Abstract
Background
Colonic tuft cells are epithelial chemosensory cells involved in barrier integrity, modulation of inflammatory responses and gut homeostasis. Recent evidence indicates an involvement of tuft cells in ulcerative colitis pathogenesis, though mechanisms remain largely unknown.
Here, we quantified the colonic tuft cell population in patients with quiescent ulcerative colitis as compared to patients without identified colonic disease (controls).
Methods
In this retrospective study, we obtained endoscopic colonic sigmoid biopsies from 14 patients with quiescent ulcerative colitis and from 17 controls. In a blinded central-reading design, we identified tuft cells by immunohistochemistry using a cyclooxygenase-1 antibody as a marker and performed a simple counting by visual inspection. Poisson regression was employed for statistics and results were adjusted for gender, age and smoking status.
Results
Ulcerative colitis patients demonstrated a 55% reduced tuft cell count in colonic mucosa compared with the control group (95% confidence limit: range 31–71%, P = 0.0002). Ulcerative colitis patients had a mean tuft cells count of 46 tuft cells/mm2 (95% CI, 36–59), while controls demonstrated a mean of 104 tuft cells/mm2 (95% CI, 79–136). No interactions of other covariates, such as age, smoking status, total duration of ulcerative colitis disease and duration of clinical remission prior to study inclusion were detected between ulcerative colitis patients and controls.
Conclusion
Quiescent ulcerative colitis patients have a relatively low number of colonic tuft cells. Further studies are warranted to explore the potential involvement of tuft cells in ulcerative colitis pathogenesis.
Keywords: cyclooxygenase 1, doublecortin-like kinase 1, inflammatory bowel disease, trichuris suis ova, ulcerative colitis
Introduction
Tuft cells were discovered more than 60 years ago, and yet their function and implication in human disease are still not understood [1]. They occupy a minor fraction of the epithelial lining of several tissues, such as the respiratory tract, ducts of the pancreas, testicular ducts and various parts of the gastrointestinal tract [2]. In adult mice, tuft cells constitute less than 1 % of the intestinal epithelial cells, where they apparently function as chemosensory cells, important for maintaining homeostasis, mucosal barrier integrity, and even orchestrating immunologic responses towards helminthic and protozoan infections [3–10].
A suspected involvement of tuft cells in various diseases is steadily attracting more attention by basic and clinical researchers. Especially, involvement in the pathogenesis of ulcerative colitis and colitis-associated cancer has been proposed [11,12].
Ulcerative colitis is an idiopathic chronic inflammatory bowel disease (IBD) characterized by latent quiescent periods exacerbated by sudden relapses of colonic mucosal inflammation with abdominal pain, increased stool frequency and bloody diarrhea [13]. The pathogenesis, including factors that trigger initiation and relapse of ulcerative colitis disease activity, is not fully understood. Interestingly, a growing body of preclinical evidence supports tuft cells to play a role in protection against certain enteric infections and inflammation, albeit investigations are still needed to show a causal and direct involvement of tuft cells in any human gastrointestinal disease [11]. So far, very few studies have focused on tuft cells in man. Based on animal studies, however, tuft cells are likely pivotal for sustaining colonic mucosal barrier integrity and alleviating inflammatory responses [7,9,11,14–16].
In terms of inflammation, tuft cells have recently been identified as the predominant colonic epithelial cell-derived source of interleukin 25 (IL-25), an important factor in the initiation of type 2 immune responses and recruitment of eosinophils [3,4,9,10,17,18]. Interestingly, IL-25 levels are lower in both serum and colonic mucosal biopsies from ulcerative colitis patients with active disease. Similar trends exist for patients with quiescent ulcerative colitis disease [19].
In this study, we test the hypothesis that patients with quiescent ulcerative colitis have an altered number of colonic tuft cells. By quantifying the colonic tuft cell populations, we compared the tuft cell counts in quiescent ulcerative colitis patients (i.e., clinical, endoscopic and histologic remission) to controls (without ulcerative colitis). Furthermore, we evaluated cyclooxygenase-1 (COX-1), cytokeratin 18 (CK18), hematopoietic prostaglandin D synthase (HPGDS) and doublecortin-like kinase 1 (Dclk-1) as potential tuft cells markers. The most complete staining of the tuft cell population was achieved with a COX-1 antibody and thus used for tuft cell identification.
Materials and methods
Study population
We included colonic sigmoid biopsies retrospectively from ulcerative colitis patients referred to endoscopy for disease management. The control group consisted of patients referred for a colonoscopy on nonspecific suspicion of colonic disease due to symptoms such as abdominal pain and altered stool pattern. Controls were deemed colon healthy based on normal findings by endoscopy and biopsy histology. Biopsies from 31 subjects (14 with quiescent ulcerative colitis and 17 controls) were included. All ulcerative colitis patients were in clinical, endoscopic and histologic remission. Clinical and endoscopic remission was defined by a total Mayo score ≤2 and no subscore >1 [20], and histologic remission by a Nancy histological index score = 0, Fig. 1 [21]. Most ulcerative colitis patients (86%, 12/14) had been in clinical remission for more than 3 months prior to study inclusion and the majority (86%, 12/14) had a Mayo endoscopic subscore of 0. Only two of the ulcerative colitis patients (14%, 2/14) had a Mayo endoscopic subscore of 1 in the rectum, and thus not at biopsy site. Controls had normal endoscopic and histological findings. The number of tuft cells was determined in a blinded fashion by simple visual counting of COX-1 immuno-positive cells in the epithelial lining of colonic biopsies from quiescent ulcerative colitis patients and controls, as shown in Fig. 2a. Patients were excluded if suffering from other acute or chronic gastrointestinal diseases; that is, colonic neoplasia or diverticulosis, celiac disease, dyspepsia, lactose intolerance and irritable bowel syndrome. Patients were also excluded if regularly treated with a nonsteroidal anti-inflammatory drug or nonselective COX-inhibitor, as COX-positive tuft cells might be affected. Other use of medication, including preventive treatment in ulcerative colitis patients, was allowed. Basic patient characteristics, including medications are listed in Table 1.
Fig. 1.
H&E staining of colonic mucosal biopsy (× 200 magnification). Representative histological features in included subject with quiescent ulcerative colitis. All ulcerative colitis patients were in histologic remission defined by a Nancy histological score of 0.
Fig. 2.
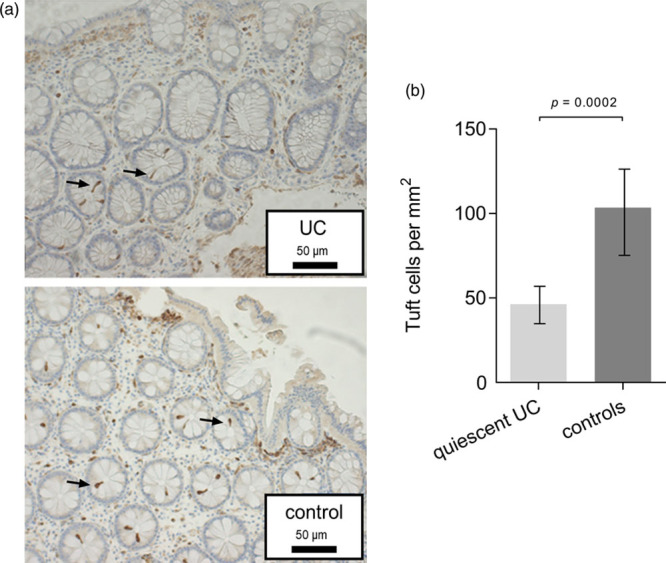
Tuft cells in human sigmoid colon. (a) Examples of immunohistochemical staining of colonic tuft cells with cyclooxygenase-1 specific antibody in a patient with quiescent ulcerative colitis and a control patient. Enlargement × 200. Arrows indicate tuft cells. (b) Colonic mucosal tuft cell numbers per square millimeter in 14 patients with quiescent ulcerative colitis compared to 17 controls. Data presented as mean tuft cells/mm2. The P value is based on Poisson regression with covariate adjustment.
Table 1.
Study population characteristics
| Ulcerative colitis | Controls | |
|---|---|---|
| Total number | 14 | 17 |
| Males/females | 9/5 | 9/8 |
| Mean age, years (range) | 39 (23–75) | 46 (20–68) |
| Smoking habit | ||
| Active/nonsmoker | 0/14 | 0/17 |
| History of maximum disease extent | ||
| Proctitis | 3 | N/A |
| Left sided colitis | 4 | N/A |
| Pancolitis | 7 | N/A |
| Disease duration, mean months (range) | 124 (3–324) | N/A |
| Remission duration, mean months (range) | 14 (2–61) | N/A |
| Medication | ||
| No treatment | 3 | 5 |
| 5-ASA | 5 | 0 |
| Anti-TNFα | 2 | 0 |
| Azathioprine | 3 | 0 |
| Antiepileptic | 1 | 0 |
| Antihistamine | 0 | 2 |
| Inhaler (β2-agonist, steroid) | 0 | 2 |
| Contraception | 0 | 1 |
| Proton pump inhibitor | 0 | 1 |
| Statins | 0 | 2 |
| Thiazides | 0 | 2 |
| Thyroid hormone | 0 | 2 |
| Vitamin/iron-supplements | 1 | 2 |
Baseline values for patients with quiescent ulcerative colitis and controls.
5-ASA, 5-aminosalicylic acid; N/A, non-applicable; TNFα, tumor necrosis factor alpha.
All endoscopies were performed and assessed by local physicians. To ensure nonbiased consistent disease activity assessment by Mayo endo subscore, all endoscopies from ulcerative colitis patients were recorded and evaluated with a blinded central reading by an external experienced gastroenterologist.
Biopsy collection and preparation
Biopsies were extracted from the sigmoid colon about 30 cm from the anal verge, on retraction of the endoscope, using standard biopsy forceps (Boston Scientific, Radical Jaw 4, outside diameter of 2.2 mm). Biopsies were fixed immediately in 4% paraformaldehyde and subsequently embedded in paraffin. Upon further preparation, biopsies were cut in 4 µm thick sections.
Immunohistochemical staining and identification of colonic tuft cells
For immunofluorescence staining, paraffin colon sections were dewaxed, rehydrated and blocked in blocking buffer (2% BSA and PBS) and incubated overnight at 4°C. Antibodies for COX-1, CK18, HPGDS and two antibodies for Dclk-1 were used. Primary antibodies were detected with AlexaFluor-conjugated secondary antibodies, Table 2. Tissue sections were mounted using ProLong Gold Antifade Mountant with DAPI (P36931, Thermo Fisher Scientific) and analyzed using an IX71 Olympus microscope and XM10 Olympus camera. For quantification of tuft cells numbers, tissue was treated as described above and incubated with anti-COX-1 overnight at 4°C followed by incubation with biotinylated secondary antibodies diluted 1:200 in blocking buffer. After washing, endogenous peroxidase was blocked with 3% H2O2 in PBS, and sections were incubated with vectastain reagents (Vectastain ABC Kit PK 4000, Vector laboratories Inc., California, USA) and stained with 3’-Diaminobenzidine solution (Cat. No. 4170, Kementec Diagnostics, Denmark) and counterstained with Mayer’s Haematoxylin (Ampliqon, Denmark).To identify a suitable tuft cell marker for the quantification studies, we applied double-labeling immunohistochemistry on human colon sections and compared three antibodies specific for tuft cell marker proteins; anti-COX-1, HPGDS and CK18 staining, Fig. 3. Furthermore, we also tested two antibodies specific for the murine Dclk-1 (ab37994 and ab31704, Abcam) on the murine and human colon.
Table 2.
Summary of the antibodies used
| Peptide target | Manufacturer/cat. no. | Host | Immunohistochemistry dilutions |
|---|---|---|---|
| COX-1 | Santa Cruz Biotechnology/sc-1752 | Goat | 1:100 |
| Cytokeratin-18 | Cayman/160013 | Mouse | 1:25 |
| Hematopoietic prostaglandin D synthase | Progen/61028 | Rabbit | 1:100 |
| Doublecortin-like kinase 1 | Abcam/ab37994 | Rabbit | 1:25 |
| Doublecortin-like kinase 1 | Abcam/ab31704 | Rabbit | 1:700 |
| Alexa fluor 488 anti-Goat IgG | Thermo Fischer Scientific/A11055 | Donkey | 1:200 |
| Alexa fluor 568 anti-Rabbit IgG | Thermo Fischer Scientific/A10042 | Donkey | 1:200 |
| Alexa fluor 568 anti-mouse IgG | Thermo Fischer Scientific/A10037 | Donkey | 1:200 |
| Alexa fluor 488 anti-Rabbit IgG | Thermo Fischer Scientific/A21206 | Donkey | 1:200 |
| Goat Biotinylated anti-rabbit IgG | Vector Labs/BA-1000 | Goat | 1:200 |
Antibodies used and their specifications.
COX-1, cyclooxygenase-1.
Fig. 3.
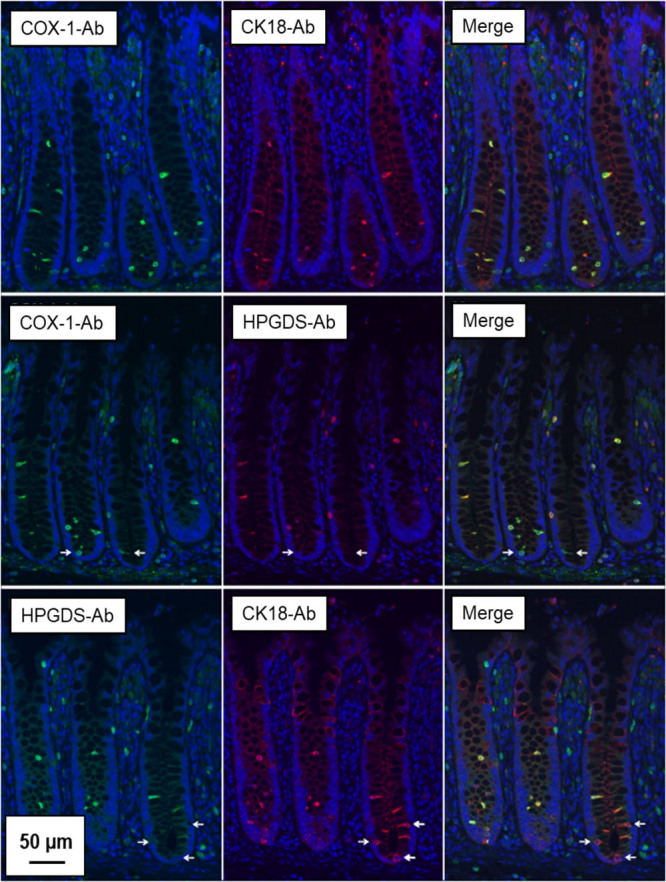
Double labelling immunofluorescence staining of tuft cell marker proteins in human colon. Representative fluorescence microscopy images of human colonic tissue sections co-stained with cyclooxygenase-1 (COX-1), hematopoietic prostaglandin D synthase (HPGDS) and cytokeratin 18 (CK18) antibodies demonstrating a high degree of overlap. Arrows indicate cryptal tuft cells stained with either COX-1 or CK18 but lacking HPGDS staining. Counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). Bar = 50 µm.
Counting of tuft cells
Images were recorded using a Zeiss Axioplan 2 plus microscope (Jena, Germany) fitted with a Photometrics CoolSNAP camera (Tucson, Arizona, USA). Colonic mucosal tuft cells were counted independently in each biopsy by the first three authors, all blinded to clinical diagnosis. A mean value from three counts was employed. Biopsy sizes were calculated, and data presented as mean counts of tuft cells per square millimeter mucosa (tuft cells/mm2) with standard deviation (± SD). The analysis was performed using Image-Pro 9.1 software.
Statistical analysis
Statistical analysis of tuft cell counts was compared between groups using Poisson regression with a log of the biopsy size as an off-set and adjustment for covariates: age, sex and smoking status. Robust standard errors were used to allow for a possible overdispersion. In the sensitivity analysis, we also tested whether the group effect depended on sex, age or smoking status by including interaction terms. Additionally, a separate analysis was performed on ulcerative colitis patients only to determine the influence of total disease duration and duration of clinical remission prior to study inclusion.
Ethical considerations
This study was approved by the scientific ethical committee of Copenhagen (H-18000856) and the Danish Data Protection Agency (P-2019-313).
Results
Immunohistochemical staining and identification of colonic tuft cells
As shown in Fig. 3, anti-COX-1 displayed a near-complete overlap with anti-CK18 in solitaire, epithelial cells of the human colonic crypts. Similarly, anti-COX-1 and anti-HPGDS showed overlap in most epithelial cells; however, a few COX-1-positive cells at the base of the crypts appeared devoid of HPGDS staining. Anti-CK18 and anti-HPGDS also displayed a large degree of overlap, and again, some CK18-positive epithelial cells at the base of the crypts appeared to lack anti-HPGDS staining. While both Dclk-1 antibodies gave positive epithelial staining in murine colon sections (data not shown), only ab37994 reacted on human colon sections. Meanwhile, we failed to obtain convincing immunolabelling compared to COX-1, HPGDS and CK18 antibodies, and Dclk-1 was therefore not used in the subsequent studies, Fig. 4. Taken together, these results show that anti-COX-1 overlap with other tuft cell marker protein antibodies; anti-HPGDS and anti-CK18 in most cases in the human colon sections and indicate that anti-COX-1 gave a more complete staining of the total tuft cell population.
Fig. 4.
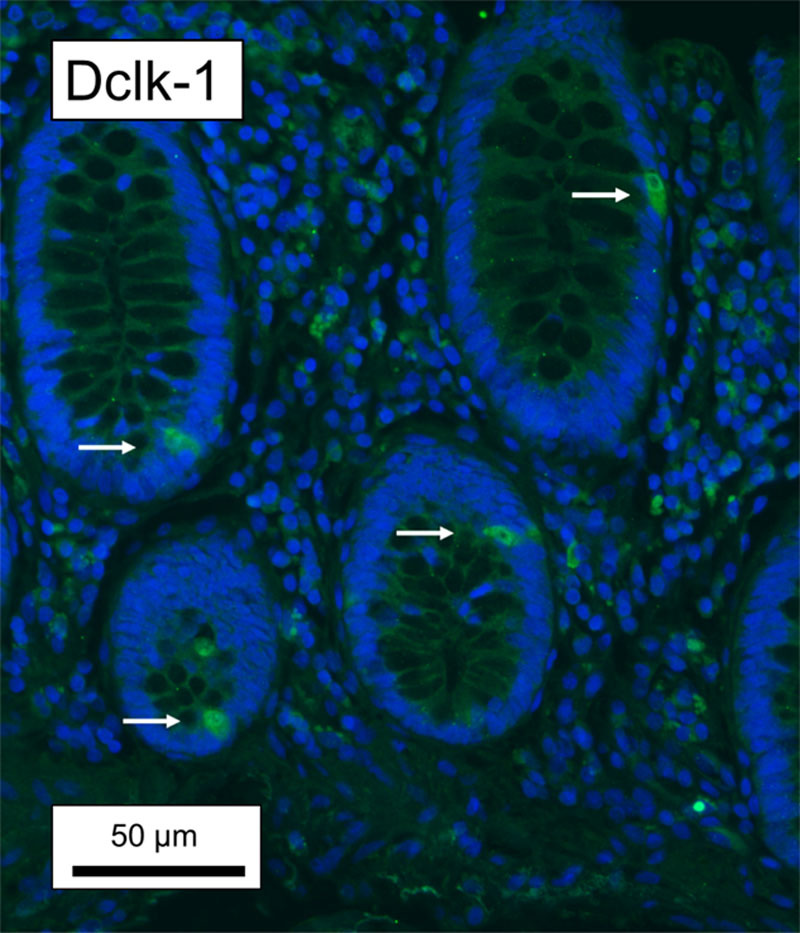
Representative fluorescence microscopy images of human colonic tissue sections co-stained with doublecortin-like kinase 1 (Dclk-1, ab37994). Arrows indicate staining of epithelial cells. Counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). Bar = 50 µm.
Colonic tuft cell population in quiescent ulcerative colitis
Biopsies from ulcerative colitis patients and controls yielded counts of 46 ± 7 tuft cells/mm2 (range 36–59) and 104 ± 21 tuft cells/mm2 (range 79–136), respectively, Fig. 2b. With covariate adjustment quiescent ulcerative colitis patients demonstrated a highly significant 55% lower number of colonic tuft cells as compared to controls (95% CI, 31–71%, P = 0.0002).
Statistical evaluation of interaction and covariance
No statistically significant associations between groups and other covariates were detected, indicating a constant group effect across values of other covariates. For ulcerative colitis patients, we found no statistically significant effects of total disease duration (P = 0.64) nor of duration of clinical remission prior to study inclusion (P = 0.095).
Discussion
Animal studies indicate that tuft cells are involved in sustaining mucosal integrity and barrier function, mediating inflammatory processes, as well as the postinflammatory mucosal healing [3,4,11,14–16]. Whether similar associations and mechanisms exist in man is largely unknown.
The present observational retrospective study provides data which shows that ulcerative colitis patients in clinical, endoscopic and histologic remission (quiescent disease stage) have a relative reduced number of colonic tuft cells. A prospective expanded study in ulcerative colitis patients with an active disease state is needed in order to further explore and test the involvement of tuft cells in ulcerative colitis pathophysiology.
Investigation of tuft cells in man has been scarce, and the mechanisms behind the observed lowered number of tuft cells in quiescent ulcerative colitis remain unknown. A genetic predisposition, an increased apoptosis of tuft cells and a reduced positive feedback circuit of proliferation from progenitor tuft cells and stem cells have all been hypothesized, but none of them confirmed [11]. Interestingly, one study suggests that number of tuft cells increase in the human stomach during inflammation, hyperplasia and metaplasia [22]. Other studies find an increased number of tuft cells in patients with diarrhea-predominant irritable bowel syndrome [23], while a loss of tuft cells in the duodenum is observed in pediatric patients with severe duodenitis [24]. Accordingly, there is a need for additional studies to examine the association and potential direct and indirect involvement of tuft cells in specific gastrointestinal diseases in active and silent phases.
Detection of human colonic tuft cells
In this study, we identified human colonic tuft cells with COX-1 antibodies, which has proven to be a valid marker for human colonic tuft cells [25,26]. Further, we compared the anti-COX-1 immunolabeling pattern with that of tuft cells markers such as HPGDS, CK18 and Dclk-1. COX-1 immunolabeling displayed substantial overlap with that obtained using specific antibodies against HPDGS and CK18, Fig. 3. In contrast, we failed to obtain convincing immunolabeling for Dclk-1 using two commercially available antibodies; ab31704 failed completely (data not shown), while ab37994 yielded an unconvincing result, Fig. 4.
Several studies using different commercially available Dclk-1 antibodies have confirmed them as excellent markers for intestinal tuft cells in mice, including colonic mucosa [5,16,25,27]. Meanwhile, except for one study by Aigbologa et al., [23] none of these markers have been claimed to be reliable markers for human colonic tuft cells [25], thus corroborating our finding with 2 Abcam Dclk-1 antibodies as unreliable markers for tuft cells in the human colonic mucosa, Fig. 4. Looking carefully at the figures in the study by Aigbologa et al., we can conclude that their data are not convincing for the rabbit Dclk-1 polyclonal antibody as a reliable marker for human colonic tuft cells. Contrary, the COX-1 antibody from Santa Cruz has turned out to be an excellent marker for human tuft cells, also in the colonic mucosa [25,26], supporting our use of this antibody as a marker for human colonic tuft cells.
Pro-biotic Trichuris suis ova treatment of ulcerative colitis with tuft cell implications
Our finding of a lowered number of tuft cells and the observed lower levels of IL-25 in circulating blood and colonic mucosal biopsies by Su et al., [19] may indicate an underlying failed immunosuppressive action eliciting part of the ulcerative colitis symptoms. Ulcerative colitis is less common in developing countries and genetic factors are not solely responsible for these differences [28,29]. Additional explanations are related to higher rates of gastrointestinal infections with parasitic agents [30]. As such, ingestion of pro-biotic Trichuris suis ovae (pig whipworm eggs, TSO) has been observed to be associated with increased numbers of IL-25 secreting intestinal tuft cells in mice, and may therefore be an interim treatment [4]. Furthermore, several studies have tested the effect on gut immunity provoked by parasitic worms (e.g. T. suis) as therapies for IBD in mouse models and in humans [31–36]. So far, the majority of clinical studies with T. suis intervention therapy has been explored in patients with Crohn’s disease and only one in ulcerative colitis patients [34,35,37]. Summers et al., find that TSO treatment is both well tolerated and effective in reducing disease activity in ulcerative colitis [36,38]. If TSO therapy for ulcerative colitis is associated with elevation of tuft cell numbers, we speculate that tuft cells are involved in TSO mode of action and as such, an efficacy and predictive biomarker for disease activity. Figure 5 illustrates a hypothetical model on preventive TSO therapy.
Fig. 5.
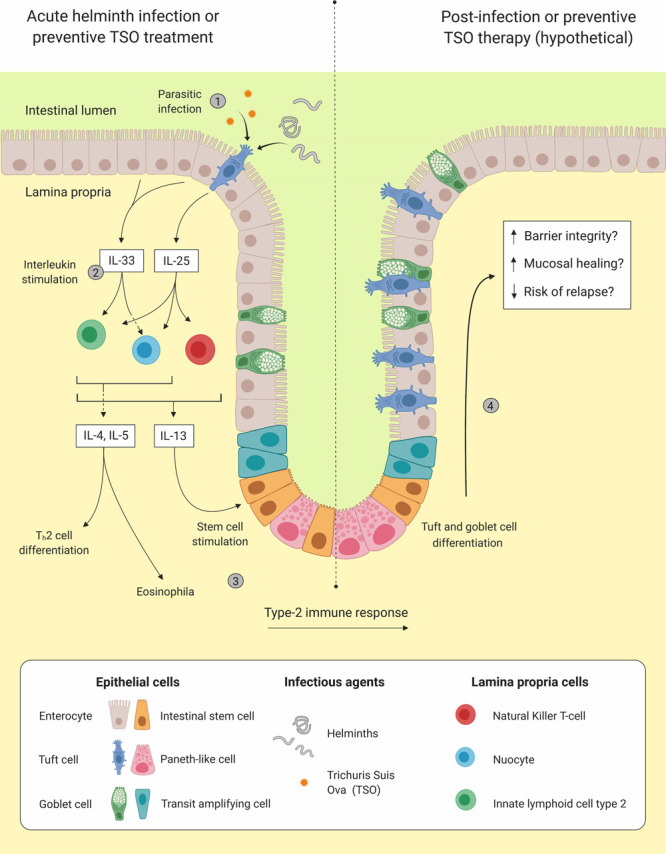
Presentation of the potential beneficial effects of an infection with helminths or preventive Trichuris suis ova (TSO) treatment in patients with ulcerative colitis. As data on human tuft cells are rare, this theory is obviously based on limited amount of evidence. Left side shows colonic mucosa of ulcerative colitis patient in quiescent disease stage before infection/preventive TSO therapy. (1) Upon helminths/TSO exposure tuft cells increase secretion of interleukin (IL)-25, while enterocytes release IL-33. (2) Both IL-25 and IL-33 stimulate lamina propria immune cells such as, type-2 innate lymphoid cells, natural killer T-cells and nuocytes to produce and release type-2 inflammatory cytokines (e.g. IL-4, IL-5 and IL-13). (3) A type-2 immune response is initiated including differentiation of T helper type 2 cells (Th2), recruitment of eosinophils and stimulation of intestinal stem cells. Right side shows potential beneficial effects of infection on colonic mucosa of ulcerative colitis patient. (4) Stimulation of intestinal stem cells leading to tuft cells and goblet cell differentiation and hyperplasia, which normalize and strengthen mucosa.
Study limitations
As some ulcerative colitis patients were referred for only a sigmoidoscopy, we might have failed to detect polyps or other uncharacteristic noncontinuous activity in the right and transverse segments of the colon.
The retrospective character of this study and the relatively low number of patients and observations per patient are also obvious inherent limitations. Also, the visual counting of tuft cells, and lack of supplementing methods, also carry some limitations.
Furthermore, despite tuft cells being the only COX-1 positive cells of the epithelial lining, COX-1 positive immune cells from the lamina propria can migrate towards the epithelium, as is seen sometimes in ulcerative colitis. If this is the case, however, it would only make the difference in tuft cell numbers even more pronounced, and therefore not influence the conclusion.
Despite results indicating tuft cell involvement in ulcerative colitis, this study is still exploratory. However, the study outcome speaks clearly in favor of further exploring tuft cells in ulcerative colitis pathogenesis and maybe even therapy eventually. As such, patients with and without active disease and effects of TSO intervention on colonic tuft cell numbers in ulcerative colitis patients could be addressed.
Conclusion
Patients with quiescent ulcerative colitis demonstrated a relative reduced number of colonic mucosal tuft cells when compared to controls without ulcerative colitis in this relatively small, observational retrospective study. Accordingly, tuft cells might be involved in ulcerative colitis pathogenesis. Finally, tuft cells are potential novel diagnostic, predictive and prognostic ulcerative colitis disease biomarkers and ultimately maybe even a therapeutic target.
Acknowledgements
A special thanks goes to pathologist Lene Buhl Riis, MD, PhD, for preparing paraffin sections of colonic biopsies. Figure 5 was created with BioRender.com. M.B.-H. was an employee at Zealand Pharma and Novo Nordisk during the study. This research was funded by Candys Foundation (2016-179); Familien Heden Nielsen’s Fond (NA); Grosserer LF Foght’s Fond (NA); and Else og Mogens Wedell-Wedellborg’s Fond (22-17-1).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Dr. Sebastian Kjærgaard, Dr. Thorbjørn S.R. Jensen and Dr. Ulrike R. Feddersen contributed equally to the writing of this article.
References
- 1.Rhodin J, Dalhamn T. Electron microscopy of the tracheal ciliated mucosa in rat. Z Zellforsch Mikrosk Anat. 1956; 44:345–412. [DOI] [PubMed] [Google Scholar]
- 2.Reid L, Meyrick B, Antony VB, Chang LY, Crapo JD, Reynolds HY. The mysterious pulmonary brush cell: a cell in search of a function. Am J Respir Crit Care Med. 2005; 172:136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Moltke J, Ji M, Liang HE, Locksley RM. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature. 2016; 529:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature. 2016; 529:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbe F, Legraverend C, Jay P. The intestinal epithelium tuft cells: specification and function. Cell Mol Life Sci. 2012; 69:2907–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schütz B, Jurastow I, Bader S, Ringer C, von Engelhardt J, Chubanov V, et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015; 6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, et al. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS One. 2013; 8:e73940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo XC, Chen ZH, Xue JB, Zhao DX, Lu C, Li YH, et al. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc Natl Acad Sci U S A. 2019; 116:5564–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ting HA, von Moltke J. The immune function of tuft cells at gut mucosal surfaces and beyond. J Immunol. 2019; 202:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo GY, Giles DA, Kronenberg M. The role of innate lymphoid cells in response to microbes at mucosal surfaces. Mucosal Immunol. 2020; 13:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steele SP, Melchor SJ, Petri WA, Jr. Tuft cells: new players in colitis. Trends Mol Med. 2016; 22:921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good H, Shin AE, Zhang L, Asfaha S. Inhibition of NF-kB signaling in DCLK1+ cells promotes colonic inflammation and colitis-associated cancer. J Can Assoc Gastroenterol. 2020; 3:37–38. [Google Scholar]
- 13.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu D, Weygant N, May R, Chandrakesan P, Madhoun M, Ali N, et al. Ablation of doublecortin-like kinase 1 in the colonic epithelium exacerbates dextran sulfate sodium-induced colitis. PLoS One. 2015; 10:e0134212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi J, Bergstrom K, Fu J, Shan X, McDaniel JM, McGee S, et al. Dclk1 in tuft cells promotes inflammation-driven epithelial restitution and mitigates chronic colitis. Cell Death Differ. 2019; 26:1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, et al. Brief report: Dclk1 deletion in tuft cells results in impaired epithelial repair after radiation injury. Stem Cells. 2014; 32:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klose CSN, Artis D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020; 30:475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider C, O’Leary CE, Locksley RM. Regulation of immune responses by tuft cells. Nat Rev Immunol. 2019; 19:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su J, Chen T, Ji XY, Liu C, Yadav PK, Wu R, et al. IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflamm Bowel Dis. 2013; 19:720–728. [DOI] [PubMed] [Google Scholar]
- 20.Vermeire S, O’Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014; 384:309–318. [DOI] [PubMed] [Google Scholar]
- 21.Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, et al. Development and validation of the Nancy histological index for UC. Gut. 2017; 66:43–49. [DOI] [PubMed] [Google Scholar]
- 22.Gumucio DL, Samuelson LC, Merchant JL. Gastric tuft cells express DCLK1, and increase in hyperplasia. Histochem Cell Biol. 2013; 136:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aigbologa J, Connolly M, Buckley JM, O’Malley D. Mucosal tuft cell density is increased in diarrhea-predominant irritable bowel syndrome colonic biopsies. Front Psychiatry. 2020; 11:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huh WJ, Roland JT, Asai M, Kaji I. Distribution of duodenal tuft cells is altered in pediatric patients with acute and chronic enteropathy. Biomed Res. 2020; 41:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011; 192:767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen TSR, Mahmood B, Damm MB, Backe MB, Dahllöf MS, Poulsen SS, et al. Combined activity of COX-1 and COX-2 is increased in non-neoplastic colonic mucosa from colorectal neoplasia patients. BMC Gastroenterol. 2018; 18:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016; 351:1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barreiro-de Acosta M, Alvarez Castro A, Souto R, Iglesias M, Lorenzo A, Dominguez-Muñoz JE. Emigration to western industrialized countries: a risk factor for developing inflammatory bowel disease. J Crohn’s Colitis. 2011; 5:566–569. [DOI] [PubMed] [Google Scholar]
- 29.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018; 390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 30.Moreels TG, Pelckmans PA. The hygiene hypothesis and inflammatory bowel diseases: role of helminths. Acta Gastroenterol Belg. 2006; 69:413–417. [PubMed] [Google Scholar]
- 31.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003; 284:G385–G391. [DOI] [PubMed] [Google Scholar]
- 32.Heylen M, Ruyssers NE, Gielis EM, Vanhomwegen E, Pelckmans PA, Moreels TG, et al. Of worms, mice and man: an overview of experimental and clinical helminth-based therapy for inflammatory bowel disease. Pharmacol Ther. 2014; 143:153–167. [DOI] [PubMed] [Google Scholar]
- 33.Villa OF, Kuhn RE. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1996; 112 (Pt 6):561–570. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Zeng LR, Chen FS, Zhu JP, Zhu MH. Trichuris suis ova therapy in inflammatory bowel disease: a meta-analysis. Medicine (Baltimore). 2018; 97:e12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schölmerich J, Fellermann K, Seibold FW, Rogler G, Langhorst J, Howaldt S, et al. ; International TRUST-2 Study Group. A randomised, double-blind, placebo-controlled trial of trichuris suis ova in active crohn’s disease. J Crohns Colitis. 2017; 11:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summers RW, Elliott DE, Qadir K, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003; 98:2034–2041. [DOI] [PubMed] [Google Scholar]
- 37.Summers RW, Elliott DE, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. 2005; 54:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summers RW, Elliott DE, Urban JF, Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005; 128:825–832. [DOI] [PubMed] [Google Scholar]


