Abstract
Context:
Energy drinks are the fastest growing product in the beverage industry. However, there is concern regarding potential for adverse effects with use.
Objective:
To evaluate the reported adverse effects of energy drink consumption.
Data Sources:
The electronic databases MEDLINE, EMBASE, and PubMed were searched for relevant studies from inception to November 2019, and pertinent data were abstracted.
Study Selection:
Only clinical studies reporting adverse events after energy drink consumption were included.
Study Design:
Systematic review.
Level of Evidence:
Level 4.
Data Extraction:
Data regarding sample size characteristics, energy drink characteristics, comparators, and all adverse events were extracted in duplicate and recorded.
Results:
A total of 32 studies and 96,549 individuals were included. Frequently reported adverse events in the pediatric population were insomnia (35.4%), stress (35.4%), and depressive mood (23.1%). Frequently reported adverse events in the adult population were insomnia (24.7%), jitteriness/restlessness/shaking hands (29.8%), and gastrointestinal upset (21.6%). Alcohol mixed with energy drinks significantly reduced the likelihood of sedation effects but increased the likelihood of stimulatory effects. Energy drink consumption significantly increased the odds of insomnia (OR, 5.02; 95% CI, 1.72-14.63) and jitteriness/activeness (OR, 3.52; 95% CI, 1.28-9.67) compared with the control group.
Conclusion:
The authors recommend that individuals avoid frequent energy drink consumption (5-7 energy drinks/week) and avoid co-consumption with alcohol; increased regulatory standards should be placed in the sale of energy drinks, particularly with regard to the pediatric population.
Keywords: energy drink, safety, adverse events, caffeine
Energy drinks are beverages formulated to improve mental and physical stimulation. Energy-enhancing ingredients, such as caffeine, taurine, herbal extracts, sugar, and B vitamins are commonly used in energy drinks.17 Energy drinks, as well as sports drinks and nutraceutical drinks, are a form of functional beverage.17 Sports drinks are typically formulated to prevent dehydration, supply carbohydrates, provide electrolytes, and be highly palatable, and they typically do not contain caffeine. They are generally designed to be consumed before or during exercise.12 Nutraceutical beverages generally contain bioactive compounds such as concentrated extracts of fruits, vegetables, teas, or herbs and are designed to promote and enhance health.43 Energy drinks may overlap into the other 2 categories depending on their ingredient composition.17
Energy drinks first appeared in Europe and Asia in the 1960s.17,41 Since its inception, the popularity of energy drinks has grown exponentially, with about 500 new brands launched worldwide in 2006. This represented a 240% increase in sales between 2004 and 2009.3,10,41 Energy drinks are the fastest growing product in the beverage industry since bottled water.17
The regulation of energy drinks has been challenging. The absence of regulatory oversight has resulted in aggressive marketing of energy drinks targeted primarily toward young adults.27,41 Malinauskas et al24 found that approximately half of college students reported consuming at least 1 energy drink per month to compensate for lack of sleep, increase energy, or mix with alcohol while partying. Several countries are now regulating the labeling, distribution, and sales of energy drinks containing significant quantities of caffeine. However, these regulatory measures differ between countries.41
Caffeine (1,3,7-trimethylxanthine) is a naturally found alkaloid. After ingestion, it is rapidly and completely absorbed from the gastrointestinal tract into the bloodstream and is readily distributed throughout the entire body. The most important mechanism of action of caffeine is the antagonism of adenosine receptors, which results in the release of norepinephrine, dopamine, and serotonin in the brain and the increase of circulating catecholamines.29 A significant adverse effect of energy drinks is the risk of caffeine intoxication.5 Common features of caffeine intoxication include restlessness, nervousness, excitement, insomnia, diuresis, gastrointestinal disturbance, muscle twitching, rambling flow of thought and speech, cardiac arrhythmias, periods of inexhaustibility, and psychomotor agitation.5 In overdose cases, hypertension, hypotension, arrhythmia, and seizures have been reported as a result of caffeine intoxication, which may result in death.47
Although there is published literature reviewing the effects of individual ingredients of energy drinks, there is a lack of evidence evaluating the potential adverse effects of energy drink consumption. The purpose of this systematic review was to evaluate and report potential adverse health effects after consumption of energy drinks.
Methods
A systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.28 A literature search was conducted using 3 electronic medical databases—PubMed, EMBASE, and MEDLINE—from inception to the second week of November 2019, to identify all relevant studies related to adverse effects of energy drinks. Medical Subject Headings (MeSH) and EMTREE terms were utilized in various combinations to increase search sensitivity. References of included studies were also reviewed for additional relevant references that met the inclusion criteria.
Studies were included in this systematic review if they were (1) level 1 to 4 evidence published in English, (2) included human patients of any age, (3) evaluated the use of energy drinks, and (4) reported adverse effects after consumption of energy drinks. Exclusion criteria included literature reviews, conference proceedings, expert opinions, case reports, technique guides, nonhuman studies, cadaveric or biomechanical studies, clinical studies that did not evaluate energy drinks, and studies in which adverse effects were not reported. The Dietary Supplement and Non-prescription Drug Consumer Protection Act defined a serious adverse event as one that results in death, a life-threatening experience, inpatient hospitalization, a persistent or significant disability or incapacity, a congenital anomaly or birth defect, or requires medical or surgical intervention.38 All serious and nonserious adverse events were included. The adverse events were grouped into one of the following categories for ease of reporting and comparison among studies: (1) cardiorespiratory events, (2) gastrointestinal events, (3) immune responses, (4) musculoskeletal events, (5) neurological events, (6) physiological events, (7) psychological events, and (8) renal-related events.
A list of citations was compiled from the literature search and duplicates were removed. Systematic screening was performed in duplicate by 2 independent reviewers from title to full-text screening stages. Discrepancies were resolved by consensus between the 2 reviewers. If a consensus could not be reached, the input of a third, senior reviewer was used to determine the final eligibility of the study. Interrater agreement was calculated using Cohen kappa coefficient (κ).26
Data were extracted in duplicate by 2 independent reviewers and recorded in a Microsoft Excel spreadsheet (Version 2016; Microsoft Corp). Data regarding authors, year of publication, study design, level of evidence, sample size characteristics, active ingredients, co-ingestants, and all adverse events were extracted. The primary outcome was adverse events after consumption of energy drinks.
Quality of included clinical studies was appraised using the Methodological Index for Non-Randomized Studies (MINORS) tool.44 Quality was appraised in duplicate by 2 independent reviewers, and discrepancies were resolved by consensus between the 2 reviewers.
A Cohen κ statistic was used to evaluate interreviewer agreement at all screening stages. Agreement was categorized a priori. Agreement was characterized as follows: κ of 0.81 to 0.99 as excellent agreement, κ of 0.61 to 0.80 as substantial agreement, κ of 0.41 to 0.60 as moderate agreement, κ of 0.21 to 0.40 as fair agreement, and κ value of 0.20 or less as slight agreement.26
Because of differences of physiology, subgroup analyses were conducted in the pediatric and the adult population. Pediatric studies were defined as studies that reported adverse events in populations less than 19 years of age, and adult studies were defined as studies that reported adverse events in populations greater than 18 years of age.
Meta-analyses were conducted on adverse events that were reported in at least 3 or more randomized controlled trials. Review Manager 5.3 (The Cochrane Collaboration, 2014) was used to perform the meta-analysis. Dichotomous data were presented as odds ratios (ORs) with a 95% CI. The χ2 and I2 statistics were used to measure the heterogeneity of results within the included studies. P < 0.05 was considered significant for the χ2 test. TheI2 test was categorized as follows: 0.0%-24.9% to indicate no heterogeneity, 25.0%-49.9% to indicate low heterogeneity; 50.0%-74.9% to indicate moderate heterogeneity; 75.0%-100.0% to indicate high heterogeneity. Additionally, the random-effects model was used due to expected clinical heterogeneity.
Results
Eligibility
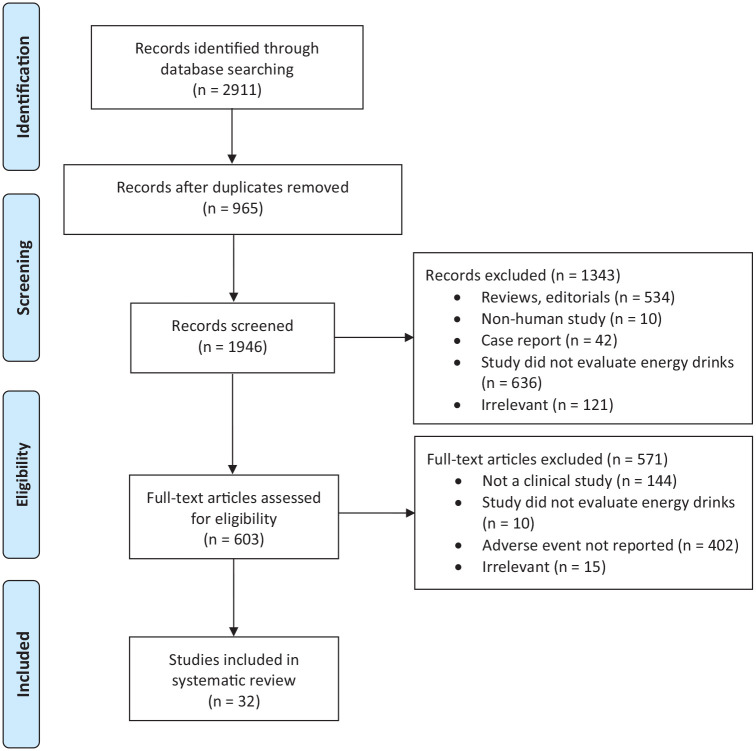
The search strategy initially identified 2911 titles for screening. After elimination of duplicates, 1946 titles and abstracts were screened. After the full-text review of 603 studies, 32 met our inclusion criteria. There was substantial agreement between reviewers at the titles and abstract screening stage (κ = 0.781; 95% CI, 0.749-0.812) and at the full-text screening stage (κ = 0.702; 95% CI, 0.581-0.822) (Figure 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) scheme of retrieved literature.
Study Characteristics
All 32 included studies were published between 2007 and 2018, of which 20 (62.5%) were published in the past 5 years (2015-2018). There were 7 studies1,2,6,14,16,23,32 that were of level 1 evidence, 1 study45 of level 2 evidence, and 24 studies4,7-9,11,13,15,18-22,24,25,30,31,33-37,39,40,42 of level 4 evidence. There were 13 comparative1,2,6,11,14-16,20,22,23,32,34,36 and 19 noncomparative studies.4,7-9,13,18,19,21,24,25,30,31,33,35,37,39,40,42,45 Among the comparative studies, 71,2,6,14,16,23,32 were randomized and 611,15,20,22,34,36 were cross-sectional in design. The mean MINORS score for comparative studies was 18.8 ± 3.0 out of 24, and the MINORS score for noncomparative studies was 9.53 ± 2.35 out of 16, indicating a fair quality of evidence. A total of 96,549 individuals who consumed energy drinks were included. The mean age was 15.2 years (range, 11-63 years), and 52.1% (49,219/94,438) were male. The studies conducted by Park et al,35 Holubcikova et al,19 and Kim et al21 evaluated a total of 85,981 pediatric individuals with a mean age of 14.9 years (range, 11-18 years), which skewed the cumulative mean age across all studies into the pediatric range (Table 1).
Table 1.
Study characteristics of included studies, in order of publication date
| Study | Country of Study | Study Design (Level of Evidence) | No. of Individuals Evaluated for Consumption of Energy Drinks | Age in Years of Sample, Mean ± SD (Range) | Proportion Male (%) | MINORS score a |
|---|---|---|---|---|---|---|
| Kim et al21 (2018) | Republic of Korea | Cross-sectional (4) | 8961 | 15.05 ± 1.74 | 5063/8961 (56.5) | 11 |
| Martins et al25 (2018) | Portugal | Cross-sectional (4) | 802 | 15.4 ± 1.5 (11-17) | 406/802 (50.6) | 8 |
| Nowak et al32 (2018) | Poland | RCT (I) | 36 | 24.8 ± 6.9 | 8/36 (22.2) | 19 |
| Nordt et al30 (2017) | USA | Cross-sectional (4) | 192 | (13-19) | 94/192 (49.0) | 8 |
| Droste et al15 (2017) | Australia | Cross-sectional—within-participant comparison (4) | 731 | 23.5 ± 5.6 | 304/731 (41.6) | 14 |
| Holubcikova et al19 (2017) | Slovakia | Cross-sectional (4) | 8977 | 13.49 ± 1.33 (11-15) | 4490/8977 (50.0) | 9 |
| Fletcher et al16 (2017) | USA | RCT–crossover study (1) | 18 | 26.7 ± 4.0 (18-40) | 12/18 (66.7) | 22 |
| Rahamathulla39 (2017) | Saudi Arabia | Cross-sectional (4) | 274 | — | 0/274 (0) | 6 |
| Bashir et al8 (2016) | USA | Cross-sectional (4) | 612 | 16 ± 2.0 (12-18) | 258/612 (42.2) | 11 |
| Park et al35 (2016) | South Korea | Cross-sectional (4) | 68,043 | 15.09 ± 1.72 (12-18) | 35,204/68,043 (51.7) | 12 |
| Costa et al13 (2016) | Australia | Cross-sectional (4) | 224 | (12-18) | — | 8 |
| Nowak and Jasionowski33 (2016) | Poland | Cross-sectional (4) | 488 | 14.3 (13-16) | 296/488 (60.7) | 8 |
| Sather and Delorey42 (2016) | USA | Cross-sectional (4) | 239 | 23.7 ± 2.0 (21-35) | 223/239 (93.3) | 10 |
| Pensa et al37 (2016) | USA | Cross-sectional (4) | 50 | 40.3 (23-63) | 48/50 (96) | 6 |
| Busuttil and Willoughby11 (2016) | Australia | Cross-sectional (4) | 42 | 26.29 ± 6.8 (15-39) | — | 12 |
| Del Coso et al14 (2016) | Spain | RCT–crossover study (1) | 13 | 23.2 ± 3.9 | 13/13 (100) | 20 |
| Reid et al40 (2015) | Trinidad and Tobago | Cross-sectional (4) | 1718 | NR | NR | 7 |
| Bonar et al9 (2015) | USA | Cross-sectional (4) | 158 | 18.8 ± 1.4 (14-20) | 78/158 (49.4) | 12 |
| Banerjee et al7 (2015) | Nepal | Cross-sectional (4) | 88 | NR | 51/88 (58.0) | 12 |
| Abian et al1 (2015) | Spain | RCT–crossover study (1) | 16 | 25.4 ± 7.3 | 16/16 (100) | 20 |
| Abian-Vicen et al2 (2014) | Spain | RCT–crossover study (1) | 16 | 14.9 ± 0.8 | 16/16 (100) | 20 |
| Kristjansson et al22 (2014) | Iceland | Cross-sectional (4) | 1521 | (10-13) | 1024/1521 (67.3) | 17 |
| Jackson et al20 (2013) | USA | Cross-sectional (4) | 90 | (18-23) | 60/90 (66.7) | 19 |
| Kurtz et al23 (2013) | USA | RCT–crossover study (1) | 20 | 23.30 ± 2.67 | 10/20 (50) | 24 |
| Hidiroglu et al18 (2013) | Turkey | Cross-sectional (4) | 127 | (16-27) | NR | 8 |
| Peacock et al36 (2012) | Australia | Cross-sectional—within-participant comparison (4) | 403 | 23.1 ± 3.8 (18-35) | 157/403 (39.0) | 20 |
| Nordt et al31 (2012) | USA | Cross-sectional (4) | 1298 | >18 | 661/1298 (52.6) | 10 |
| Astorino et al6 (2012) | USA | RCT–crossover study (1) | 15 | 19.5 ± 1.1 | 0/15 (0) | 18 |
| Alsunni and Badar4 (2011) | Saudi Arabia | Cross-sectional (4) | 412 | 21.4 ± 2.1 | 282/412 (68.4) | 8 |
| Steinke et al45 (2009) | USA | Prospective study (2) | 15 | 25.9 ± 5.9 (20-39) | 7/15 (46.7) | 15 |
| O’Brien et al34 (2008) | USA | Cross-sectional (4) | 697 | 20.3 ± 2.2 (17-30) | 331/697 (47.5) | 20 |
| Malinauskas et al24 (2007) | USA | Cross-sectional (4) | 253 | NR | 107/253 (42.3) | 12 |
MINORS, Methodological Index for Non-Randomized Studies; NR, not reported; RCT, randomized controlled trial.
The global ideal MINORS score is 16 for noncomparative studies and 24 for comparative studies.
Energy Drink Brand, Frequency, Co-ingestants, and Reason for Consumption
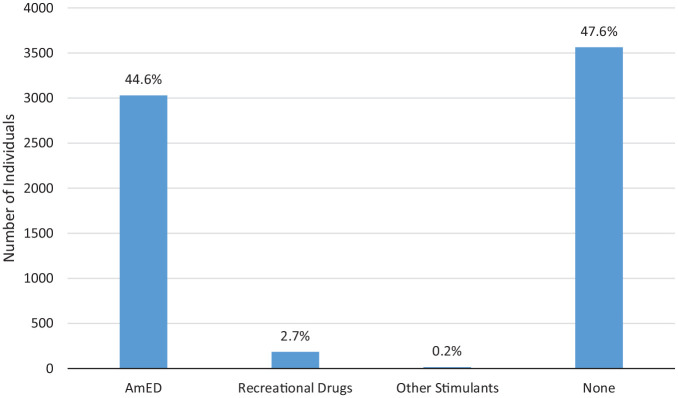
The brand of energy drink consumed was reported in 8 studies.1,2,6,14,23,37,39,40 The most commonly consumed energy drink reported was Red Bull (27.2%; 294/1080), followed by Monster (23.1%; 250/1080) and Full Throttle (7.1%; 77/1080). Frequency of consumption was reported in 15 studies.4,7,8,13,15,19,21,22,30,31,33,35,37,40,42 Among 92,006 individuals reporting the frequency of energy drink consumption, 76.7% reported <1 energy drink/week (70,597/92,006)7,8,13,22 (Figure 2). Co-ingestants were reported in 13 studies.2,9,11,15,18,20,25,30,31,33,36,37,40 Among a total of 6796 individuals evaluated, 3232 reported co-ingestants (47.6%), with mixed alcohol use being the most frequent (44.6%; 3030/6796) (Figure 3). Reasons for energy drink consumption were reported in 12 studies.4,9,18,24,25,30,31,33,34,39,40,42 The most common reasons for energy drink consumption were to increase energy/relieve fatigue (24.5%; 2640/10757), to stay awake or to counter insufficient sleep (15.7%; 1694/10,757), and for academic-related reasons (eg, for concentration during studies; 14.1%; 1520/10,757).
Figure 2.
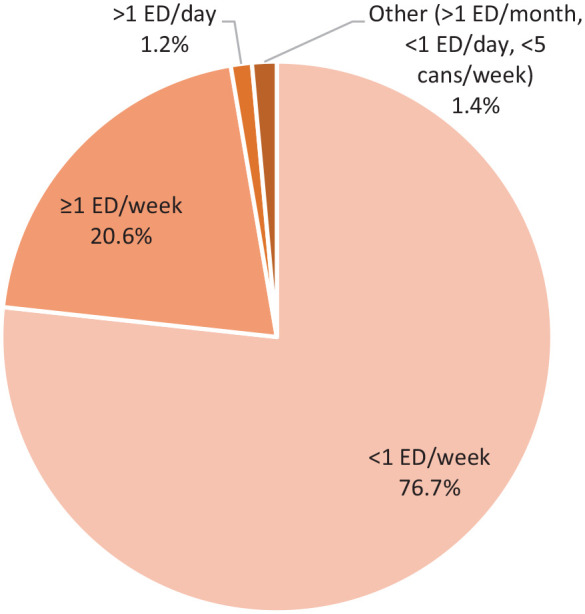
Frequencies of energy drink consumption (n = 92,006). ED, energy drinks.
Figure 3.
Co-ingestants reported with consumption of energy drinks (n = 6796). AmED, alcohol mixed with energy drinks.
Appendix Table A1 (available in the online version of this article) summarizes all energy drink brands, frequency, co-ingestants, and reasons for consumption across all included studies.
Adverse Events
All reported adverse events in the 32 included studies can be found in Appendix Table A2 (available online). Elevated heart rate (tachycardia) was the most frequently reported cardiorespiratory event (26.2%; 1016/3878), followed by heart palpitations (20.0%; 853/4255), and chest pain (10.3%; 240/2340). Gastrointestinal events include abdominal pain/stomachache (14.6%; 1822/12497) and gastrointestinal upset, which includes nausea, vomiting, and diarrhea (18.7%; 705/3774). An allergic reaction was reported in 1.9% (12/620). A cumulative rate of 14.0% of individuals reported either muscle tension, pain, soreness, twitching, and/or backache (1400/9979). Headaches were the most frequently reported neurological event (18.4%; 3191/17,331), followed by dizziness (12.3%; 1290/10,093), and tremors (11.4%; 508/4449). Frequently reported physiological events include insomnia/sleeping-related symptoms (34.5%; 29,445/85,373), jitteriness/restlessness/shaking hands (25.1%; 1164/4631), and jolt and crash episodes (22.6%; 900/3979). Frequently reported psychological events were stress (35.4%; 24,114/68,133), depressive mood (23.0%; 17,817/77,317), and suicidal ideation/plan/attempt (19.8%; 15,278/77,004). Increased urination was the most frequently reported renal event (13.0%; 381/2945), followed by kidney pain (0.8%; 2/258).
Subgroup Analyses of Adverse Events in the Pediatric and Adult Populations
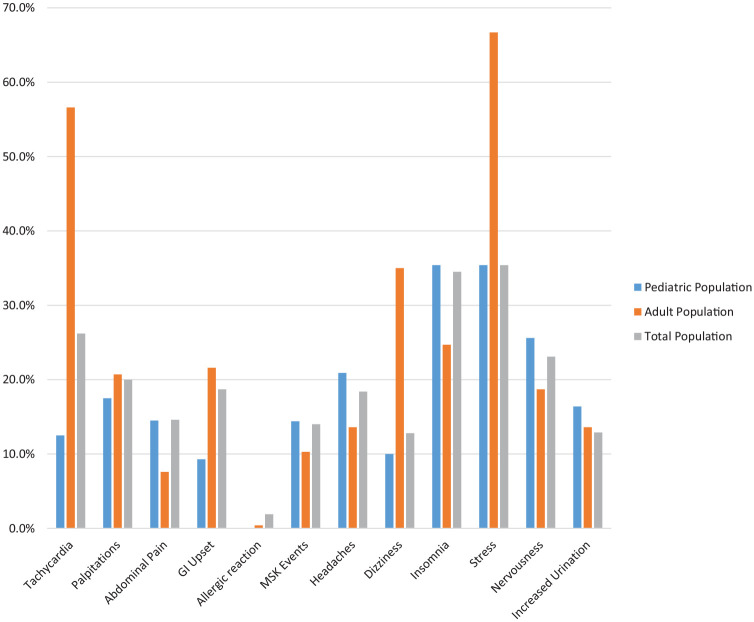
Table 2 lists all adverse events after energy drink consumption that were reported in the pediatric, adult, and total population. Figure 4 shows the rates of the most frequently reported adverse event per organ system in all 3 populations. Formal statistical analyses and meta-analyses were not conducted due to lack of comparative studies between pediatric and adult populations.
Table 2.
Reported adverse events after consumption of energy in the pediatric, adult, and total population, in order of frequency in total population
| Organ System | Adverse Event | Rate in Pediatric Population (%) | Rate in Adult Population (%) | Rate in Total Population (%) |
|---|---|---|---|---|
| Cardiovascular events | Tachycardia | 127/1016 (12.5) | 558/986 (56.6) | 1016/3878 (26.2) |
| Palpitations | 174/995 (17.5) | 578/2788 (20.7) | 853/4255 (20.0) | |
| Chest pain | 154/786 (19.6) | 70/1424 (4.9) | 240/2340 (10.3) | |
| Dyspnea | 134/784 (17.1) | 54/477 (11.3) | 192/1391 (13.8) | |
| Arrhythmia | 7/488 (1.4) | 25/258 (9.7) | 32/746 (4.3) | |
| GI events | Abdominal pain | 1691/11,637 (14.5) | 26/340 (7.6) | 1822/12,497 (14.6) |
| GI upset | 83/893 (9.3) | 604/2793 (21.6) | 705/3774 (18.7) | |
| Low appetite | 256/1483 (17.3) | NR | 256/1483 (17.3) | |
| Increased saliva | NR | 49/350 (14.0) | 49/350 (14.0) | |
| Immune response | Allergic reaction | NR | 1/258 (0.4) | 12/620 (1.9) |
| MSK events | Muscle tension/pain/soreness/twitching | 1266/8803 (14.4) | 105/1018 (10.3) | 1400/9979 (14.0) |
| Neurological events | Headaches | 2501/11,972 (20.9) | 392/2889 (13.6) | 3191/17,331 (18.4) |
| Dizziness | 885/8875 (10.0) | 395/1130 (35.0) | 1290/10,093 (12.8) | |
| Tremors | 130/1606 (8.1) | 232/1125 (20.6) | 508/4449 (11.4) | |
| Slurred speech | NR | 355/1110 (32.0) | 355/1110 (32.0) | |
| Walking difficulties | NR | 329/1107 (29.7) | 329/1107 (29.7) | |
| Decreased coordination | NR | 270/731 (36.9) | 270/731 (36.9) | |
| Visual disturbances | 31/213 (14.6) | 72/627 (11.5) | 103/840 (12.3) | |
| Seizures | 2/192 (1.0) | 17/1556 (1.1) | 21/1836 (1.1) | |
| Physiological events | Insomnia/sleeping related symptoms | 28,371/80,173 (35.4) | 767/3109 (24.7) | 29,445/85,373 (34.5) |
| Jitteriness/restlessness/shaking hands | 37/192 (19.3) | 730/2447 (29.8) | 1164/4631 (25.1) | |
| Jolt and crash | 30/488 (6.1) | 448/1362 (32.9) | 900/3979 (22.6) | |
| Rapid speech | 74/214 (34.6) | 381/1106 (34.4) | 455/1320 (34.5) | |
| Dehydration | 123/592 (20.8) | 80/497 (16.1) | 203/1089 (18.6) | |
| Fatigue | 3/488 (0.6) | 170/781 (21.8) | 175/1396 (12.5) | |
| Weakness | 172/596 (28.9) | NR | 172/596 (28.9) | |
| Heat intolerance | NR | 68/429 (15.9) | 77/517 (14.9) | |
| Psychological events | Stress | 24,054/68,043 (35.4) | 60/90 (66.7) | 24,114/68,133 (35.4) |
| Depressive mood | 17,757/76,859 (23.1) | 60/458 (13.1) | 17,817/77,317 (23) | |
| Suicidal ideation/plan/attempt | 15,278/77,004 (19.8) | NR | 15,278/77,004 (19.8) | |
| Agitation/anxiety/nervousness | 2801/10,937 (25.6) | 331/1771 (18.7) | 3452/14,946 (23.1) | |
| Irritability | 2489/8866 (28.1) | 62/643 (9.6) | 2733/11,385 (24) | |
| Renal-related events | Increased urination | 97/592 (16.4) | 37/273 (13.6) | 381/2945 (12.9) |
| Kidney pain | NR | 2/258 (0.8) | 2/258 (0.8) |
GI, gastrointestinal; MSK, musculoskeletal; NR, not reported.
Figure 4.
Rates of the most frequently reported adverse event per organ system in the pediatric, adult, and total populations. GI, gastrointestinal; MSK, musculoskeletal.
A total of 10 studies evaluating a total of 89,836 individuals reported adverse events after energy drink consumption in the pediatric population (age range, 11-19 years).2,8,13,19,21,22,25,30,33,35 Palpitations were the most frequently reported cardiorespiratory adverse event (17.5%; 174/995), followed by chest pain (19.6%; 154/786), dyspnea (17.1%; 134/784), and tachycardia (12.5%; 127/1016). Frequently reported gastrointestinal events were abdominal pain (14.5%; 1691/11,637), low appetite (17.3%; 256/1483), and gastrointestinal upset (9.3%; 83/893). A cumulative rate of 14.4% of individuals reported muscle soreness or backache (1266/8803). Headaches were the most frequently reported neurological event (20.9%; 2501/11,972), followed by dizziness (10.0%; 885/8875) and tremors (8.1%; 130/1606). Frequently reported physiological events were insomnia/sleeping-related symptoms (35.4%; 28,371/80,173) followed by weakness (28.9%; 172/596) and dehydration (20.8%; 123/592). Increased urination was the only renal related adverse event reported (16.4%; 97/592). Adverse immune responses were not reported in the pediatric population. Stress, depressive mood, and suicidal ideation/plan/attempt were the most frequently reported psychological adverse events with cumulative rates of 35.4% (24,054/68,043), 23.1% (17,757/76,859), and 19.8% (15,278/77,004), respectively. Kim et al21 reported that rates of suicidal ideation and attempts in participants who consumed energy drinks more than once daily were higher relative to those who consumed 3 to 6 and 1 to 2 times weekly. Park et al35 reported that energy drink intake was significantly associated with suicide plan and suicide attempt, with more frequent use of energy drinks (≥5 times/week) conferring a higher risk than with less frequent use (1-4 times/week).
A total of 14 studies evaluating a total of 3356 individuals reported adverse events after energy drink consumption in the adult population (age range, 18-63 years). Palpitations were the most frequently reported cardiorespiratory adverse event (20.7%; 578/2788), followed by tachycardia (56.6%; 558/986) and chest pain (4.9%; 70/1424). Frequently reported gastrointestinal events were gastrointestinal upset (21.6%; 604/2793), increased salivation (14%; 49/350), and abdominal pain (7.6%; 26/340). Allergic reactions were reported in 0.4% of individuals (1/258). A cumulative rate of 10.3% of individuals reported muscle soreness or backache (105/1018). Dizziness was the most frequently reported neurological event (35.0%; 395/1130), followed by headaches (13.6%; 392/2889) and slurred speech (32.0%; 355/1110). Frequently reported physiological events were insomnia/sleeping-related symptoms (24.7%; 767/3109) followed by jitteriness/restlessness/shaking hands (29.8%; 730/2447) and jolt and crash episodes (32.9%; 448/1362). Agitation/anxiety/nervousness, irritability, stress, and depressive mood were the most frequently reported psychological events with rates of 18.7% (331/1771), 9.6% (62/643), 66.7% (60/90), and 13.1% (60/458), respectively. Suicidal ideation/plan/attempt was not reported in the adult population. Increased urination was the more frequently reported renal-related event (13.6%; 37/273).
Randomized Controlled Trials Evaluating Energy Drinks Versus Control
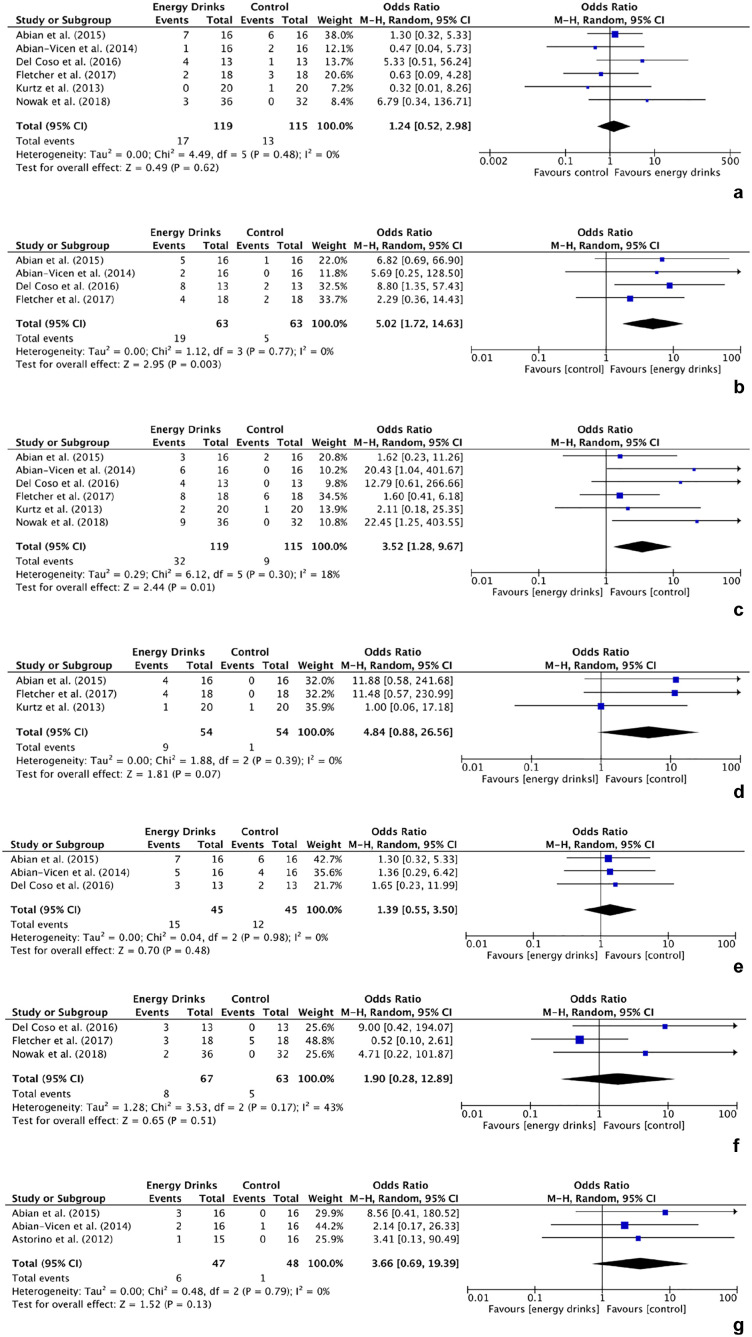
Of the total, 7 studies conducted randomized controlled trials evaluating the effects of energy drinks compared with a control.1,2,6,14,16,23,32 Controls included water, a nonenergy caffeinated drink, a decaffeinated shot, and Canada Dry Gingerale (caffeine- and taurine-free).6,16,23,32 Meta-analyses were conducted on the following adverse events: (1) headaches, (2) insomnia, (3) jitteriness/activeness, (4) tachycardia/palpitations, (5) muscular pain, (6) anxiety/nervousness, and (7) abdominal pain. Statistical analyses showed that energy drinks significantly increased the odds of insomnia (OR, 5.02; 95% CI, 1.72-14.63) and jitteriness/activeness (OR, 3.52; 95% CI, 1.28-9.67) compared with the control group (P < 0.05). The odds of headaches (OR, 1.24; 95% CI, 0.52-2.98), tachycardia/palpitations (OR, 4.84; 95% CI, 0.88-26.56), muscular pain (OR, 1.39; 95% CI, 0.55-3.50), anxiety/nervousness (OR, 1.90; 95% CI, 0.28-12.89), and abdominal pain (OR, 3.66; 95% CI, 0.69-19.39) were greater in the energy drink group compared with the control group, but the results were not significant (Figure 5).
Figure 5.
Meta-analysis forest plots evaluating the odds of (a) headaches, (b) insomnia, (c) jitteriness/activeness, (d) tachycardia/palpitations, (e) muscular pain, (f) anxiety/nervousness, and (g) abdominal pain with energy drink consumption compared with control. M-H, Mantel-Haenszel test.
Alcohol Mixed With Energy Drinks Versus Alcohol Alone
Three cross-sectional studies compared adverse events after consumption of alcohol mixed with energy drinks (AmED) and of alcohol alone15,34,36 (Appendix Table A3 available online). Droste et al15 and Peacock et al36 conducted within-participant comparisons and reported that the odds of experiencing several physiological and psychological stimulant adverse effects were significantly greater during the AmED sessions compared with alcohol-only sessions. However, the odds of experiencing several physiological and psychological sedation outcomes were significantly greater in the alcohol-only sessions compared with AmED sessions.15,36 O’Brien et al34 conducted a cross-sectional study on 4271 college students (AmED, n = 697; alcohol only, n = 2189; nondrinkers, n = 1351) and reported that students who reported consuming AmED had significantly higher prevalence of alcohol-related consequences. These consequences included being physically hurt or injured (OR, 2.25; 95% CI, 1.70-2.96), riding with an intoxicated driver (OR, 2.20; 95% CI, 1.81-2.68), taking advantage of another sexually (OR, 2.18; 95% CI, 1.34-3.55), requiring medical treatment (OR, 2.17; 95% CI, 1.24-3.80), and being taken advantage of sexually (OR, 1.77; 95% CI, 1.23-2.55).34
Discussion
This systematic review evaluated many relevant topics regarding energy drink consumption that have not been discussed in recent literature. Although relatively high rates of adverse events were reported, very few met the criteria to be considered a serious adverse event. According to the Dietary Supplement and Non-prescription Drug Consumer Protection Act, the definition of a serious adverse event included requiring medical attention.38 Only 3 studies reported the proportion of individuals who required medical attention.9,21,34 Among 9816 individuals, 165 required medical attention (1.7%). It is also important to note that reported adverse events after energy drink consumption do not necessarily reflect a causal relationship and many of the studies lacked control groups. This meta-analysis demonstrates that energy drinks significantly increased the odds of insomnia and jitteriness/activeness compared with the control group (P < 0.05). Additionally, the odds of headaches, tachycardia/palpitations, muscular pain, anxiety/nervousness, and abdominal pain were greater in the energy drink group compared with the control group, but the results were not significant.
Many of the adverse effects of energy drinks are related to caffeine intoxication, a clinical syndrome included in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition.5 Common features of caffeine intoxication include restlessness, nervousness, excitement, insomnia, diuresis, gastrointestinal disturbance, muscle twitching, rambling flow of thought and speech, cardiac arrhythmias, periods of inexhaustibility, and psychomotor agitation.5 Nawrot et al29 concluded that among the healthy adult population, a moderate daily caffeine intake of ≤400 mg (equivalent to 6.5 mg/kg per day for a 65-kg person) was not associated with any adverse events.29 Energy drinks can vary widely in caffeine content, ranging from 50 to 505 mg per can or bottle.41
The most commonly reported energy drink consumption frequency was <1 energy drinks/week (76.4%; 70,597/92,368), followed by ≥1 energy drinks/week (20.5%; 18,942/92,368) and >1 energy drinks/day (1.2%; 1138/92,368). Several studies reported increased risk of various adverse effects as energy drink consumption increased. The rates of suicidal ideation and attempts were significantly higher in participants who consumed energy drinks more than once a day, followed by those who consumed 3 to 6 drinks a week and 1 to 2 drinks a week.21 Similarly, Park et al35 found that after adjusting for confounders, participants who used energy drinks frequently (≥5 energy drinks/week) were more likely to experience adverse effects, including sleep dissatisfaction, perceived stress, and persistent depressive mood compared with moderate and infrequent energy drink consumers.35
Alcohol was the most frequent co-ingestant. A meta-analysis analyzing the consumption of alcohol mixed with energy drinks revealed that consumers who mixed alcohol with energy drinks drank significantly more alcohol than alcohol-only consumers, a finding consistent with Peacock et al36 and Verster et al.46 Additionally, the stimulant effects of energy drinks may counteract the depressant effects of alcohol, as sedation outcomes were found to be significantly less in the group that mixed alcohol with energy drinks compared with the alcohol-only group.15 However, mixing alcohol with energy drinks does not affect subjective intoxication.46
This review found that the majority of energy drink consumers were younger than 19 years of age, and the most common reasons for energy drink consumption were to increase energy/relieve fatigue, to stay awake or to counter insufficient sleep, and for academic-related reasons. In the pediatric population in particular, stress, depressive mood, and suicidal ideation/plan/attempt were the most frequently reported psychological adverse events, while agitation/anxiety/nervousness, irritability, and stress were the most frequently reported psychological events in the adult population. Suicidal ideation/plan/attempt was not reported in the adult population and is an area of future research. It is important to note that this review did not conduct statistical analyses of adverse events between pediatric and adult populations due to lack of comparative studies. Therefore, further research comparing the adverse effects between the 2 populations is required to provide definitive conclusions.
Based on the findings of this systematic review and meta-analysis, recommendations that can be made are: (1) individuals should avoid frequent energy drink consumption (5-7 energy drinks/week), (2) individuals should avoid co-consumption with alcohol, and (3) increased regulatory standards should be placed in the sale of energy drinks, particularly with regard to the pediatric population.
The strengths of this systematic review include a search strategy using multiple large medical databases, screening and data extraction conducted in duplicate with a high level of interrater agreement, a large sample size, and a mean MINORS score indicating fair quality of evidence of included studies. This systematic review is limited by the level of evidence of the included studies, as the studies were primarily cross-sectional in design. Additionally, the majority of the population was younger than 19 years, which greatly skewed the mean age of all included individuals into the pediatric range. Last, significant heterogeneity was present between included studies, particularly with regard to brand of energy drink, active ingredients, age, and the reported adverse events. Future research in this area should include large randomized controlled trials with baseline equivalence of characteristics.
Conclusion
Energy drink consumption significantly increases the odds of insomnia and jitteriness/activeness compared with the control group. Increased frequency of energy drink consumption and co-ingestion of energy drinks with alcohol significantly increased the risk of adverse events. Recommendations that can be made are avoiding frequent energy drink consumption (5-7 energy drinks/week), avoiding co-consumption with alcohol, and increasing regulatory standards in the sale of energy drinks, particularly with regard to the pediatric population.
Supplemental Material
Supplemental material, sj-docx-1-sph-10.1177_1941738120949181 for Energy Drinks and Their Adverse Health Effects: A Systematic Review and Meta-analysis by Ibrahim M. Nadeem, Ajaykumar Shanmugaraj, Seaher Sakha,, Nolan S. Horner, Olufemi R. Ayeni and Moin Khan in Sports Health: A Multidisciplinary Approach
Footnotes
The following author declared potential conflicts of interest: M.K. is a paid associate editor for Sports Health.
References
- 1. Abian P, Del Coso J, Salinero JJ, et al. The ingestion of a caffeinated energy drink improves jump performance and activity patterns in elite badminton players. J Sports Sci. 2015;33:1042-1050. [DOI] [PubMed] [Google Scholar]
- 2. Abian-Vicen J, Puente C, Salinero JJ, et al. A caffeinated energy drink improves jump performance in adolescent basketball players. Amino Acids. 2014;46:1333-1341. [DOI] [PubMed] [Google Scholar]
- 3. Ali F, Rehman H, Babayan Z, Stapleton D, Joshi DD. Energy drinks and their adverse health effects: a systematic review of the current evidence. Postgrad Med. 2015;127:308-322. [DOI] [PubMed] [Google Scholar]
- 4. Alsunni AA, Badar A. Energy drinks consumption pattern, perceived benefits and associated adverse effects amongst students of University of Dammam, Saudi Arabia. J Ayub Med Coll Abbottabad. 2011;23:3-9. [PubMed] [Google Scholar]
- 5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 6. Astorino TA, Matera AJ, Basinger J, Evans M, Schurman T, Marquez R. Effects of Red Bull energy drink on repeated sprint performance in women athletes. Amino Acids. 2012;42:1803-1808. [DOI] [PubMed] [Google Scholar]
- 7. Banerjee I, Pugazhandhi B, Banerjee I, Sathian B, Nagpal P, Roy B. Is energy drink safe? A cross sectional study on the effects of energy drink on medical students from a medical school of Nepal. Nepal J Epidemiol. 2015;5:444-450. [Google Scholar]
- 8. Bashir D, Reed-Schrader E, Olympia RP, et al. Clinical symptoms and adverse effects associated with energy drink consumption in adolescents. Pediatr Emerg Care. 2016;32:751-755. [DOI] [PubMed] [Google Scholar]
- 9. Bonar EE, Cunningham RM, Polshkova S, Chermack ST, Blow FC, Walton MA. Alcohol and energy drink use among adolescents seeking emergency department care. Addict Behav. 2015;43:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burrows T, Pursey K, Neve M, Stanwell P. What are the health implications associated with the consumption of energy drinks? A systematic review. Nutr Rev. 2013;71:135-148. [DOI] [PubMed] [Google Scholar]
- 11. Busuttil M, Willoughby S. A survey of energy drink consumption among young patients presenting to the emergency department with the symptom of palpitations. Int J Cardiol. 2016;204:55-56. [DOI] [PubMed] [Google Scholar]
- 12. Coombes JS, Hamilton KL. The effectiveness of commercially available sports drinks. Sports Med. 2000;29:181-209. [DOI] [PubMed] [Google Scholar]
- 13. Costa BM, Hayley A, Miller P. Adolescent energy drink consumption: an Australian perspective. Appetite. 2016;105:638-642. [DOI] [PubMed] [Google Scholar]
- 14. Del Coso J, Portillo J, Salinero JJ, Lara B, Abian-Vicen J, Areces F. Caffeinated energy drinks improve high-speed running in elite field hockey players. Int J Sport Nutr Exerc Metab. 2016;26:26-32. [DOI] [PubMed] [Google Scholar]
- 15. Droste N, Peacock A, Bruno R, et al. Combined use of alcohol and energy drinks: dose relationship with self-reported physiological stimulation and sedation side effects. Addict Behav. 2017;71:68-74. [DOI] [PubMed] [Google Scholar]
- 16. Fletcher EA, Lacey CS, Aaron M, Kolasa M, Occiano A, Shah SA. Randomized controlled trial of high-volume energy drink versus caffeine consumption on ECG and hemodynamic parameters. J Am Heart Assoc. 2017;6:e004448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heckman MA, Sherry K, de Mejia EG. Energy drinks: an assessment of their market size, consumer demographics, ingredient profile, functionality, and regulations in the United States. Compr Rev Food Sci Food Saf. 2010;9:303-317. [DOI] [PubMed] [Google Scholar]
- 18. Hidiroglu S, Tanriover O, Unaldi S, Sulun S, Karavus M. A survey of energy-drink consumption among medical students. J Pak Med Assoc. 2013;63:842-845. [PubMed] [Google Scholar]
- 19. Holubcikova J, Kolarcik P, Madarasova Geckova A, Reijneveld SA, van Dijk JP. Regular energy drink consumption is associated with the risk of health and behavioural problems in adolescents. Eur J Pediatr. 2017;176:599-605. [DOI] [PubMed] [Google Scholar]
- 20. Jackson DAE, Cotter BV, Merchant RC, et al. Behavioral and physiologic adverse effects in adolescent and young adult emergency department patients reporting use of energy drinks and caffeine. Clin Toxicol. 2013;51:557-565. [DOI] [PubMed] [Google Scholar]
- 21. Kim J-S, Kim K, Seo Y. Associations between Korean adolescents’ energy drink consumption and suicidal ideation and attempts. Arch Psychiatr Nurs. 2018;32:331-336. [DOI] [PubMed] [Google Scholar]
- 22. Kristjansson AL, Sigfusdottir ID, Mann MJ, James JE. Caffeinated sugar-sweetened beverages and common physical complaints in Icelandic children aged 10-12 years. Prev Med. 2014;58:40-44. [PubMed] [Google Scholar]
- 23. Kurtz AM, Leong J, Anand M, Dargush AE, Shah SA. Effects of caffeinated versus decaffeinated energy shots on blood pressure and heart rate in healthy young volunteers. Pharmacotherapy. 2013;33:779-786. [DOI] [PubMed] [Google Scholar]
- 24. Malinauskas BM, Aeby VG, Overton RF, Carpenter-Aeby T, Barber-Heidal K. A survey of energy drink consumption patterns among college students. Nutr J. 2007;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martins A, Ferreira C, Sousa D, Costa S. Consumption patterns of energy drinks in Portuguese adolescents from a city in northern Portugal. Acta Med Port. 2018;31:207-212. [DOI] [PubMed] [Google Scholar]
- 26. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276-282. [PMC free article] [PubMed] [Google Scholar]
- 27. Miller KE. Energy drinks, race, and problem behaviors among college students.J Adolesc Health. 2008;43:490-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nawrot P, Jordan S, Eastwood J, Rotstein J, Hugenholtz A, Feeley M. Effects of caffeine on human health. Food Addit Contam. 2003;20:1-30. [DOI] [PubMed] [Google Scholar]
- 30. Nordt SP, Claudius I, Rangan C, et al. Reasons for energy drink use and reported adverse effects among adolescent emergency department patients. Pediatr Emerg Care. 2017;33:770-773. [DOI] [PubMed] [Google Scholar]
- 31. Nordt SP, Vilke GM, Clark RF, et al. Energy drink use and adverse effects among emergency department patients. J Community Health. 2012;37:976-981. [DOI] [PubMed] [Google Scholar]
- 32. Nowak D, Gośliński M, Nowatkowska K. The effect of acute consumption of energy drinks on blood pressure, heart rate and blood glucose in the group of young adults. Int J Environ Res Public Health. 2018;15:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nowak D, Jasionowski A. Analysis of consumption of energy drinks by a group of adolescent athletes. Int J Environ Res Public Health. 2016;13:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Brien MC, McCoy TP, Rhodes SD, Wagoner A, Wolfson M. Caffeinated cocktails: energy drink consumption, high-risk drinking, and alcohol-related consequences among college students. Acad Emerg Med. 2008;15:453-460. [DOI] [PubMed] [Google Scholar]
- 35. Park S, Lee Y, Lee JH. Association between energy drink intake, sleep, stress, and suicidality in Korean adolescents: energy drink use in isolation or in combination with junk food consumption. Nutr J. 2016;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peacock A, Bruno R, Martin FH. The subjective physiological, psychological, and behavioral risk-taking consequences of alcohol and energy drink co-ingestion. Alcohol Clin Exp Res. 2012;36:2008-2015. [DOI] [PubMed] [Google Scholar]
- 37. Pensa MA, Galusha DH, Stowe MH, Lefkowitz RY, Redlich CA. Patterns of energy drink use and associated symptoms among a population of Connecticut factory workers. J Occup Environ Med. 2016;58:e188-e190. [DOI] [PubMed] [Google Scholar]
- 38. Porter DV. Dietary Supplement and Nonprescription Drug Consumer Protection Act (P.L.109-462). Congressional Research Service, Library of Congress; 2014. [Google Scholar]
- 39. Rahamathulla MP. Prevalence, side effects and awareness about energy drinks among the female university students in Saudi Arabia. Pak J Med Sci. 2017;33:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reid SD, Ramsarran J, Brathwaite R, Lyman S. Energy drink usage among university students in a Caribbean country: patterns of use and adverse effects. J Epidemiol Glob Health. 2015;5:103-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks—a growing problem. Drug Alcohol Depend. 2009;99:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sather TE, Delorey DR. Energy beverage consumption among naval aviation candidates. Aerosp Med Hum Perform. 2016;87:557-564. [DOI] [PubMed] [Google Scholar]
- 43. Shahidi F, Weerasinghe DK. Nutraceutical beverages: an overview. In: Shahidi F, Weerasinghe DK, eds. Nutraceutical Beverages: Chemistry, Nutrition, and Health Effects. American Chemical Society; 2003:1-5. [Google Scholar]
- 44. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [DOI] [PubMed] [Google Scholar]
- 45. Steinke L, Lanfear DE, Dhanapal V, Kalus JS. Effect of “energy drink” consumption on hemodynamic and electrocardiographic parameters in healthy young adults. Ann Pharmacother. 2009;43:596-602. [DOI] [PubMed] [Google Scholar]
- 46. Verster JC, Benson S, Johnson SJ, Alford C, Godefroy SB, Scholey A. Alcohol mixed with energy drink (AMED): a critical review and meta-analysis. Hum Psychopharmacol. 2018;33:e2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamamoto T, Yoshizawa K, Kubo S, et al. Autopsy report for a caffeine intoxication case and review of the current literature. J Toxicol Pathol. 2015;28:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sph-10.1177_1941738120949181 for Energy Drinks and Their Adverse Health Effects: A Systematic Review and Meta-analysis by Ibrahim M. Nadeem, Ajaykumar Shanmugaraj, Seaher Sakha,, Nolan S. Horner, Olufemi R. Ayeni and Moin Khan in Sports Health: A Multidisciplinary Approach