Supplemental digital content is available in the text.
Key Words: pharmacokinetics, asenapine, transdermal, patch, schizophrenia
Abstract
Purpose/Background
The asenapine transdermal system (HP-3070) is the first antipsychotic patch approved in the United States for treatment of adults with schizophrenia.
Methods/Procedures
Three phase 1, open-label, randomized studies characterized the pharmacokinetic (PK) profile of HP-3070 by assessing its relative bioavailability compared with sublingual asenapine, its single-/multiple-dose PK and dose proportionality, and the effects of application site, ethnicity, and external heat on bioavailability. Two studies were conducted in healthy subjects, and 1 was conducted in adults with schizophrenia.
Findings/Results
During single HP-3070 administration, asenapine concentrations increased gradually over approximately 12 hours and remained steady until the patch was removed 24 hours after application. Asenapine area under the curve values at HP-3070 3.8 and 7.6 mg/24 hours doses were similar to those for sublingual asenapine 5 and 10 mg twice-daily doses, respectively, whereas peak exposure (maximum observed plasma concentration) was significantly lower. During daily application of HP-3070, steady-state PK was reached within approximately 72 hours after initiating daily dosing and was characterized by peak-to-trough asenapine plasma concentration ratio of approximately 1.5. HP-3070 PK was dose proportional in the dose range studied, not affected by administration site, and similar across the studied ethnic groups. Application of external heat increased the rate of asenapine absorption (time to reach maximum observed plasma concentration) but did not significantly affect peak and total exposure.
Implications/Conclusions
HP-3070 exhibited a dose-dependent PK profile unaffected by site of administration or ethnicity. HP-3070 showed a predictable absorption profile with limited variability, with an area under the curve similar to that of sublingual asenapine. Based on these PK metrics, HP-3070 steadily delivers asenapine with lower peaks and troughs than sublingual administration of asenapine.
The Food and Drug Administration–approved schizophrenia treatment options include oral, sublingual, and long-acting injectable medications. Although the need for multiple types of formulations has been acknowledged in the literature1,2 and a range of formulation options have been explored,2 a transdermal delivery option for antipsychotic medications had not been available until recently.3
Asenapine, a second-generation antipsychotic, was originally commercially available only as a sublingual tablet administered twice daily with instructions that the tablet be placed under the tongue and allowed to dissolve.4 Adverse effects related to the sublingual administration of asenapine can include oral ulcers, hypoesthesia, or dysgeusia.3,5 These adverse effects, combined with restrictions on eating or drinking for 10 minutes after administration, can create a barrier to adherence or patient cooperation, making sublingual formulations unsuitable for some patients.6,7
Transdermal antipsychotics are a relatively recent innovation in the treatment of schizophrenia. Available transdermal options for treatment of schizophrenia include Lonasen Tape, a transdermal formulation of blonanserin available in Japan, and HP-3070, a transdermal formulation of asenapine available in the United States.8–10 HP-3070 has demonstrated efficacy and safety in a phase 3 trial in adults with schizophrenia.11
Here, we describe 2 single-dose studies (study 1, study 2) and a multiple ascending-dose study (study 3) that were conducted to address the following objectives: characterize the pharmacokinetic (PK) profile of the asenapine transdermal system HP-3070 and evaluate the safety and tolerability of HP-3070 in both healthy adults and adults with schizophrenia. The efficacy and clinical implications of treatment with HP-3070 have been addressed elsewhere.11
MATERIALS AND METHODS
The 3 single-center, phase 1, open-label studies were conducted in the United States (study 1) and in the United Kingdom (studies 2 and 3), in compliance with the International Conference on Harmonization E6 Guideline for Good Clinical Practice. The study protocols, amendments, and informed consent forms for all 3 studies were approved by an independent ethics committee/institutional review board for each site (study 1, PPD Institutional Review Board, Austin, Texas; study 2, National Research Ethics Service Committee London and the Medicines and Healthcare Products Regulatory Agency, London, United Kingdom; study 3, Research Ethics Committee London, London, United Kingdom). All subjects provided written informed consent before any study-related procedures were initiated.
Trial Design, Participants, and Study Treatments
Study 1
Study 1 was a randomized, single-dose, 3-way crossover study enrolling 18 healthy subjects aged 18 to 45 years. Primary objectives were to compare the PK profiles of asenapine after HP-3070 and sublingual asenapine administration and assess the effect of locally applied external heat on the rate and extent of asenapine absorption after HP-3070 application. Each subject received 3 treatments in random order: a single 24-hour application of HP-3070 1.9 mg/24 hours (10 cm2) to the upper arm in the presence of a 40°C heating pad constantly applied for 8 hours; a single 24-hour application of HP-3070 1.9 mg/24 hours (10 cm2) to the upper arm without external heat application; and a single dose of sublingual asenapine 5 mg. During each treatment period, subjects were admitted to the testing site (PPD Phase 1 Clinic, Austin, Texas) and, depending on treatment assignment, discharged on either day 4 (after receiving sublingual asenapine) or day 6 (after receiving HP-3070).
Study 2
Study 2 (EudraCT 2013-003567-63) was a single-dose, 5-period crossover study whose primary objective was to evaluate the effect of different patch application sites on the relative bioavailability of HP-3070 3.8 mg/24 hours. A secondary objective was to evaluate potential ethnic differences in asenapine PK in Japanese and White populations after application of HP-3070 3.8 mg/24 hours. Forty healthy adult male and female subjects aged 20 to 40 years (20 Japanese, 20 White) were enrolled, admitted to the testing site (Richmond Pharmacology Ltd, London, United Kingdom), and received a single 24-hour HP-3070 patch administration of 3.8 mg/24 hours (20 cm2) at each of the following sites in random sequence: abdomen, hip, upper arm, upper back, and upper chest (Supplementary Fig. 1. Supplemental Digital Content, http://links.lww.com/JCP/A735). Subjects remained at the testing site until after completion of all assessments on day 6.
Study 3
Study 3 (EudraCT 2014-000806-36) was a multiple ascending-dose study with a primary objective of evaluating the PK profile of HP-3070 1.9, 3.8, 5.7, and 7.6 mg/24 hours after multiple 24-hour applications. Eligible subjects (N = 24) were 18- to 65-year-old male or female patients meeting the diagnostic criteria for schizophrenia or schizoaffective disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (no other active axis I diagnoses were permitted) with a Positive and Negative Syndrome Scale (PANSS) total score of 70 or less. Subjects were ineligible if they lacked the capacity to provide consent for research or were judged by the investigator to exhibit a significant risk of suicidal or violent ideation or behavior.
At the time of inclusion, all patients were being treated for schizophrenia with a stable dose of oral medication (except for 1 patient who had received an injectable antipsychotic), which was stopped 7 to 21 days before initial application of the HP-3070 patch. All subjects were given the option to undergo the initial 7-day washout period as inpatients or as outpatients under the care of their psychiatrists. Subjects were admitted to the testing site (Richmond Pharmacology Ltd, Croydon, United Kingdom) for the entire treatment period. Each patient received once-daily, 24-hour, single-patch administration of the following 4 ascending doses of study treatment applied in rotation to the upper arm, upper chest, upper back, and hip areas (Supplementary Fig. 1. Supplemental Digital Content, http://links.lww.com/JCP/A735): 1.9 mg/24 hours (patch size 10 cm2), days 1 to 7; 3.8 mg/24 hours (20 cm2), days 8 to 14; 5.7 mg/24 hours (30 cm2), days 15 to 21; and 7.6 mg/24 hours (40 cm2), days 22 to 28. All patch doses were compositionally identical, with dosage determined by patch size.
For all 3 studies, anatomical sites containing tattoos, scar tissue, infections, or other skin abnormalities were avoided. Study subjects were not permitted to shave patch application sites.
Assessments
Pharmacokinetics
For study 1, blood samples for PK assessments of HP-3070 treatment were collected before patch application and at 2, 4, 6, 8, 10, 12, 14, 16, 20, 24, 26, 28, 32, 36, 48, 60, 72, 96, and 120 hours after application, and blood samples for PK assessments of sublingual asenapine treatment were collected before dose and at 0, 10, and 20 minutes and 0.5, 0.75, 1, 1.25, 1.5, 2, 4, 6, 8, 10, 12, 14, 24, 36, 48, 60, and 72 hours after dose. For study 2, blood samples for PK assessments were collected before dose and at 2, 4, 8, 12, 16, 20, 24, 26, 28, 32, 36, 48, 60, 72, 96, and 120 hours after dose. For study 3, blood samples for PK assessments were collected before dose on day 1 to day 28; 2, 4, 6, 12, 16, and 20 hours after dose on day 7, 14, 21, and 28; and 24 hours after the last dose (day 29 or early termination). Analytic methods for assessment of asenapine in plasma samples are detailed in Supplementary Document 1 (Supplemental Digital Content, http://links.lww.com/JCP/A735).
All plasma concentrations for asenapine and the actual blood sampling times were subjected to noncompartmental PK analysis. Pharmacokinetic parameter calculations were performed primarily using Phoenix WinNonlin Version 6.3 or higher (Pharsight, Inc). Pharmacokinetic parameters analyzed included maximum observed plasma concentration (Cmax); Cmax at steady state (Cmax,ss); minimum concentration at steady state (Cmin,ss); time to reach maximum observed plasma concentration (tmax); tmax at steady state (tmax,ss); area under the plasma concentration-time curve calculated from time zero to t hours by the log-linear trapezoidal rule, where t is the last time point with a concentration above the lower limit of quantification (AUC0–t); AUC from time zero to time τ (ie, 24 hours) at steady state (AUC0–24,ss); AUC from time 0 to 8 hours (AUC0–8); AUC from 0 to infinity, calculated as AUC0–t + Ct/λz (AUC0–inf); percentage of estimated part of the calculation of AUC0–inf (%AUC); terminal elimination rate constant, determined by regression analysis of the log-linear segment of the plasma concentration-time curve (λz); and elimination half-life, calculated as ln2/λz (t1/2). For each study, the used patches were also collected and analyzed to determine the amount of residual drug remaining. Details of the analysis of residual drug in the used patches are supplied in Supplementary Document 1 (Supplemental Digital Content, http://links.lww.com/JCP/A735).
Dermal Assessments
Dermal evaluations included assessments for the presence and severity of skin irritation and discomfort, patch adhesion, and adhesive residue. Skin irritation at the patch site was judged by visual inspection and graded according to the dermal response scale (grades 0–7)12 and Other Effects Observation scale (numerical equivalent grades 0–3; Supplementary Table 1A. Supplemental Digital Content, http://links.lww.com/JCP/A735), which were combined to calculate a skin irritation score. Discomfort was reported by subjects and rated according to the experience of discomfort scale (grades 0–4; Supplementary Table 1B. Supplemental Digital Content, http://links.lww.com/JCP/A735). In this study, patch adhesion was rated according to an estimate of the percentage of patch surface in contact with skin and scored using a 5-point scale (0–4; Supplementary Table 1C. Supplemental Digital Content, http://links.lww.com/JCP/A735). The amount of adhesive residue remaining at the patch application site was graded according to an adhesive residue scale (grades 0–3; Supplementary Table 1D. Supplemental Digital Content, http://links.lww.com/JCP/A735).
In study 1, skin irritation was assessed immediately before patch application, upon removal, and 30 minutes, 1, 12, and 24 hours after removal. Patch adhesion and discomfort were assessed 2, 4, 8, 12, and 24 (immediately before removal) hours after patch application. In study 2, skin irritation was assessed each period immediately before patch application, upon removal, and 30 minutes, 24, 48, 72, and 96 hours after removal. Adhesion and discomfort were evaluated each period at 2, 4, and 12 hours after patch application and before patch removal. In study 3, skin irritation was assessed immediately before patch application and 30 minutes, 1 hour, and 24 hours after removal, and adhesion and discomfort were evaluated at 2, 4, 12, and 24 (immediately before patch removal) hours after application.
For each study, adhesive residue at the application site was assessed immediately after patch removal.
Safety
Systemic safety was assessed for each study using treatment-emergent adverse event (TEAE) monitoring, clinical laboratory test results, vital sign measurements, physical examination findings, cardiac Holter monitoring, electrocardiogram (ECG), and the Columbia-Suicide Severity Rating Scale.13
Statistical Analysis/Data Analyses
For all studies, mean plasma asenapine concentration-time profiles were presented graphically and compared, and asenapine PK parameters were summarized using descriptive statistics.
In study 1, no formal sample size calculation was performed; however, a sample of 18 subjects was determined to be adequate based on previously completed phase 1 studies for HP-3070.
In study 2, assuming that the relative to reference application site ratios for all asenapine PK parameters were within 0.9 and 1.1, the required number of subjects for bioequivalence assessment (bioequivalence limit 80%–125%) was estimated to be 32 at a power of 80% and type I error α value of 5%. Assuming a dropout rate of approximately 20%, the total number of enrolled subjects was approximately 40. Bioequivalence across 5 application sites with respect to asenapine AUC0–t, AUC0–inf, and Cmax was assessed by analysis of variance using SAS PROC MIXED, fitting a linear mixed-effect model to the natural log (ln)-transformed parameters with sequence, application site, and period as fixed effects and subject nested within sequence as a random effect.
For study 3, sample size was determined using type I error (α = 0.05) and type II error (power = 80%). Because the r value was 4 (where r = highest dose/lowest dose) in this study, dose proportionality would be confirmed if the 90% confidence intervals (CIs) for β (slope) fell entirely within the critical region (0.839, 1.161). The intrasubject coefficient of variation was assumed to be 25% or less (based on previous phase 1 studies), and the proposed sample size needed to be 18 subjects to demonstrate dose proportionality based on criteria for β. Assuming a dropout rate of approximately 25%, the sample size needed was 24. Dose proportionality was assessed by comparing Cmax,ss, Cmin,ss, and AUC0–24,ss across each dose level using a power model with mixed effects.14 The estimated slope (β1) was reported along with the 90% CIs. In a post hoc analysis of study 3 data, observed plasma concentrations were compared with the predicted plasma concentrations via a model-independent superposition method.
Statistical analyses and reporting for studies 1, 2, and 3 were performed using SAS software Version 9.1.3 or higher (SAS Institute, Inc, Cary, NC).
RESULTS
Subject Characteristics and Disposition
Demographic data for all 3 studies are available in Supplementary Table 2 (Supplemental Digital Content, http://links.lww.com/JCP/A735).
Study 1
Eighteen male and female subjects with a mean age of 26.3 years (range, 20–41 years) were enrolled. All subjects were included in the safety and PK sets. Three subjects discontinued the study: 1 subject withdrew consent, 1 was discontinued because of a protocol violation (subject failed to disclose a history of migraines, an exclusionary criterion for the study), and 1 discontinued because of physician's decision (subject presented with an unrelated fever at the second period check-in).
Study 2
Twenty White and 20 Japanese male and female subjects were enrolled. The mean age was 29.5 years (range, 20–38 years). All 40 subjects completed all study periods.
Study 3
Twenty-four male and female subjects with a mean age of 38.5 years (range, 19–63 years) were enrolled. Twenty-two subjects were included in the PK set (2 withdrew consent without complete PK data for at least 1 dose level). All 24 subjects were included in the safety analysis set.
General PK Characteristics
Single-Dose PK (Study 1)
After a single 24-hour application of HP-3070 1.9 mg/24 hours, mean plasma asenapine concentration increased gradually over time, reaching a maximum between 12 and 24 hours that was sustained throughout the duration of the 24-hour patch wear time (Fig. 1). After a single sublingual administration of asenapine 5 mg, the plasma asenapine concentration-time profile demonstrated a rapid absorption phase, with a median tmax of 1.25 hours, compared with the median tmax of 16.1 hours observed after HP-3070 1.9 mg/24 hours application. Terminal half-life (t1/2) of asenapine after HP-3070 1.9 mg/24 hours removal was 33.9 hours, and asenapine t1/2 after sublingual administration of asenapine 5 mg was 17.7 hours. The AUC values after a single application of HP-3070 1.9 mg/24 hours and a single dose of sublingual asenapine 5 mg were similar, whereas peak exposure (Cmax) for HP-3070 1.9 mg/24 hours was significantly lower than that seen with sublingual asenapine 5 mg.
FIGURE 1.
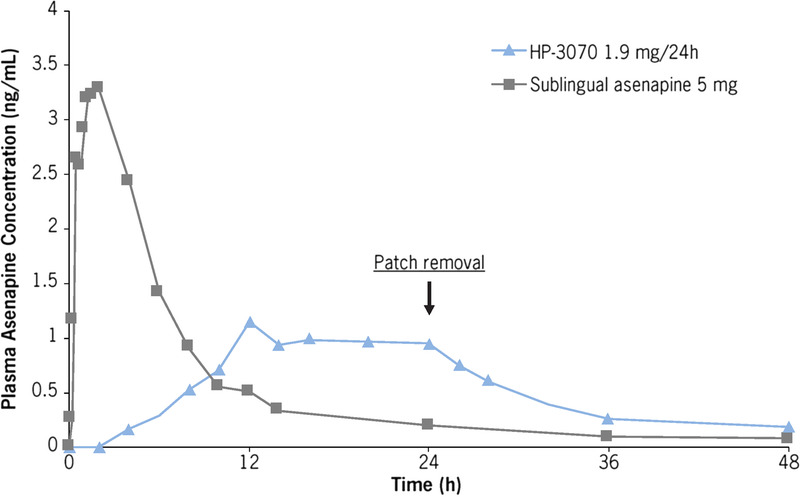
Study 1: Mean plasma asenapine concentration-time profiles after a single application of HP-3070 1.9 mg/24 hours and a single dose of sublingual asenapine 5 mg.
Multiple-Dose PK (Study 3)
With daily dosing of HP-3070 1.9 mg/24 hours, plasma asenapine concentrations reached steady state approximately 72 hours after first application of HP-3070. Plasma asenapine concentrations increased with ascending HP-3070 dose (every 7 days) and reached steady state approximately 48 hours after each dose escalation. The maximum observed plasma concentration at steady state (mean Cmax,ss) was approximately 1.5 times higher than the minimum observed concentration at steady state (mean Cmin,ss). For all dose levels, the median times to maximum observed plasma concentration at steady state (tmax,ss) were similar (range, 12–16 hours). Summary statistics of steady-state PK parameters for asenapine in plasma are shown in Table 1.
TABLE 1.
Study 3: Mean (%CV) Steady-State Asenapine PK Parameters After Multiple Applications at Each Dose in Patients With Schizophrenia
| Treatment, mg/24 h | n | AUC0–24, ng•h/mL | Cmax,ss, ng/mL | tmax,ss,* h | Cmin,ss, ng/mL | |
|---|---|---|---|---|---|---|
| HP-3070 | 1.9 mg/24 h | 22 | 22.3 (28.2) | 1.14 (30.3) | 16.0 (2.0–23.8) | 0.745 (32.4) |
| 3.8 mg/24 h | 21 | 45.8 (24.7) | 2.26 (23.9) | 16.0 (2.0–24.0) | 1.57 (25.8) | |
| 5.7 mg/24 h | 20 | 69.3 (20.0) | 3.40 (18.6) | 14.0 (2.0–23.8) | 2.33 (20.6) | |
| 7.6 mg/24 h | 20 | 96.2 (18.7) | 4.68 (17.3) | 12.0 (4.0–24.0) | 3.25 (22.5) | |
| Sublingual asenapine | 5 mg BID | 28 | 53.2 (38)† | 4.23 (45) | 1.75 (51) | N/A |
| 10 mg BID | 25 | 86.8 (53)† | 6.56 (51) | 1.96 (48) | N/A | |
*Median (range) for HP-3070 and mean (%CV) for sublingual asenapine.
†Mean AUC0–24 for sublingual asenapine was calculated as double the reported mean AUC0–12 and presented in this manner to facilitate comparisons.15
In a post hoc analysis of study 3, observed plasma asenapine concentrations were compared with simulated plasma asenapine concentrations by superposition method using single-dose PK data. Observed plasma asenapine concentrations after multiple dosing were consistent with simulated concentrations, suggesting linear PK behavior with no marked changes in PK and/or unexpected accumulation after multiple HP-3070 dosing (Fig. 2).
FIGURE 2.
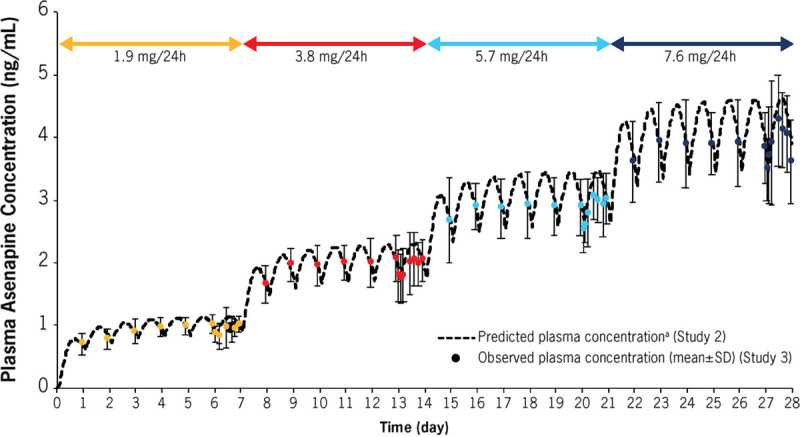
Study 2 and study 3: Comparison of observed and predicted concentration after multiple dosing of HP-3070. Data shown as mean ± SD. aObtained by superposition method using mean asenapine concentration and mean t1/2 for HP-3070 3.8 mg/24 hours (study 2, White subject data).
Relative Bioavailability (Study 1, Study 3)
In study 1, bioavailability of the HP-3070 1.9 mg/24 hours patch relative to sublingual asenapine 5 mg was determined. Comparison of asenapine relative exposure after HP 3070 1.9 mg/24 hours patch (without heat) treatment and sublingual asenapine 5 mg treatment showed that the 90% CIs of the treatment ratios (geometric mean ratios [GMRs]) of AUC0–t and AUC0–inf fell within the 0.8 to 1.25 limit, demonstrating statistically equivalent total asenapine exposure between the 2 treatments.
In study 3, AUC0–24 values observed after multiple dosing of HP-3070 (1.9, 3.8, 5.7, and 7.6 mg/24 hours) were compared with the reported mean AUC0–12 (doubled to correspond to AUC0–24) observed after twice daily (BID) dosing of sublingual asenapine 5 mg (10 days) and 10 mg (5 days).15 Similar AUC0–24 values were confirmed between HP-3070 3.8 mg/24 hours and sublingual asenapine 5 mg BID and between HP-3070 7.6 mg/24 hours and sublingual asenapine 10 mg BID, whereas Cmax for HP-3070 was lower than the Cmax values for sublingual asenapine at the corresponding doses (Table 1; Supplementary Fig. 2. Supplemental Digital Content, http://links.lww.com/JCP/A735). In addition, plasma concentration-time profiles for HP-3070 were compared with those reported for sublingual asenapine.15 The PK of sublingual asenapine displayed higher peak-to-trough fluctuations at steady state in comparison with those observed after HP-3070 doses of 3.8, 5.7, and 7.6 mg/24 hours (Fig. 3).15
FIGURE 3.
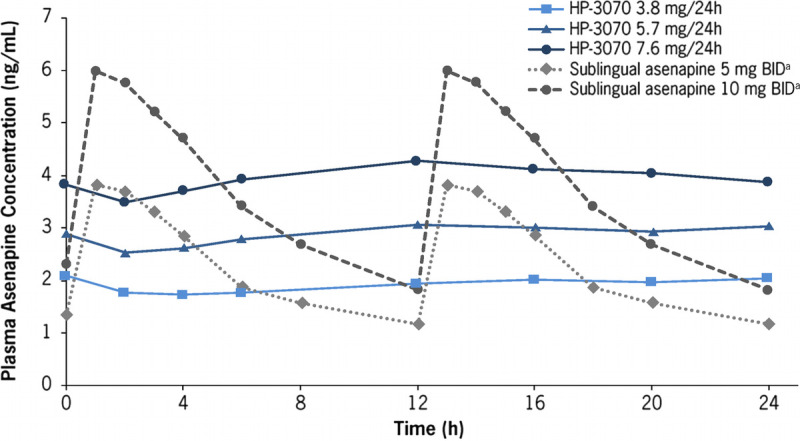
Study 3: Mean plasma asenapine concentration-time profiles for HP-3070 3.8, 5.7, and 7.6 mg/24 hours at steady state and for sublingual asenapine 5 and 10 mg BID in patients with schizophrenia. aPlasma concentrations for sublingual asenapine were depicted based on the reported time-concentration profiles for sublingual asenapine, which represent a 12-hour time course and are repeated for comparison purposes.15
Dose Proportionality (Study 3)
Asenapine AUC0–24,ss, Cmax,ss, and Cmin,ss after application of HP-3070 1.9, 3.8, 5.7, and 7.6 mg/24 hours are shown in Table 1. Estimated β1 and 90% CIs across doses for AUC0–24,ss (Supplementary Fig. 2A. Supplemental Digital Content, http://links.lww.com/JCP/A735), Cmax,ss (Supplementary Fig. 2B. Supplemental Digital Content, http://links.lww.com/JCP/A735), and Cmin,ss (β = 1.09, 90% CI = 1.03–1.14) fell within the predefined criteria of 0.839 to 1.161, confirming dose proportionality for asenapine across the range of HP-3070 doses.
Influence of Intrinsic and Extrinsic Factors on HP-3070 PK (Study 1, Study 2)
Plasma concentrations were similar after single applications of HP-3070 3.8 mg/24 hours to 5 different application sites (abdomen, hip, upper arm, upper back, and upper chest; Fig. 4A, Table 2). The 90% CIs of the GMRs for asenapine by patch application site were within the 80% to 125% bioequivalence limit for Cmax, AUC0–t, and AUC0–inf, indicating bioequivalence of asenapine exposure among the different application sites. The only observed exception was Cmax for the abdomen compared with the upper arm, for which the lower boundary was slightly outside the criterion (Table 2).
FIGURE 4.
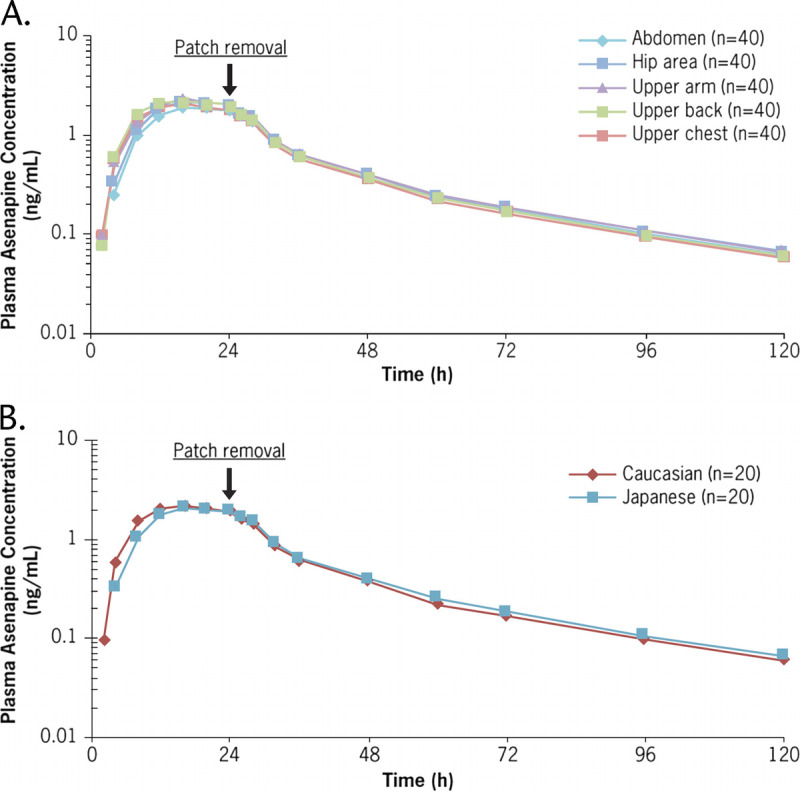
Study 2: Mean plasma asenapine concentration-time profiles by (A) site of application and (B) ethnic group after a single dose of HP-3070 3.8 mg/24 hours.
TABLE 2.
Study 2: Asenapine PK Parameters and Statistical Analysis of Relative Exposure by Application Site and Ethnic Group
| n | Cmax, ng/mL | AUC0–t, ng•h/mL | AUC0–inf, ng•h/mL | tmax, Median (Range), h | t1/2, Mean (%CV), h | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (%CV) | GMR (90% CI) | Mean (%CV) | GMR (90% CI) | Mean (%CV) | GMR (90% CI) | |||||
| Application site | Abdomen | 40 | 2.02 (25.6) | 81.9 (77.6* to 86.5) | 60.3 (22.3) | 85.8 (81.7 to 90.0) | 63.2 (22.5%) | 85.9 (81.9 to 90.2) | 20.0 (8.00–28.0) | 30.2 (17.9) |
| Hip area | 40 | 2.30 (23.6) | 93.5 (88.6 to 98.7) | 66.7 (20.7) | 95.1 (90.6 to 99.8) | 69.9 (20.2) | 95.6 (91.1 to 100) | 20.0 (8.00–28.0) | 31.3 (25.1) | |
| Upper back | 40 | 2.41 (20.3) | 98.8 (93.6 to 104) | 69.7 (17.8) | 100 (95.3 to 105) | 72.5 (18.1) | 99.6 (94.9 to 104) | 16.0 (8.00–24.0) | 30.5 (20.7) | |
| Upper chest | 40 | 2.15 (18.8) | 88.2 (83.6 to 93.1) | 63.6 (17.7) | 91.2 (86.9 to 95.8) | 66.2 (18.1) | 90.9 (86.6 to 95.3) | 16.0 (8.00–24.0) | 29.7 (23.1) | |
| Upper arm | 40 | 2.46 (23.8) | 70.1 (20.3) | 73.2 (20.2) | 16.0 (8.00–28.0) | 30.5 (23.5) | ||||
| Ethnic group | White | 20 | 2.31 (24.5) | 67.4 (21.3) | 70.3 (21.0) | 16.0 (8.0–28.0) | 31.0 (24.1) | |||
| Japanese | 20 | 2.23 (22.3) | 97.4 (88.3 to 107) | 64.7 (19.2) | 96.3 (88.5 to 105) | 67.7 (19.6) | 96.5 (88.7 to 105) | 16.0 (8.0–28.0) | 29.9 (19.7) | |
Reference data for GMR estimation were upper arm for application site and White for ethnic group.
*90% CI not entirely within the critical region.
After a single application of HP 3070 3.8 mg/24 hours in White and Japanese subjects, asenapine plasma concentrations were similar for the 2 ethnic groups (Fig. 4B, Table 2). The median asenapine tmax was 16.0 hours for both White and Japanese subjects, and the mean asenapine t1/2 was 31.0 hours for White subjects and 29.9 hours for Japanese subjects (Fig. 4B, Table 2). The percent coefficient of variations (%CVs) for Cmax, AUC0–t, and AUC0–inf ranged from 22.3% to 24.5% for Cmax, from 19.2% to 21.3% for AUC0–t, and from 19.6% to 21.0% for AUC0–inf, confirming that the variability of asenapine exposure was similar among the 2 populations (Table 2).
When a heating pad was applied for 8 consecutive hours on the HP-3070 1.9 mg/24 hours patch (upper arm), a faster asenapine absorption rate was observed (Fig. 5). The median tmax without an external heat source was approximately 16 hours; the median tmax with an external heat source was approximately 8 hours (Table 3). The mean asenapine partial AUC as measured by AUC0–8 was also significantly greater in the presence of 8 hours of external heat (5.37 ng•h/mL) versus without (1.46 ng•h/mL; Table 3). However, peak and overall asenapine exposure, measured as Cmax and AUC0–inf, respectively, were not significantly affected by external heat application (Table 3).
FIGURE 5.
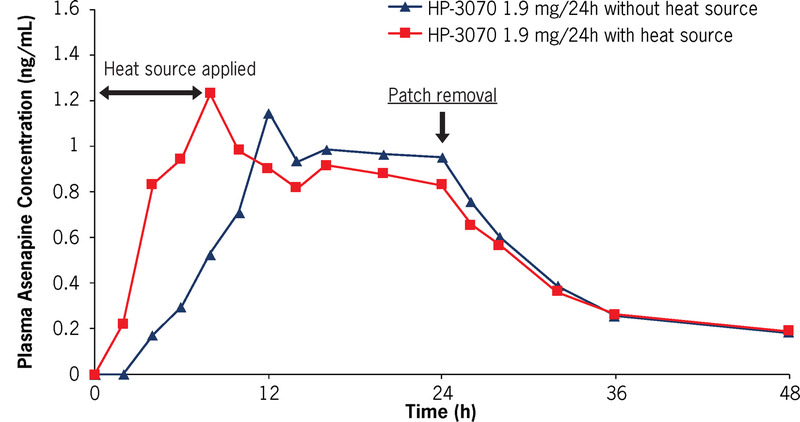
Study 1: Mean plasma asenapine concentration-time profiles after a single dose of HP-3070 1.9 mg/24 hours with and without heat source.
TABLE 3.
Study 1: Mean (%CV) Asenapine PK Parameters in Healthy Subjects
| HP-3070 1.9 mg/24 h No Heat Application (n = 17) | HP-3070 1.9 mg/24 h 8-h Heating Pad Application (n = 16) | Sublingual Asenapine 5 mg (n = 16) | |
|---|---|---|---|
| AUC0–8, ng•h/mL | 1.46 (54.8) | 5.37 (49.8) | N/A |
| AUC0–t, ng•h/mL | 29.7 (23.5) | 33.0 (28.3) | 26.0 (20.6) |
| AUC0–inf, ng•h/mL | 32.8 (25.7)* | 35.7 (29.2) | 27.8 (18.9) |
| Cmax, ng/mL | 1.27 (67.3) | 1.34 (33.0) | 3.96 (27.8) |
| tmax, h† | 16.1 (12.0–24.0) | 8.00 (4.00–24.0) | 1.25 (0.500–2.00) |
| t1/2, h | 33.9 (59.0) | 27.1 (45.8) | 17.7 (53.0) |
*n = 16.
†Median (range).
Apparent Dose
The percent of drug released and apparent dose of asenapine maleate delivered from the patch were determined after patch removal for each study. Across studies, an average of approximately 60% of the drug was released from the patch over 24 hours. Results are shown in Supplementary Table 3 (Supplemental Digital Content, http://links.lww.com/JCP/A735).
Dermal Assessments and Patch Adhesion
Across all 3 studies, 81 subjects received at least 1 HP-3070 patch. With respect to skin irritation and discomfort, HP-3070 transdermal patches were generally well tolerated across all studies (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). In addition, no patient withdrew from the studies because of skin irritation or discomfort. More than 90% of patches remained adhered throughout the 24-hour wear time, and in only 2 instances did the patch completely detach. In most cases, adhesive residue was not observed on the skin after patch removal, with only light adhesive residue noted occasionally.
Skin Irritation and Discomfort
For this study, a clinically meaningful degree of irritation is defined as a combined irritation score (dermal response + other effects) of 3 or greater (erythema and papules). No subject in study 1 reported a meaningful degree of skin irritation or patch discomfort at any point in the study, regardless of whether heat was applied (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735).
One subject in study 2 reported a combined skin irritation score of 3 or greater when the HP-3070 3.8 mg/24 hours patch was applied to the upper back (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). In study 2, a discomfort score of 0 (no discomfort) was reported for the upper back and upper arm application sites at all evaluation time points. A score of 1 (mild discomfort) was reported when HP-3070 was applied to the upper chest (1 instance), hip area (3 instances), and abdomen (4 instances). Most White and Japanese subjects enrolled reported no discomfort at most evaluation time points. Among White subjects, 6 instances of mild discomfort were reported after patch application, and among Japanese subjects, 3 instances of mild discomfort were reported (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735).
In study 3, no meaningful degree of irritation was reported for the 1.9 or 3.8 mg/24 hours doses (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). A combined skin irritation score of 3 was observed for 1 subject (4.8%) at the 5.7 mg/24 hours dose level only, and a score of 4 (definite edema) was observed for 1 subject at both the 5.7 mg/24 hours (4.8%) and 7.6 mg/24 hours (5.0%) doses (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). Among each study 3 treatment group, 75% or more of the subjects reported a discomfort score of 0 (no discomfort) and mild discomfort was reported by 16 subjects (18.4%). Moderate but tolerable discomfort was reported in 2 subjects (10%) who received HP-3070 7.6 mg/24 hours.
Patch Adhesion
For most subjects, patch adherence was greater than 90% (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). No premature patch detachments occurred during study 1. All patches in the HP-3070 without heat treatment group had a flawless adhesion score at all time points, whereas 2 subjects (12.5%) who received an HP-3070 patch with heat applied for 8 hours experienced 75% or greater to less than 90% adhesion (adhesion score = 1), with some edges lifting off the skin (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). No subjects experienced a partial detachment, defined as an adhesion score of 2 or 3.
In study 2, patch adhesion was rated as 90% or greater adhered (essentially no lift off the skin) for most patches at most time points (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). Only 1 subject experienced a meaningful degree of detachment at the abdomen site (≥50% to <75% adhered). No difference in patch adhesion was seen between White and Japanese subjects and among the upper back, abdomen, upper chest, hip, and upper arm application sites. In study 3, a multiple-dose study, adhesion scores of 0 (≥90% adhered) were reported by 95.9%, 91.4%, 87.4%, and 79.8% of the subjects for patch formulations of 1.9, 3.8, 5.7, and 7.6 mg/24 hours, respectively (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). Partial detachments (<75% adhered but not detached) occurred during less than 5% of individual patch applications. Complete detachment from the skin was observed for one 1.9 mg/24 hours patch and one 5.7 mg/24 hours patch.
Adhesive Residue
In study 1, the most severe recorded adhesive residue score was 1 (light residue), occurring in 1 subject (5.9%) after removal of HP-3070 1.9 mg/24 hours without heat (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). No adhesive residue was observed in the subjects receiving HP-3070 with heat. In study 2, the most severe adhesive residue score observed was 1 (light residue), seen in 5 subjects (3.1%) after removal of the HP-3070 3.8 mg/24 hours patch from any site (Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). In study 3, for the 1.9 mg/24 hours dose group, the most severe adhesive residue score observed was 2 (medium residue), occurring in 2 subjects (8.3%). No adhesive residue scores greater than 1 were reported for any other study 3 dosing group.
Safety and Tolerability
Across all studies, most TEAEs determined by investigators to be related to the study drug among subjects receiving HP-3070 were rated mild in severity (93.8% [60/64], 82.7% [62/75], and 75.8% [25/33] of TEAEs were mild in studies 1, 2, and 3, respectively). No deaths or serious TEAEs were reported during any of the studies, and no subjects discontinued any study because of TEAEs. Across all treatment groups in all 3 studies, the most frequently reported TEAEs determined by investigators to be related to study treatment were somnolence (11.9%), application site erythema (reported as application site irritation in study 2 and application site discoloration and reaction in study 3, 7.4%), dizziness (4.7%), headache (3.3%), insomnia (3.3%), and fatigue (2.7%; Supplementary Table 4. Supplemental Digital Content, http://links.lww.com/JCP/A735). Three of the reported instances of somnolence and 4 of the instances of dizziness deemed related to study medication were moderate in severity, and the rest were mild. All reported instances of application site TEAEs related to study treatment were mild.
No clinically significant findings were observed with respect to vital signs, cardiac monitoring, or ECG results in any of the studies. In studies 2 and 3, there were no findings of relevance with respect to Columbia-Suicide Severity Rating Scale assessments. In study 3, there were no clinically significant findings for PANSS assessments. For 3 study 3 subjects, an increase in pulse rate was observed during the study, and tachycardia was observed for 3 subjects during 24-hour cardiac Holter evaluation. For 1 subject, tachycardia was reported as a TEAE considered related to the study medication. Except for an unrelated, mild TEAE of pyrexia that 1 study 2 subject presented with at check-in to the second period, none of the subjects experienced a TEAE associated with abnormal vital signs, physical examinations, or 12-lead ECGs.
DISCUSSION
HP-3070 is the first transdermal patch approved for use in patients with schizophrenia in the United States. In healthy volunteers, single-dose administration of HP-3070 1.9 mg/24 hours resulted in a lower peak asenapine concentration compared with a single dose of sublingual asenapine 5 mg, with a similar overall systemic exposure, as measured by AUC. After initiating daily dosing of HP-3070, steady-state plasma concentrations were achieved in approximately 72 hours, and stable plasma asenapine concentrations with minimal peak-to-trough fluctuations (~1.5 fold) were observed. In contrast, peak-to-trough ratios during steady-state dosing with sublingual asenapine have been reported to be approximately 2.6 to 3.15,16
Dose proportionality in asenapine exposure at steady state was established in the range of HP-3070 doses from 1.9 to 7.6 mg/24 hours. In PK studies of sublingual asenapine, increasing the dose from 5 to 10 mg BID resulted in sublinear (1.7×) increases in both exposure and maximum concentration.15
The AUC for daily dosing of HP-3070 3.8 mg/24 hours was comparable with the AUC reported for sublingual asenapine 5 mg BID, and the AUC after daily HP-3070 7.6 mg/24 hours was similar to that observed after sublingual asenapine 10 mg BID.15 These comparisons with sublingual asenapine exposure informed the design of the phase 3 clinical efficacy trial of HP-3070, in which doses of 3.8 and 7.6 mg/24 hours established the efficacy and safety profile of HP-3070 in patients with schizophrenia.11 In that randomized, placebo-controlled clinical trial of HP-3070 in adult patients with schizophrenia, both HP-3070 doses demonstrated significant improvement in PANSS total scores and Clinical Global Impression–severity of illness scores after 6 weeks of treatment compared with placebo.
Because it has been well established that drug absorption after transdermal administration can be affected by both site of application and external heating on transdermal products, those external factors were assessed for HP-3070.17,18 Consistent asenapine exposure, as measured by AUC, among 5 different application sites (upper arm [reference site], upper chest, upper back, abdomen, and hip area) was demonstrated, indicating that HP-3070 can provide similar drug exposure across multiple application sites. This equivalence offers patients and caregivers application options to account for patient comfort and preference and minimizes skin safety issues, such as skin irritation or discomfort. Application of external heat on the HP-3070 patch caused an increase in the rate of asenapine absorption (tmax) but did not significantly affect the Cmax, AUC0–t, or AUC0–inf of asenapine. The clinical relevance of this is reflected in the instructions contained in product labeling, in which patients are advised to avoid exposing patches that they are wearing to direct external heat sources, such as hair dryers, heating pads, electric blankets, and heated water beds. It was also observed that asenapine PK after HP-3070 3.8 mg/24 hours treatment was similar between White and Japanese subjects, indicating no PK differences across these ethnic groups.
HP-3070 treatment resulted in no unexpected dermal safety concerns, with an application site reaction profile similar to what has already been observed with transdermal patches for other psychiatric conditions.11,19–22 In the present studies, most subjects did not report any skin irritation or discomfort. Skin irritation and discomfort seemed to worsen at the higher dose levels in study 3, which were the last doses applied as part of the ascending-dose study design. The observed increase in irritation and discomfort was consistent with repeated use of application sites, as any lingering irritation from a previous patch may have been present when the next patch was applied. To minimize this issue during clinical use, patients should be encouraged to rotate application sites regularly.3 Minimal variability in irritation or discomfort was observed between patch application sites, with no irritation or discomfort reported in the presence or absence of external heat in study 1. Patch adhesion was consistently good, with more than 90% adhesion for most of the subjects and a low rate of partial detachments in each study. Among the subjects receiving study treatment in all studies, complete patch detachment occurred in only 2 instances. Adhesive residue was also infrequently observed overall.
The most frequently reported nondermal TEAEs were somnolence, dizziness, headache, insomnia, and fatigue. Somnolence and dizziness are among the adverse reactions most commonly observed with sublingual asenapine.10,23,24 In the present PK study, the incidence rates of somnolence and dizziness observed in subjects receiving HP-3070 were similar to or lower than those occurring in subjects receiving sublingual asenapine (study 1). Approximately 50% of the subjects who received sublingual asenapine experienced somnolence, whereas no subjects reported somnolence after receiving HP-3070, although somnolence was reported at rates of 5% to 18% among subjects receiving HP-3070 in the other 2 studies. Similarly, dizziness was experienced by approximately 35% of the subjects receiving sublingual asenapine compared with 9% of the subjects receiving HP-3070. In this study, equivalent total asenapine exposure between the HP 3070 1.9 mg/24 hours patch and sublingual asenapine 5 mg BID was confirmed, although peak exposure for HP-3070 was substantially lower than that for sublingual asenapine. Steadier plasma concentration evidenced by lower peak-to-trough variability has been associated in the literature with better tolerability,25 but no head-to-head study of HP-3070 and sublingual asenapine in patients with schizophrenia has been conducted, and further data would be needed to confirm such an assertion.
In conclusion, a single application of HP-3070 results in asenapine exposure similar to those observed with approved BID doses of sublingual asenapine but with lower Cmax and with consistent plasma levels over the dosing interval. Repeated daily doses of HP-3070 result in dose-proportional steady-state plasma levels of asenapine with minimal peak-to-trough fluctuations. These findings, taken together with results from the pivotal phase 3 study of HP-3070, which established efficacy and safety,11 suggest that HP-3070 is an additional treatment option for patients with schizophrenia.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Yoshinobu Hayakawa, MPharm, for his contributions to this article. Medical writing and editorial assistance were provided by Michelle L. Jones, PhD, MWC, and Anthony DiLauro, PhD, of PharmaWrite, LLC, and were funded by Hisamitsu Pharmaceutical Co, Inc. This article was prepared according to the International Society for Medical Publication Professionals' “Good Publication Practice for Communicating Company-Sponsored Medical Research: The GPP3 Guidelines.”
AUTHOR DISCLOSURE INFORMATION
K.S., M.C., M.K., and B.S. received nonfinancial support from Hisamitsu Pharmaceutical Co, Inc, during the conduct of the study and personal fees from Noven Pharmaceuticals, Inc, outside the submitted work. T.T. received nonfinancial support from Hisamitsu Pharmaceutical Co, Inc, and personal fees from Hisamitsu Pharmaceutical Co, Inc, outside the submitted work. L.C. received nonfinancial support from Hisamitsu Pharmaceutical Co, Inc, and personal fees from Noven Pharmaceuticals, Inc, during the conduct of the study; personal fees from Acadia, Alexza, Alkermes, Allergan, AstraZeneca, Avanir, BioXcel, Boehringer Ingelheim, Bristol-Myers Squibb, Eisai, Eli Lilly, Forum, Genentech, Impel, Indivior, Intra-Cellular Therapies, Janssen, Jazz, Lundbeck, Luye, Medivation, Meiji, Merck, Mylan, Neurocrine, Novartis, Noven, Osmotica, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Sage, Shire, Sunovion, Takeda, Teva, Valeant, and Vanda, outside the submitted work; stocks (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer purchased more than 10 years ago; and royalties from Springer Healthcare (book), UpToDate (reviewer), and Wiley (Editor-in-Chief through end 2019, International Journal of Clinical Practice).
This study was supported by Hisamitsu Pharmaceutical Co, Inc.
Footnotes
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.psychopharmacology.com).
Contributor Information
Katsumi Suzuki, Email: KSuzuki@noven.com.
Mariacristina Castelli, Email: mcastelli@noven.com.
Marina Komaroff, Email: mkomaroff@noven.com.
Takaaki Terahara, Email: takaaki_terahara@hisamitsu.co.jp.
Leslie Citrome, Email: nntman@gmail.com.
REFERENCES
- 1.Brissos S Veguilla MR Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4:198–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suresh A, Narayan R, Nayak UY. Recent advances in the development of asenapine formulations. Expert Opin Drug Deliv. 2020;17:1377–1393. [DOI] [PubMed] [Google Scholar]
- 3.Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic. Int J Clin Pract. 2009;63:1762–1784. [DOI] [PubMed] [Google Scholar]
- 5.Landbloom R Mackle M Wu X, et al. Asenapine for the treatment of adults with an acute exacerbation of schizophrenia: results from a randomized, double-blind, fixed-dose, placebo-controlled trial with olanzapine as an active control. CNS Spectr. 2017;22:333–341. [DOI] [PubMed] [Google Scholar]
- 6.Citrome L. Asenapine review, part II: clinical efficacy, safety and tolerability. Expert Opin Drug Saf. 2014;13:803–830. [DOI] [PubMed] [Google Scholar]
- 7.Abruzzo A Cerchiara T Luppi B, et al. Transdermal delivery of antipsychotics: rationale and current status. CNS Drugs. 2019;33:849–865. [DOI] [PubMed] [Google Scholar]
- 8.Harvey PD, Nakamura H, Murasaki M. Blonanserin versus haloperidol in Japanese patients with schizophrenia: a phase 3, 8-week, double-blind, multicenter, randomized controlled study. Neuropsychopharmacol Rep. 2019;39:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwata N Ishigooka J Naoi I, et al. Long-term safety and efficacy of blonanserin transdermal patches in Japanese patients with schizophrenia: a 52-week open-label, multicenter study. CNS Drugs. 2020;34:103–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou M Derakhshanian S Rath A, et al. Asenapine transdermal patch for the management of schizophrenia. Psychopharmacol Bull. 2020;50:60–82. [PMC free article] [PubMed] [Google Scholar]
- 11.Citrome L Walling DP Zeni CM, et al. Efficacy and safety of HP-3070, an asenapine transdermal system, in patients with schizophrenia: a phase 3, randomized, placebo-controlled study. J Clin Psychiatry. 2021;82:20m13602. [DOI] [PubMed] [Google Scholar]
- 12.Berger RS, Bowman JP. A reappraisal of the 21-day cumulative irritation test in man. Cutan Ocul Toxicol. 1982;1:109–115. [Google Scholar]
- 13.Posner K Brown GK Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith BP Vandenhende FR DeSante KA, et al. Confidence interval criteria for assessment of dose proportionality. Pharm Res. 2000;17:1278–1283. [DOI] [PubMed] [Google Scholar]
- 15.Chapel S Hutmacher MM Haig G, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49:1297–1308. [DOI] [PubMed] [Google Scholar]
- 16.de Greef R Maloney A Olsson-Gisleskog P, et al. Dopamine D2 occupancy as a biomarker for antipsychotics: quantifying the relationship with efficacy and extrapyramidal symptoms. AAPS J. 2011;13:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastore MN Kalia YN Horstmann M, et al. Transdermal patches: history, development and pharmacology. Br J Pharmacol. 2015;172:2179–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao J Ghosh P Li SK, et al. Heat effects on drug delivery across human skin. Expert Opin Drug Deliv. 2016;13:755–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Citrome L, Zeni CM, Correll CU. Patches: established and emerging transdermal treatments in psychiatry. J Clin Psychiatry. 2019;80:18nr12554. [DOI] [PubMed] [Google Scholar]
- 20.Poewe WH Rascol O Quinn N, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 2007;6:513–520. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson Study Group . A controlled trial of rotigotine monotherapy in early Parkinson's disease. Arch Neurol. 2003;60:1721–1728. [DOI] [PubMed] [Google Scholar]
- 22.Citrome L, Goldberg JF, Portland KB. Placing transdermal selegiline for major depressive disorder into clinical context: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord. 2013;151:409–417. [DOI] [PubMed] [Google Scholar]
- 23.Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psychiatry. 2007;68:1492–1500. [DOI] [PubMed] [Google Scholar]
- 24.Kane JM Cohen M Zhao J, et al. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30:106–115. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan JJ Reilly KR Fu DJ, et al. Comparison of the peak-to-trough fluctuation in plasma concentration of long-acting injectable antipsychotics and their oral equivalents. Innov Clin Neurosci. 2012;9:17–23. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


