Abstract
Background:
Aristolochic acids (AA) and arsenic are chemical carcinogens associated with urothelial carcinogenesis. Here we investigate the combined effects of AA and arsenic towards the risk of developing upper tract urothelial carcinoma (UTUC).
Methods:
Hospital-based (n=89) and population-based (2,921 cases, 11,684 controls) Taiwanese UTUC cohorts were used to investigate the association between exposure to AA and/or arsenic and the risk of developing UTUC. In the hospital cohort, AA exposure was evaluated by measuring aristolactam-DNA adducts in the renal cortex and by identifying A>T TP53 mutations in tumors. In the population cohort, AA exposure was determined from prescription health insurance records. Arsenic levels were graded from 0–3 based on concentrations in well water and the presence of arseniasis-related diseases.
Results:
In the hospital cohort, 43, 26, and 20 patients resided in grade 0, 1+2, and 3 arseniasis-endemic areas, respectively. Aristolactam-DNA adducts were present in >90% of these patients indicating widespread AA exposure. A>T mutations in TP53 were detected in 28%, 44% and 22% of patients residing in grade 0, 1+2, and 3 arseniasis-endemic areas, respectively. Population studies revealed that individuals who consumed more AA-containing herbs had a higher risk of developing UTUC in both arseniasis-endemic and non-endemic areas. Logistic regression showed an additive effect of AA and arsenic exposure on the risk of developing UTUC.
Conclusions:
Exposure to both AA and arsenic acts additively to increase the UTUC risk in Taiwan.
Impact:
This is the first study to investigate the combined effect of AA and arsenic exposure on UTUC.
Keywords: aristolactam, DNA adduct, herbs, transitional cell carcinoma, TP53
Introduction
Arseniasis-associated environmental diseases have been reported in Chile, West Bengal, Bangladesh and Taiwan.(1–3) The incidence of bladder urothelial cancer (UC) is related, in a dose-dependent manner, to arsenic exposure from drinking artesian well water.(4–6) Based on the Taiwan Cancer Registry (TCR), residents of arseniasis-endemic areas have a high risk of developing upper tract urothelial carcinoma (UTUC), as well as bladder UC.(7) The incidence of UTUC decreases with increasing distance from arseniasis-endemic areas.
Aristolochic acids (AA) are carcinogenic and nephrotoxic constituents of Aristolochia plants. Exposure to these herbs, either as contaminants of wheat flour in the Balkans, or through their use in the practice of traditional Chinese medicine, particularly throughout East Asia, has emerged as a global concern. (8–12) Our previous studies showed that the unusually high incidence of UTUC in Taiwan is closely related to AA exposure.(10) More than 39% of Taiwanese adults were prescribed AA-containing regimens between 1997 and 2003,(13) underscoring the magnitude of this problem. The widespread use of herbal regimens in Taiwan suggests that AA may be a confounding factor in assessing the etiology of UTUC in residents of arseniasis-endemic areas. The current investigation was undertaken to establish whether the high incidence of UTUC in Taiwan should be attributed to exposure to one of these two carcinogens or is a result of the combined effects of both.
In previous studies,(14,15) Chen et al. classified the degree of arseniasis in Taiwanese villages and towns (Figure 1) into four grades (from 0 to 3) based on the prevalence of arsenic-associated diseases and the concentration of arsenic in artesian well water from these areas. There was a significant positive correlation between the arseniasis grade and the age-adjusted (world population in 2000) incidence of UTUC (Figure 2).(7) Residents in the grade 3 zone had almost twice the risk of UTUC compared with those in grade 0.
Figure 1. Endemic arseniasis in Taiwan.
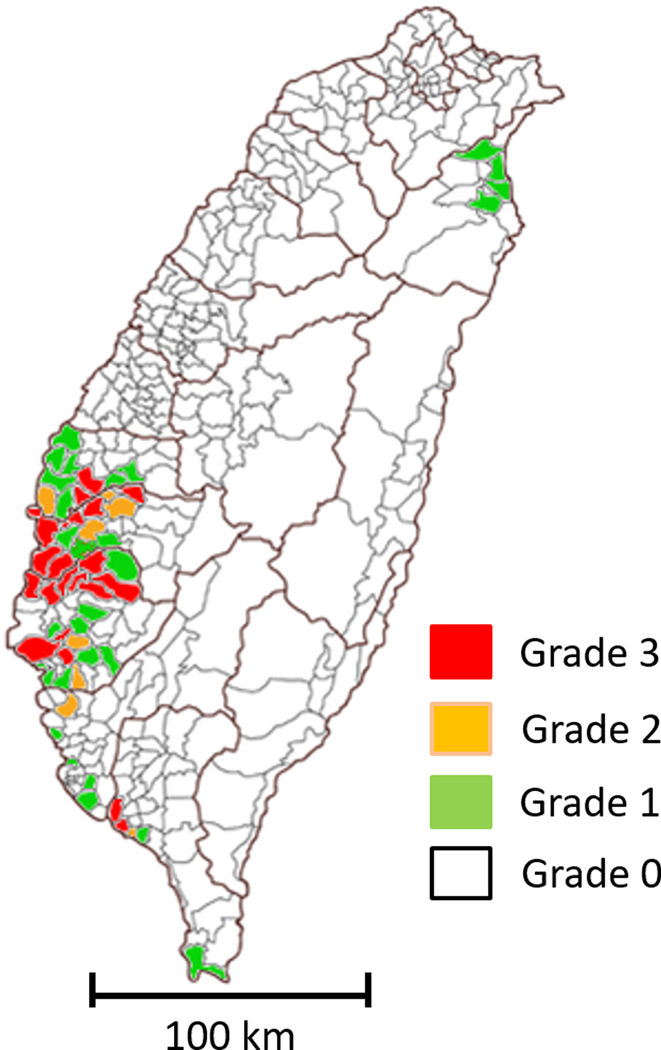
As previously described, arseniasis levels for all villages/towns in Taiwan were graded from 0 to 3 based on increasing arsenic concentrations in artesian well water from residential areas and the presence of arseniasis-related blackfoot disease or skin lesions.
Figure 2. Age-adjusted incidence of upper urinary tract urothelial carcinoma in Taiwan, in areas stratified by arseniasis grading between 1979 and 2010.(14,15).
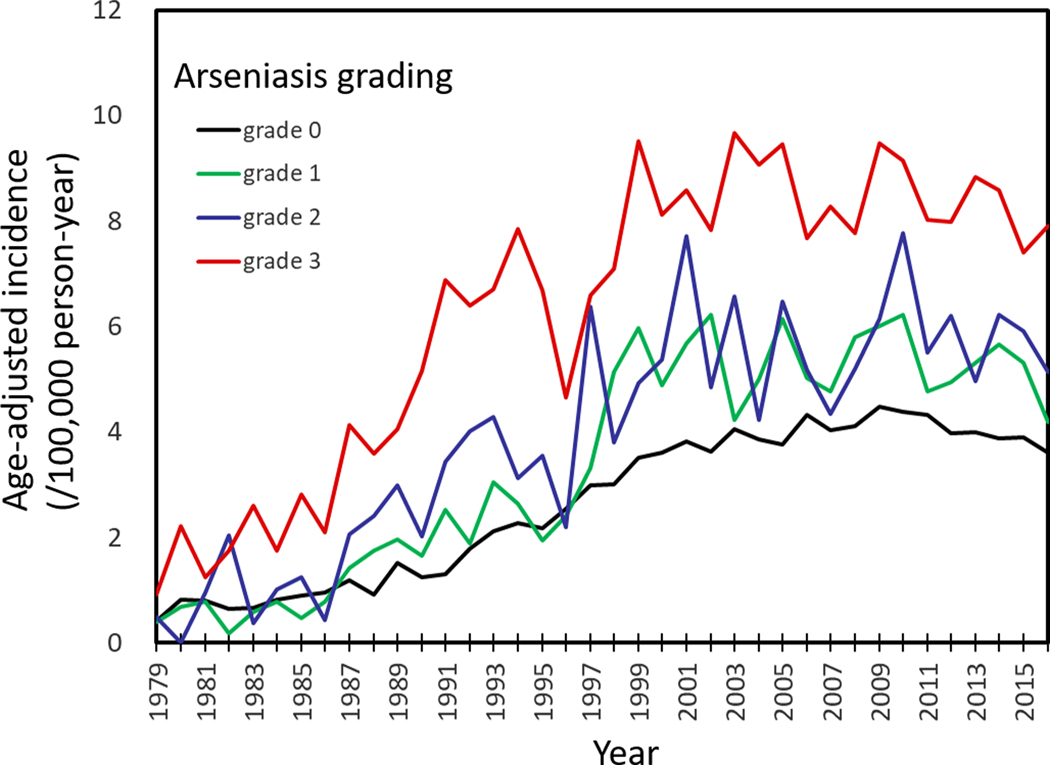
The incidence rates shown here were from both males and females. Arseniasis grading was classified from 0 (least exposure) to 3 (highest exposure). Age-adjustment was made against the year 2000 world population.
However, studies relating arsenic exposure to the high prevalence of UTUC in Taiwan were conducted prior to the discovery that AA is an important upper tract urothelium carcinogen in humans.(1,10) The goal of the present study was to assess the relative contributions of AA and arsenic to the development of UTUC in Taiwanese patients. To do so, we used a case-control study to investigate the impact of AA on the risk of UTUC in a population-based cohort from graded arseniasis areas. As supportive evidence, we evaluated the association of arseniasis and AA exposure in a hospital-based UTUC patient cohort. This is the first study to investigate the combined effect of AA and arsenic exposure on UTUC.
Materials and Methods
A hospital-based UTUC patient cohort (N=89) and a population-based cohort from the TCR with UTUC cases (N=2,921) and matched control (N=11,684) subjects were included in the study.
Hospital-based UTUC patient cohort
A total of 89 UTUC patients who had a radical nephroureterectomy with bladder cuff resection at National Taiwan University Hospital (NTUH) or National Cheng Kung University Hospital (NCKUH) between September 2007 and December 2013 were enrolled retrospectively. The study was approved by the Research Ethics Committees at NTUH, NCKUH and the Human Subjects Institutional Review Board at Stony Brook University. All patients provided signed informed consent prior to donating tissue samples. Patients who received systemic chemotherapy or radiotherapy before tissue donation were excluded from the study. None of the subjects in the cohort reported a history suggestive of occupational exposure to aromatic amines.
Arseniasis grading
A well-established arseniasis grading system (grade 0 to 3) was used in this study.(5,14) The grading system is based both on arseniasis-related clinical conditions and estimated arsenic exposure in each town/village in Taiwan (Figure 1). Three factors were used to grade arseniasis: (1) an arsenic concentration of more than 350 ng/ml in the artesian well water in the town/village; (2) the presence of clinical cases of Blackfoot Disease (BFD); and (3) presence of children with skin lesions characteristic of chronic arseniasis. The grade 3 zone, where all three factors listed above are present, represents the highest level of arseniasis. The grade 2 zone is defined as towns/villages with factors 1 and 3, but not factor 2, i.e. no BFD cases. The grade 1 zone refers to towns/villages with only factor 1, but without BFD or skin lesions. Grade 0 areas are residential areas where the arsenic concentration of well water is 350 ng/ml or less.
Laboratory tests
Tissue collection, 32P-postlabeling DNA adduct analysis(16), mass spectrometry adduct analysis(17,18), and TP53 gene sequencing(10) were described previously. This information also is available in Supplementary Materials and Methods and Supplementary Table 1.
Clinical data collection for the hospital-based UTUC cohort
Information related to smoking behavior, occupational exposure, detailed residential history, AA exposure, and history of radiotherapy or chemotherapy was collected using a standardized questionnaire. Patients who had had lived in arseniasis-endemic towns for any length of time prior to diagnosis were classified as being exposed to arsenic. Estimated glomerular filtration rate calculated with the Modification of Diet in Renal Disease equation was used for staging chronic kidney disease (CKD).(19) All surgical tissue specimens were reviewed by a single pathologist at NTUH (CT Shun). Tumors were graded according to the WHO 2004 classification and staged using the TNM 2002 classification. Multi-site tumors were defined as UCs that were present in two or more of the following sites: renal pelvis, upper ureter, lower ureter and urinary bladder.
Population-based national cohort with matched controls
The national cohort data obtained from the National Health Insurance Research Database (NHIRD) are described in detail in Supplementary Materials and Methods. Subjects with newly diagnosed UTUC (ICD-9-CM codes 189.1–189.2) between January 1, 2004 and December 31, 2011 (N=6,445) were identified in the NHIRD Catastrophic Illness Registry. The flow diagram of inclusions and exclusions were shown in Supplementary figure 1. The index dates for cases and matched controls were the date of UTUC diagnosis. A total of 1,562 UTUC patients diagnosed with other cancers (ICD-9-CM codes 140–208) prior to the index date were excluded. For each case, four matching controls without a cancer diagnosis prior to the index date were randomly selected from the 2005 Longitudinal Health Insurance Dataset (LHID2005) (supplementary materials). Controls were matched with each UTUC case by sex, age (ten age groups, each spanning at least 5 years from <40 to ≥80), insurance premium, administrative region of residency, urbanization level of residential areas (from level I referring to the most urbanized communities, to level IV referring to the least urbanized communities), arseniasis grades, and comorbidities defined by the following diagnoses within 12 months before the index date: renal insufficiency (ICD-9-CM codes: 585), hepatitis (ICD-9-CM codes: 070), coronary artery disease (CAD) (ICD-9-CM codes: 414), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes: 490–496), diabetes mellitus (DM) (ICD-9-CM codes: 249–250) and hyperlipidemia (ICD-9-CM codes: 272). Matching controls could not be identified in 1,045 cases of UTUC who had synchronous comorbidities and characters, including renal insufficiency (N=492), hepatitis (N=132), CAD (N=259), COPD (N=197), DM (N=401), hyperlipidemia (N=266), and residing in BFD areas (N=36). The other 897 UTUC cases with insufficient matching controls were excluded because of similar reasons, including renal insufficiency (N=272), hepatitis (N=47), CAD (N=188), COPD (N=141), DM (N=280), hyperlipidemia (N=222), and residing in BFD areas (N=30). The final case-control analysis included 2,921 cases and 11,684 controls (four times the number of cases).
Estimation of AA exposure from Aristolochia-containing herbal products
According to the Committee on Chinese Medicine and Pharmacy, Ministry of Health and Welfare, Executive Yuan in Taiwan, the following Chinese herbal medicinal products administered prior to November 2003 may have contained AA: Guan Mu Tong (stem Aristolochia manshuriensis), Guang Fangchi (root Aristolochia fangchi), Ma Dou Ling (fruit Aristolochia contorta or Aristolochia debilis), Qing Mu Xiang (root Aristolochia debilis), Tian Xian Teng (leaf and stem Aristolochia contorta, Aristolochia debilis), and Xi Xin (Asarum sieboldii).(20) In Taiwan, importation of these AA-containing herbal products was banned in November 2003 due to cumulative evidence linking these herbs with the development of CKD and UTUC. These herbal products are used either as single products or as components of mixed herbal formulas found in traditional Chinese medicines.
We estimated both the total amounts of herbs consumed for each mixture of the Chinese herbal products, and the total dose of AA-containing herbs consumed between January 1, 1997 and December 31, 2003. To allow for an adequate induction time to develop UTUC after exposure, we calculated the cumulative dose for each herb prescribed to a subject for up to one year prior to the UTUC diagnosis date. We also calculated the estimated cumulative dose of AA for each case and control subject using the following estimated average doses of AA per gram of Guan Mu Tong (2.59 mg), Guang Fangchi (2.04 mg), Ma Dou Ling (0.63 mg), Qing Mu Xiang (0.009 mg), Tian Xian Teng (0.026 mg), and Xi Xin (0.042).(21–23)
Statistical methods
Contingency tables were constructed for comparisons using the Chi-square test. The Kruskal-Wallis rank sum test was used to compare medians between groups. We used conditional logistic regression to assess the risk of UTUC in areas of graded arseniasis stratified by the estimated cumulative dose of AA from all AA-containing herbal products. Crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of UTUC were generated adjusting for covariates including aspirin, non-steroidal anti-inflammatory drugs, and acetaminophen. The combined effects of AA and arsenic exposure on the risk of developing UTUC were assessed using a logistic regression model with details shown in Supplementary Materials and Methods.(24,25) The excess risk of UTUC contributed by arsenic was calculated from the median incidence rates of UTUC from 1990 to 2016. The excess risk of UTUC contributed by AA was calculated based on the population-based case-control study. The proportional contribution to UTUC development is represented by the ratio between excess risks associated with arsenic and AA. All these analyses were conducted using SAS statistical software (version 9.2; SAS Institute, Cary, NC). All tests were two-tailed with a p-value <0.05 considered as statistically significant.
Results
Population-based national cohort
Based on the TCR database, there was a positive correlation between UTUC incidence and arseniasis grade (Figure 2). The average age-adjusted UTUC incidence rates over the past 10 years were 3.92, 4.71, 5.31, and 8.35 per 100,000 person-years for arseniasis grades 0, 1, 2, and 3 areas, respectively. Increased UTUC incidence in the high arseniasis grade areas was observed in both males and females (Figure 3). There was also a significant correlation between bladder UC incidence and arseniasis grade (Figure 3). In contrast, there was no association between the arseniasis grade and the incidence of renal cell carcinoma (Figure 3).
Figure 3. Age-adjusted incidence of bladder cancer, upper tract urothelial carcinoma (UTUC) and renal cell carcinoma (RCC) for either males or females stratified by arseniasis grading in Taiwan.
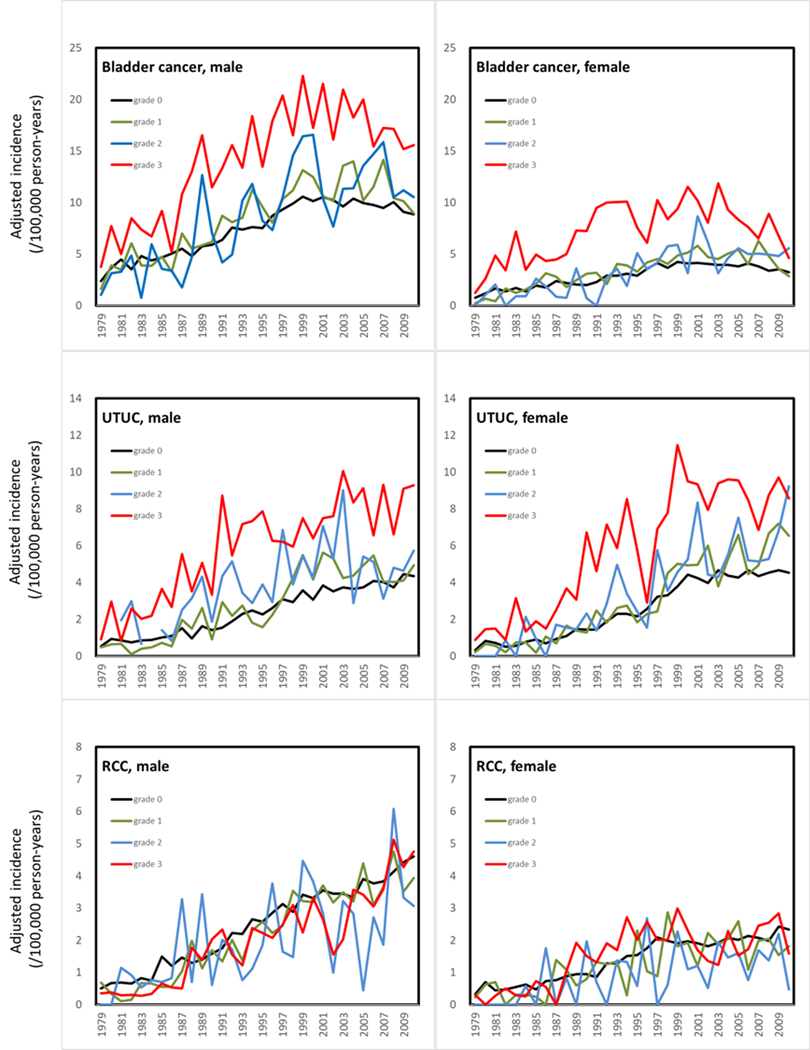
Arseniasis grades were classified from 0 (least exposure) to 3 (highest exposure).(14,15) Levels of arseniasis were positively associated with the incidence rates of both bladder cancer and UTUC in Taiwan, but not with RCC.
Consumption of AA-containing herbal medicines
Among the 2,921 cases and 11,684 controls in the population-based national cohort, 49.5% and 57.7% of subjects, respectively, had never consumed herbal medicinal products containing AA (p<0.001, Table 1). There was a significant dose-response relationship between the amount of AA-containing herbal products consumed and the risk of UTUC.
Table 1.
Association between the risk of upper tract urothelial carcinoma and consumption of aristolochic acid-containing herbal medicines in arseniasis-endemic and non-endemic areas
| Controlsa | Cases | P-value | Crude OR | 95%CI | P-value | Adjusted ORb | 95%CI | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||||||||
| Whole population | Subject number | 11684 | 2921 | |||||||||||
| AA consumption (mg) | <0.0001 | |||||||||||||
| 0 | 6744 | 57.72 | 1447 | 49.54 | 1.00 | 1.00 | ||||||||
| 1–100 | 4241 | 36.30 | 1154 | 39.51 | 1.28 | 1.18 | 1.40 | <0.0001 | 1.26 | 1.15 | 1.38 | <.0001 | ||
| 101–200 | 377 | 3.23 | 167 | 5.72 | 2.10 | 1.73 | 2.54 | <0.0001 | 2.05 | 1.69 | 2.49 | <.0001 | ||
| > 200 | 322 | 2.76 | 153 | 5.24 | 2.24 | 1.83 | 2.74 | <0.0001 | 2.21 | 1.80 | 2.70 | <.0001 | ||
| P-value for trend < 0.0001 | P-value for trend < 0.0001 | |||||||||||||
| Arsenic grade 1–3 areas | Subject number | 2252 | 563 | |||||||||||
| AA consumption (mg) | 0.0002 | |||||||||||||
| 0 | 1353 | 60.08 | 282 | 50.09 | 1.00 | 1.00 | ||||||||
| 1–100 | 723 | 32.10 | 220 | 39.08 | 1.49 | 1.22 | 1.83 | 0.0001 | 1.48 | 1.20 | 1.81 | 0.0002 | ||
| 101–200 | 81 | 3.60 | 26 | 4.62 | 1.61 | 1.01 | 2.57 | 0.0458 | 1.57 | 0.98 | 2.51 | 0.0587 | ||
| > 200 | 95 | 4.22 | 35 | 6.22 | 1.82 | 1.20 | 2.75 | 0.0046 | 1.80 | 1.19 | 2.72 | 0.0056 | ||
| P-value for trend < 0.0001 | P-value for trend < 0.0001 | |||||||||||||
| Arsenic grade 0 areas | Subject number | 9432 | 2358 | |||||||||||
| AA consumption (mg) | <0.0001 | |||||||||||||
| 0 | 5391 | 57.16 | 1165 | 49.41 | 1.00 | 1.00 | ||||||||
| 1–100 | 3518 | 37.30 | 934 | 39.61 | 1.24 | 1.12 | 1.37 | <.0001 | 1.22 | 1.10 | 1.34 | 0.0001 | ||
| 101–200 | 296 | 3.14 | 141 | 5.98 | 2.22 | 1.79 | 2.74 | <.0001 | 2.17 | 1.76 | 2.69 | <.0001 | ||
| > 200 | 227 | 2.41 | 118 | 5.00 | 2.41 | 1.91 | 3.04 | <.0001 | 2.36 | 1.87 | 2.98 | <.0001 | ||
| P-value for trend < 0.0001 | P-value for trend < 0.0001 | |||||||||||||
AA, aristolochic acid; AA consumption is defined as the cumulative dose of AA from 1997 to 2003 or to one year prior to the diagnosis of upper urinary tract urothelial carcinoma.
Factors for matching controls: sex, age, economic status, renal insufficiency, hepatitis, chronic obstructive pulmonary disease, diabetes mellitus, hyperlipidemia
adjusted for use of aspirin, NSAIDs, and acetaminophen
The relative excess risk due to interaction (RERI) = 0.12, 95% CI. −0.13 to 0.39, p = 0.339
The combined effect of AA and arseniasis in the population-based cohort
In arseniasis grade 1–3 areas, the odds ratios for developing UTUC were 1.48 (95% CI 1.20–1.81), 1.57 (95% CI 0.98–2.51), and 1.80 (95% CI 1.19–2.72) for those who consumed an estimated cumulative dose of 1–100, 101–200 and >200 mg of AA, respectively, compared with individuals who had not consumed AA-containing herbal products. Subjects who lived in the arseniasis grade 0 zone had an elevated risk of UTUC if they had taken AA-containing products compared with those who did not. The increased risk of UTUC from consuming AA-containing herbs was similar among residents living in the arseniasis grade 0 and 1–3 zones. Interaction analysis revealed that the combined effect of AA and arsenic exposure on the risk of developing UTUC was neither synergistic or antagonist, but additive (the relative excess risk due to interaction = 0.12, 95% CI −0.13 to 0.39, p = 0.339).
The relative contribution of AA and arsenic to UTUC risk
Based on the median incidence ratio of UTUC among arseniasis areas and odds ratio of UTUC risk among the people taking graded doses of AA, residents of arseniasis grade 3 areas who had ingested more than 200 mg of AA had the highest risk (relative risk = 3.98) compared with residents of grade 1 areas who had not consumed AA. The relative contributions of AA and arsenic to UTUC risk are shown in Supplementary Table 2 and Supplementary Figure 2. In general, AA contributes less in regions with higher grades of arsenic exposure.
Hospital-based UTUC patient cohort
Among the 89 UTUC patients (40 males and 49 females), 43 (48.3%), 26 (29.2%), and 20 (22.5%) subjects lived in the arseniasis grade 0, 1 and 2, and 3 zones, respectively. The median age of patients in each cohort was 70.0, 68.5 and 66.5 years, respectively. Most (76.4%) of the UTUC tumors occurred in the renal pelvis, followed by lower ureter (25.8%) and upper ureter (18.0%). A total of 19 (21.3%) patients had synchronous bladder UC, defined as bladder cancer diagnosed within 3 months of nephroureterectomy for UTUC. One-third of the patients in the UTUC cohort had synchronous multiple tumor locations. There were no significant differences among residents based on arseniasis grade in terms of age, smoking history, chronic kidney disease (CKD) stage, tumor grade, UC stage, tumor location, synchronous bladder UC or multi-site tumor locations (Table 2).
Table 2.
Demographics, tumor characteristics, aristolochic acid exposure and TP53 mutation patterns in the hospital-based UTUC patient cohort stratified by arseniasis grading (N=89).
| Arseniasis grading | Grade 0 | Grade 1+2 | Grade 3 | P value | |||
|---|---|---|---|---|---|---|---|
| Patient number (%) | 43 | (48%) | 26 | (29%) | 20 | (23%) | |
| Median age (years, range) | 70 (41–84) | 69 (35–85) | 67 (49–81) | 0.802 | |||
| Gender | 0.59 | ||||||
| Male | 18 | 41.9% | 11 | 42.3% | 11 | 55.0% | |
| Female | 25 | 58.1% | 15 | 57.7% | 9 | 45.0% | |
| Smoking history | 0.327a | ||||||
| Yes | 6 | 14.0% | 7 | 26.9% | 2 | 10.0% | |
| No | 37 | 86.0% | 19 | 73.1% | 18 | 90.0% | |
| CKD stage | 0.989a | ||||||
| 0–2 | 18 | 41.9% | 11 | 42.3% | 9 | 47.4% | |
| 3 | 17 | 39.5% | 11 | 42.3% | 7 | 36.8% | |
| 4 | 2 | 4.7% | 2 | 7.7% | 1 | 5.3% | |
| 5 | 6 | 14.0% | 2 | 7.7% | 2 | 10.5% | |
| ESRD | 1.0a | ||||||
| Yes | 4 | 9.3% | 2 | 7.7% | 2 | 10.5% | |
| No | 39 | 90.7% | 24 | 92.3% | 17 | 89.5% | |
| Tumor location | |||||||
| Renal pelvis | 33 | 76.7% | 21 | 80.8% | 14 | 70.0% | 0.693 |
| Upper ureter | 9 | 20.9% | 2 | 7.7% | 5 | 25.0% | 0.249a |
| Lower ureter | 11 | 25.6% | 9 | 34.6% | 3 | 15.0% | 0.340a |
| Synchronous bladder cancer | 9 | 20.9% | 6 | 23.1% | 4 | 20.0% | 1.0a |
| Multiple tumor location | 14 | 32.6% | 7 | 26.9% | 9 | 45.0% | 0.427 |
| Grade | 0.782a | ||||||
| High | 36 | 83.7% | 23 | 88.5% | 18 | 90.0% | |
| Low | 7 | 16.3% | 3 | 11.5% | 2 | 10.0% | |
| Stage | 0.342a | ||||||
| Ta-1N0M0 | 22 | 51.2% | 10 | 38.5% | 11 | 55.0% | |
| T2–4N0M0 | 13 | 30.2% | 14 | 53.8% | 7 | 35.0% | |
| Nodal or metastatic | 8 | 18.6% | 2 | 7.7% | 2 | 10.0% | |
| AL-DNA adduct | 0.341a | ||||||
| Yes | 42 | 97.7% | 25 | 96.2% | 18 | 90.0% | |
| No | 1 | 2.3% | 1 | 3.8% | 2 | 10.0% | |
| TP53 mutation pattern | 0.557a | ||||||
| Wild type | 20 | 50.0% | 10 | 40.0% | 11 | 61.1% | |
| A>T transversion | 11 | 27.5% | 11 | 44.0% | 4 | 22.2% | |
| Mutations other than A>T | 9 | 22.5% | 4 | 16.0% | 3 | 16.7% | |
CKD, chronic kidney disease; ESRD, end-stage renal disease; AL-DNA, aristolactam-DNA;
Fisher’s exact test
Aristolactam-DNA adduct analysis in hospital-based UTUC patients stratified by arseniasis grade
Mass spectrometry or post-labeling procedures established the presence of aristolactam-DNA adducts in renal cortical tissue samples in 85 (96%) of the 89 patients studied. This result confirms widespread exposure to AA in Taiwan and is consistent with our previous observations.(10,26,27) Moreover, in 98%, 96%, and 90% of UTUC patients living in arseniasis grade 0, 1 and 2, and 3 areas, respectively, aristolactam-DNA adducts in renal cortical tissues (Table 2) were identified (p=0.34).
Mutational analysis in tumor tissues
TP53 mutations were observed in 50%, 60% and 38.9% of UTUC tissues from patients living in arseniasis grade 0, 1 and 2, and 3 areas, respectively (Table 2). A>T transversion, the most prevalent mutation associated with AA-induced carcinogenesis, was identified in 27.5%, 44.0%, and 22.2% of UTUC patients living in arseniasis grade 0, 1 and 2, and 3 areas, respectively. There were no significant differences in the frequencies of other types of TP53 mutations among patients from different arseniasis grades (Figure 4).
Figure 4. TP53 mutational patterns in UTUC stratified by arseniasis grading in Taiwan.
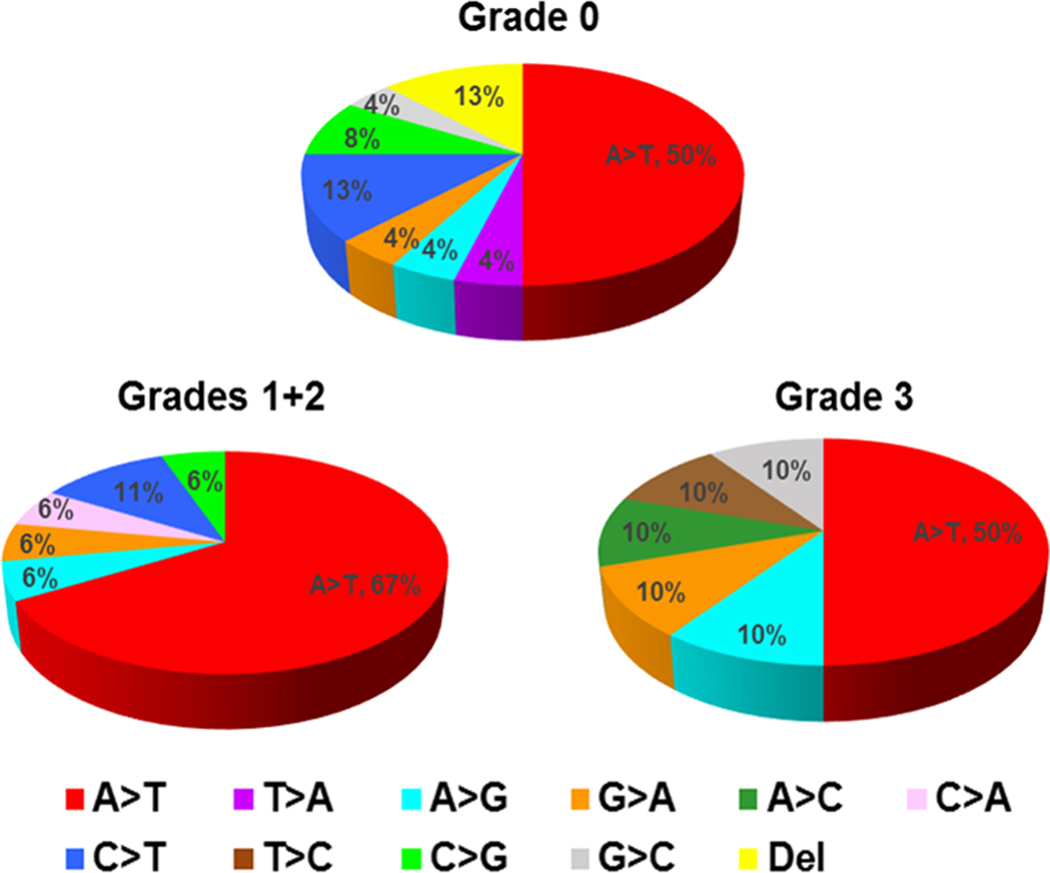
Arseniasis grades were classified from 0 (least exposure) to 3 (highest exposure).(14,15) The most common mutation for all arseniasis grades was the A>T transversion, which is part of the signature mutation for aristolochic acid carcinogenesis and suggests that an etiology other than arseniasis may play a role in cancer development.
Contribution of other carcinogens
We explored whether carcinogens other than AA and arsenic could contribute to the high incidence of UTUC in arseniasis-endemic areas. Previous studies revealed possible synergy between smoking-derived carcinogens and arsenic in inducing bladder UC.(6,28–30) However, a history of smoking was reported by only 16.9% of the 89 patients in our hospital-based UTUC cohort, and the proportion of smokers were comparable in residents of arseniasis grade 0 (14.0%) and grade 1+2+3 (19.6%) zones (not significantly different by χ2). These findings suggest that cigarette-smoking was not a significant factor in the high incidence of UTUC in arseniasis-endemic areas. This conclusion was further supported by population-based data showing proportions of smokers among residents of Tainan and Chayi, known arseniasis-endemic areas, and the whole population of Taiwan to be similar (Supplementary Figure 3). Furthermore, industrial contact with urothelial carcinogens was unlikely to play a role in this study as none of the subjects in the hospital-based UTUC cohort reported a history suggestive of occupational exposure to aromatic amines.
Discussion
Arseniasis-endemic areas are present in Bangladesh, Pakistan, Taiwan, Thailand, Vietnam, Cambodia, the United States, Canada, Chile, and Argentina.(1) Populations living in these endemic areas may also be exposed to other carcinogens, such as AA. Taiwan is the only country where the effects of exposure to arsenic and AA are-well characterized.(4,10) Accordingly, the Taiwanese population was chosen as a model to study the impact of arsenic and AA on UTUC. Our study is the first to investigate the interaction between arsenic and AA on the risk of developing UTUC, a relationship that appears to be additive. The results of the current study can provide useful information to researchers investigating co-exposure in other arseniasis-endemic areas in the world.
In earlier studies, we reported that AA-containing herbal medicines were responsible for the high incidence of UTUC observed throughout Taiwan.(10) AA carcinogenesis was evaluated with the use of two robust biomarkers that represent AA exposure (aristolactam-DNA adducts in renal cortex) and its carcinogenic effect (A>T transversions in tumor TP53 gene).(10,31) Using these two biomarkers in the present study, we found that the vast majority of UTUC patients in Taiwan had been exposed to AA, irrespective of where they lived, as aristolactam-DNA adducts were identified in the renal cortex of 94.4% of the hospital-based cohort with UTUC; and A>T mutations in TP53 were found in almost one-third of the urothelial tumors from this cohort. The latter analysis may underestimate the carcinogenic effects of AA as mutations in other cancer driver genes were not examined.(9,27,32)
In the current study, we demonstrate that residents of arseniasis-endemic areas in Taiwan face a higher risk of UTUC compared with those living in non-endemic locations. Furthermore, our population-based data showed that consumption of AA-containing herbal products increased the risk of UTUC in arseniasis-endemic areas compared with residents in the same areas who did not consume these herbs. A>T mutations in TP53 and aristolactam-DNA adducts were present at comparable frequencies in tissues from residents of arseniasis-endemic and non-endemic areas, providing evidence of comparable exposure to AA independent of location.
There are no published data addressing the interaction between arsenic and AA on the risk of developing cancer. One study from Taiwan analyzed the risk of developing UC and renal dysfunction by adjusting for exposure to arsenic and suggested a possible interaction between arsenic and AA.(33) As predicted, since both arsenic and AA substantially increase the risk of UTUC in Taiwan, this population provides a suitable model to assess the interaction between the two carcinogens. The epidemiologic evidence obtained from our population-based cohort, along with molecular data from our hospital-based cohort, revealed that the carcinogenic effects of AA and arsenic on UTUC are independent of one another, such that their combined action was neither synergistic nor antagonistic, but additive. Currently there are no data to support whether one chemical is more potent than the other in terms of carcinogenic effects.
The mechanisms responsible for the carcinogenic effect of arsenic in UC have not been defined. We were unable to find any mechanistic studies (in vitro or in vivo) investigating the combined effect of AA and arsenic. Although arsenic is not a mutagenic agent, it may act through inhibition of DNA repair,(34) sister chromatid exchange,(35) or gene amplification.(36) Also, arsenic may stimulate cell proliferation by affecting signal transduction pathways and MAPK cascades.(37–39) In contrast, AA initiates carcinogenesis by the formation of DNA adducts in a broad spectrum of genes.(27,32) The additive effects on the risk of UTUC as measured by our epidemiologic and molecular analyses confirmed that arsenic and AA act independently from each other.
Our study did not address the combined effects of the two carcinogens on tumor phenotype or the clinical outcomes of UTUC. In earlier studies we reported that bladder cancer patients from arseniasis-endemic areas had more advanced tumor stages and worse survival outcomes compared with patients from non-endemic areas.(4) However, no data were available for UTUC. With the use of TP53 A>T mutations, we previously demonstrated that patients with AA-associated UTUC had more advanced tumor stage and increased UTUC recurrence rates compared with non-AA-associated UTUC.(26) Nevertheless, the bladder recurrence rate and overall survival were similar between AA-induced and non-AA-induced UTUC patients.(26) The current study showed no significant differences in UTUC phenotype among areas based on arseniasis grade.
In the current study, we observed a large difference in the AA exposure rates between the two UTUC cohorts: 50.5% as estimated from the health insurance database compared with 94.4% as detected by aristolactam-DNA adducts. There are several possible reasons for this apparent discrepancy. For example, traditional Chinese medicine prescriptions documented in the health insurance records represent only one route of exposure to AA-containing herbs. But these and other AA-containing herbs may be purchased over-the-counter or via the internet, which would not be recorded in the health insurance database. In addition, there are various AA-containing herbal regimens that are not banned in Taiwan. (40) Finally, the limited seven-year period used for calculating AA doses may contribute to an underestimation of AA exposure in the Taiwanese population.
To investigate the effect of arsenic and AA in UTUC development without significant bias, our population-based case-control study was constructed with the adjustment of the possible factors which contributed to the frequency of physician visit. Therefore, a significant portion of UTUC cases were excluded because of insufficient number or lack of matching controls. The included cases had fewer proportion of renal insufficiency, hepatitis, DM, CAD, COPD, hyperlipidemia than did the overall cases (supplementary table 4). Fortunately, the major topic, arseniasis, did not differ between the included and overall cases. Our investigation of effects of arsenic and AA in carcinogenesis of UTUC would be not biased in most of Taiwan population, but could not extend to the people with multiple comorbidities.
Our study has several limitations. First, the number of UTUC cases in the hospital-based cohort was not sufficient to assess all clinical parameters in the multivariable analysis. Nevertheless, it served to prove our hypothesis that most UTUC patients in arseniasis-endemic areas have also been exposed to AA. Second, this is a retrospective analysis; however, selection bias may have been partially mitigated by recruiting consecutive UTUC patients. Furthermore, molecular analyses of tissue samples obtained prior to this study may have reduced recall bias. Third, using residential areas to assign arseniasis grading may be imprecise. Since all artesian wells in arseniasis-endemic areas are now closed and prohibited for use, the current arsenic concentrations in these wells are unknown. Nevertheless, this limitation was compensated for by the association between the incidence of arsenic-related disease and arseniasis grade. Fourth, biologic evidence of arsenic exposure was not assessed because nail and/or hair samples were not available. Fifth, residential information in the case-control study is limited to the records provided at the time of registration. Sixth, many UTUC cases were excluded due to the lack or insufficiency of matched controls for patients with multiple comorbidities.
In conclusion, our study revealed that exposure to AA via consumption of Aristolochia herbs significantly increases the risk of UTUC in both non-arseniasis-endemic areas and arseniasis-endemic areas of Taiwan. The combined effects of AA and arsenic towards the risk of developing UTUC appear to be additive.
Supplementary Material
Acknowledgements
This study was supported, in part, by a research grant [104-2314-B-002-132-] to CHC and a research grant [104-2314-B-002-121-MY3] to YSP, both from the National Science Council, Taiwan. This study was also supported by [R01CA220367] to RJT from the National Cancer Institute and [R01ES019564] to RJT from the National Institute of Environmental Health Sciences. Mass spectrometry was supported by Cancer Center Support Grant [CA077598] from the National Cancer Institute. KT and SW were recipients of Stony Brook Medicine International Research Fellowships. APG, VSS, KH, MM, and KGD gratefully acknowledge the financial support provided by Marsha and Henry Laufer.
Financial support:
CHC: a research grant [104-2314-B-002-132-] from the National Science Council, Taiwan
YSP: a research grant [104-2314-B-002-121-MY3] to YSP from the National Science Council, Taiwan.
RJT: a research grant [R01CA220367] from the National Cancer Institute and [R01ES019564] from the National Institute of Environmental Health Sciences. Mass spectrometry was supported by Cancer Center Support Grant [CA077598] from the National Cancer Institute.
APG, VSS, KH, MM, and KGD: financial support provided by Marsha and Henry Laufer.
Abbreviations:
- UC
urothelial carcinoma
- UTUC
upper tract urothelial carcinoma
- TCR
Taiwan Cancer Registry
- AA
aristolochic acid
- CKD
chronic kidney disease
- NHIRD
National Health Insurance Research Database
- LHID2005
Longitudinal Health Insurance Dataset
- BFD
blackfoot disease
Footnotes
Conflict of interest:
The authors declare no potential conflict of interest.
References
- 1.Chen CJ. Arseniasis in the World: From Endemic to Pandemic. In: Chen C-J, editor. Health hazards of Environmental Arsenic Poisoning From Epidemic to Pandemic. USA: World Scientific Publishing Co.; 2011. p 1. [Google Scholar]
- 2.Ramos O, Carrizales L, Yanez L, Mejia J, Batres L, Ortiz D, et al. Arsenic increased lipid peroxidation in rat tissues by a mechanism independent of glutathione levels. Environmental health perspectives 1995;103 Suppl 1:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai SM, Wang TN, Ko YC. Mortality for certain diseases in areas with high levels of arsenic in drinking water. Archives of environmental health 1999;54(3):186–93 doi 10.1080/00039899909602258. [DOI] [PubMed] [Google Scholar]
- 4.Chen CH, Chiou HY, Hsueh YM, Chen CJ, Yu HJ, Pu YS. Clinicopathological characteristics and survival outcome of arsenic related bladder cancer in taiwan. The Journal of urology 2009;181(2):547–52; discussion 53 doi 10.1016/j.juro.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, et al. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer research 1995;55(6):1296–300. [PubMed] [Google Scholar]
- 6.Steinmaus C, Yuan Y, Bates MN, Smith AH. Case-control study of bladder cancer and drinking water arsenic in the western United States. American journal of epidemiology 2003;158(12):1193–201. [DOI] [PubMed] [Google Scholar]
- 7.Bureau of Helath Promotion DoHT. January 1. The incidence of renal pelvic and ureteral tumor in Taiwan. <https://cris.hpa.gov.tw/pagepub/Home.aspx?itemNo=cr.q.10>. Accessed 2020 January 1.
- 8.Ng AWT, Poon SL, Huang MN, Lim JQ, Boot A, Yu W, et al. Aristolochic acids and their derivatives are widely implicated in liver cancers in Taiwan and throughout Asia. Science translational medicine 2017;9(412) doi 10.1126/scitranslmed.aan6446. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Liang Y, Guan B, Shi Y, Gong Y, Li J, et al. Aristolochic acid mutational signature defines the low-risk subtype in upper tract urothelial carcinoma. Theranostics 2020;10(10):4323–33 doi 10.7150/thno.43251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CH, Dickman KG, Moriya M, Zavadil J, Sidorenko VS, Edwards KL, et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proceedings of the National Academy of Sciences of the United States of America 2012;109(21):8241–6 doi 10.1073/pnas.1119920109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grollman AP, Jelakovic B. Role of environmental toxins in endemic (Balkan) nephropathy. October 2006, Zagreb, Croatia. Journal of the American Society of Nephrology : JASN 2007;18(11):2817–23 doi 10.1681/ASN.2007050537. [DOI] [PubMed] [Google Scholar]
- 12.Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). The New England journal of medicine 2000;342(23):1686–92 doi 10.1056/NEJM200006083422301. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh SC, Lin IH, Tseng WL, Lee CH, Wang JD. Prescription profile of potentially aristolochic acid containing Chinese herbal products: an analysis of National Health Insurance data in Taiwan between 1997 and 2003. Chinese medicine 2008;3:13 doi 10.1186/1749-8546-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung FC, Tsai CC, Chen KP. [Characteristics of the artesian well water in the endemic area of blackfoot disease. I High alkalinity in the water of a well]. Taiwan yi xue hui za zhi Journal of the Formosan Medical Association 1976;75(6):358–62. [PubMed] [Google Scholar]
- 15.Chen KPW, H. Y; Wu TC Epidemiologic studies on Blackfoot disease in Taiwan: 3. Physicochemical characteristics of drinking water in endemic Blackfoot disease areas. Coll Med Natl Taiwan Univ 1962;8. [Google Scholar]
- 16.Hashimoto K, Zaitseva IN, Bonala R, Attaluri S, Ozga K, Iden CR, et al. Sulfotransferase-1A1-dependent bioactivation of aristolochic acid I and N-hydroxyaristolactam I in human cells. Carcinogenesis 2016;37(7):647–55 doi 10.1093/carcin/bgw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun BH, Rosenquist TA, Nikolic J, Dragicevic D, Tomic K, Jelakovic B, et al. Human formalin-fixed paraffin-embedded tissues: an untapped specimen for biomonitoring of carcinogen DNA adducts by mass spectrometry. Anal Chem 2013;85(9):4251–8 doi 10.1021/ac400612x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun BH, Rosenquist TA, Sidorenko V, Iden CR, Chen CH, Pu YS, et al. Biomonitoring of aristolactam-DNA adducts in human tissues using ultra-performance liquid chromatography/ion-trap mass spectrometry. Chemical research in toxicology 2012;25(5):1119–31 doi 10.1021/tx3000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. American journal of kidney diseases : the official journal of the National Kidney Foundation 2007;50(1):21–35 doi 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Lai MN, Lai JN, Chen PC, Hsieh SC, Hu FC, Wang JD. Risks of kidney failure associated with consumption of herbal products containing Mu Tong or Fangchi: a population-based case-control study. American journal of kidney diseases : the official journal of the National Kidney Foundation 2010;55(3):507–18 doi 10.1053/j.ajkd.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 21.Jong TT, Lee MR, Hsiao SS, Hsai JL, Wu TS, Chiang ST, et al. Analysis of aristolochic acid in nine sources of Xixin, a traditional Chinese medicine, by liquid chromatography/atmospheric pressure chemical ionization/tandem mass spectrometry. Journal of pharmaceutical and biomedical analysis 2003;33(4):831–7. [DOI] [PubMed] [Google Scholar]
- 22.Chuang MSH YH; Chang HC; Lin JH; Liao CH Studies on Adulteration and Misusage of Marketed Akebiae Caulis. Taiwan: Department of Health; 2001. Report nr 20. 104–19 p. [Google Scholar]
- 23.Deng JS. Quality Evaluation of Fang-Ji and Analysis of Marker Constituents. Institute of Chinese Pharmaceutical Sciences, China Medical University; 2002. [Google Scholar]
- 24.VanderWeele T KM. A tutorial on interaction with SAS and Stata code. Epidemiol Method 2014(3):33–72. [Google Scholar]
- 25.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology 1992;3(5):452–6 doi 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Chen CH, Dickman KG, Huang CY, Moriya M, Shun CT, Tai HC, et al. Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: clinical characteristics and outcomes. International journal of cancer Journal international du cancer 2013;133(1):14–20 doi 10.1002/ijc.28013. [DOI] [PubMed] [Google Scholar]
- 27.Hoang ML, Chen CH, Sidorenko VS, He J, Dickman KG, Yun BH, et al. Mutational signature of aristolochic acid exposure as revealed by whole-exome sequencing. Science translational medicine 2013;5(197):197ra02 doi 10.1126/scitranslmed.3006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pu YS, Yang SM, Huang YK, Chung CJ, Huang SK, Chiu AW, et al. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicology and applied pharmacology 2007;218(2):99–106 doi 10.1016/j.taap.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Karagas MR, Tosteson TD, Morris JS, Demidenko E, Mott LA, Heaney J, et al. Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer causes & control : CCC 2004;15(5):465–72 doi 10.1023/B:CACO.0000036452.55199.a3. [DOI] [PubMed] [Google Scholar]
- 30.Bates MN, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Kalman D, et al. Case-control study of bladder cancer and exposure to arsenic in Argentina. American journal of epidemiology 2004;159(4):381–9. [DOI] [PubMed] [Google Scholar]
- 31.Grollman AP, Shibutani S, Moriya M, Miller F, Wu L, Moll U, et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proceedings of the National Academy of Sciences of the United States of America 2007;104(29):12129–34 doi 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poon SL, Pang ST, McPherson JR, Yu W, Huang KK, Guan P, et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool. Science translational medicine 2013;5(197):197ra01 doi 10.1126/scitranslmed.3006086. [DOI] [PubMed] [Google Scholar]
- 33.Lai MN, Wang SM, Chen PC, Chen YY, Wang JD. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. Journal of the National Cancer Institute 2010;102(3):179–86 doi 10.1093/jnci/djp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li JH, Rossman TG. Inhibition of DNA ligase activity by arsenite: a possible mechanism of its comutagenesis. Mol Toxicol 1989;2(1):1–9. [PubMed] [Google Scholar]
- 35.Lerda D Sister-chromatid exchange (SCE) among individuals chronically exposed to arsenic in drinking water. Mutation research 1994;312(2):111–20 doi 10.1016/0165-1161(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 36.Lee TC, Tanaka N, Lamb PW, Gilmer TM, Barrett JC. Induction of gene amplification by arsenic. Science 1988;241(4861):79–81 doi 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- 37.Trouba KJ, Wauson EM, Vorce RL. Sodium arsenite-induced dysregulation of proteins involved in proliferative signaling. Toxicology and applied pharmacology 2000;164(2):161–70 doi 10.1006/taap.1999.8873. [DOI] [PubMed] [Google Scholar]
- 38.Huang C, Ma WY, Li J, Goranson A, Dong Z. Requirement of Erk, but not JNK, for arsenite-induced cell transformation. The Journal of biological chemistry 1999;274(21):14595–601 doi 10.1074/jbc.274.21.14595. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Martindale JL, Holbrook NJ, Liu Y. Tumor promoter arsenite activates extracellular signal-regulated kinase through a signaling pathway mediated by epidermal growth factor receptor and Shc. Mol Cell Biol 1998;18(9):5178–88 doi 10.1128/mcb.18.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chinese medicine division DoH, Kong Hong. 2004. September 4. Aristolochic acid-containing herbs banned in Hong Kong. <https://www.cmchk.org.hk/cmp/news/cmp_stop_c.pdf>. Accessed 2020 September 4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


