watkins SC, McMenamin PG, Hendricks RL.. Stratification of antigen-presenting cells within the normal cornea. Ophthalmol Eye Dis. 2009; 1: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leppin K, Behrendt A-K, Reichard M, et al.. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci. 2014; 55: 3603–3615. [DOI] [PubMed] [Google Scholar]
- 6. Mayer WJ, Mackert MJ, Kranebitter N, et al.. Distribution of antigen presenting cells in the human cornea: correlation of in vivo confocal microscopy and immunohistochemistry in different pathologic entities. Curr Eye Res. 2012; 37: 1012–1018. [DOI] [PubMed] [Google Scholar]
- 7. Lee EJ, Rosenbaum JT, Planck SR.. Epifluorescence intravital microscopy of murine corneal dendritic cells. Invest Ophthalmol Vis Sci. 2010; 51: 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR.. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukocyte Biol. 2003; 74: 172–178. [DOI] [PubMed] [Google Scholar]
- 9. Rosenberg ME, Tervo TM, Muller LJ, Moilanen JA, Vesaluoma MH.. In vivo confocal microscopy after herpes keratitis. Cornea. 2002; 21: 265–269. [DOI] [PubMed] [Google Scholar]
- 10. Mastropasqua L, Nubile M, Lanzini M, et al.. Epithelial dendritic cell distribution in normal and inflamed human cornea: in vivo confocal microscopy study. Am J Ophthalmol. 2006; 142: 736–744. [DOI] [PubMed] [Google Scholar]
- 11. Klitsch A, Evdokimov D, Frank J, et al.. Reduced association between dendritic cells and corneal sub-basal nerve fibers in patients with fibromyalgia syndrome. J Peripher Nerv Syst. 2019; 25: 9–18. [DOI] [PubMed] [Google Scholar]
- 12. Yamaguchi T, Calvacanti BM, Cruzat A, et al.. Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2014; 55: 7457–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villani E, Garoli E, Termine V, Pichi F, Ratiglia R, Nucci P.. Corneal confocal microscopy in dry eye treated with corticosteroids. Optom Vis Sci. 2015; 92: e290–e295. [DOI] [PubMed] [Google Scholar]
- 14. Bitirgen G, Tinkir Kayitmazbatir E, Satirtav G, Malik RA, Ozkagnici A. In vivo confocal microscopic evaluation of corneal nerve fibers and dendritic cells in patients with Behçet's Disease. Front Neurol. 2018; 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stettner M, Hinrichs L, Guthoff R, et al.. Corneal confocal microscopy in chronic inflammatory demyelinating polyneuropathy. Ann Clin Transl Neurol. 2016; 3: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferdousi M, Romanchuk K, Mah JK, et al.. Early corneal nerve fibre damage and increased Langerhans cell density in children with type 1 diabetes mellitus. Sci Rep. 2019; 9: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bitirgen G, Turkmen K, Malik RA, Ozkagnici A, Zengin N.. Corneal confocal microscopy detects corneal nerve damage and increased dendritic cells in Fabry disease. Sci Rep. 2018; 8: 12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mikolajczak J, Zimmermann H, Kheirkhah A, et al.. Patients with multiple sclerosis demonstrate reduced subbasal corneal nerve fibre density. Mult Scler J. 2017; 23: 1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petropoulos IN, Kamran S, Li Y, et al.. Corneal confocal microscopy: an imaging endpoint for axonal degeneration in multiple sclerosis. Invest Ophthalmol Vis Sci. 2017; 58: 3677–3681. [DOI] [PubMed] [Google Scholar]
- 20. Bitirgen G, Akpinar Z, Malik RA, Ozkagnici A.. Use of corneal confocal microscopy to detect corneal nerve loss and increased dendritic cells in patients with multiple sclerosis. JAMA Ophthalmol. 2017; 135: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson AJ, Banwell BL, Barkhof F, et al.. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 22. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983; 33: 1444. [DOI] [PubMed] [Google Scholar]
- 23. Rudick R, Antel J, Confavreux C, et al.. Recommendations from the national multiple sclerosis society clinical outcomes assessment task force. Ann Neurol. 1997; 42: 379–382. [DOI] [PubMed] [Google Scholar]
- 24. Benedict RH, DeLuca J, Phillips G, et al.. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Multiple Sclerosis J. 2017; 23: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Teunissen MB, Wormmeester J, Krieg SR, et al.. Human epidermal Langerhans cells undergo profound morphologic and phenotypical changes during in vitro culture. J Invest Dermatol. 1990; 94: 166–173. [DOI] [PubMed] [Google Scholar]
- 26. Tavakoli M, Boulton AJ, Efron N, Malik RA.. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Contact Lens Anterior Eye. 2011; 34: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leppin K, Behrendt AK, Reichard M, et al.. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Invest Ophthalmol Vis Sci. 2014; 55: 3603–3615. [DOI] [PubMed] [Google Scholar]
- 28. Rajabally YA, Stettner M, Kieseier BC, Hartung H-P, Malik RA.. CIDP and other inflammatory neuropathies in diabetes—diagnosis and management. Nat Rev Neurosci. 2017; 13: 599. [DOI] [PubMed] [Google Scholar]
- 29. Cruzat A, Witkin D, Baniasadi N, et al.. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011; 52: 5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seyed-Razavi Y, Chinnery HR, McMenamin PG.. A novel association between resident tissue macrophages and nerves in the peripheral stroma of the murine cornea. Invest Ophthalmol Vis Sci. 2014; 55: 1313–1320. [DOI] [PubMed] [Google Scholar]
- 31. Lambrecht BN. Immunologists getting nervous: neuropeptides, dendritic cells and T cell activation. Respir Res. 2001; 2: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters EM, Ericson ME, Hosoi J, et al.. Neuropeptide control mechanisms in cutaneous biology: physiological and clinical significance. J Invest Dermatol. 2006; 126: 1937–1947. [DOI] [PubMed] [Google Scholar]
- 33. Yu FX, Gao N, Sun H.. Hyperglycemia targets sensory nerve-dendritic cell interactions, resulting in diabetic corneal neuropathy. Invest Ophthalmol Vis Sci. 2014; 55: 4706–4706. [Google Scholar]
- 34. Marsovszky L, Resch MD, Németh J, et al.. In vivo confocal microscopic evaluation of corneal Langerhans cell density, and distribution and evaluation of dry eye in rheumatoid arthritis. Innate Immun. 2013; 19: 348–354. [DOI] [PubMed] [Google Scholar]
- 35. Marsovszky L, Resch MD, Visontai Z, Németh J.. Confocal microscopy of epithelial and Langerhans cells of the cornea in patients using travoprost drops containing two different preservatives. Pathol Oncol Res. 2014; 20: 741–746. [DOI] [PubMed] [Google Scholar]