Keywords: vitamin D, mortality, critical care, mechanical ventilation, thromboembolism
Abstract
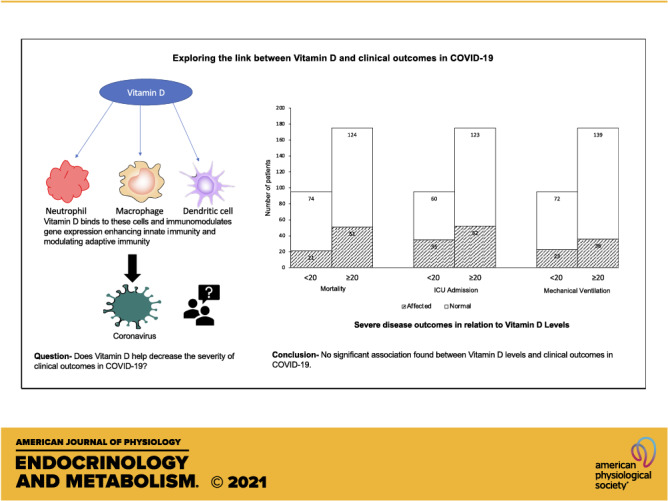
The immunomodulating role of vitamin D might play a role in COVID-19 disease. We studied the association between vitamin D and clinical outcomes in COVID-19 patients. This is a retrospective cohort study on COVID-19 patients with documented vitamin D levels within the last year. Vitamin D levels were grouped as ≥ 20 ng/mL or < 20 ng/mL. Main outcomes were mortality, need for mechanical ventilation, new DVT or pulmonary embolism, and ICU admission. A total of 270 patients (mean ± SD) age, 63.81 (14.69) years); 117 (43.3%) males; 216 (80%) Blacks; 139 (51.5%) in 65 and older age group were included. Vitamin D levels were less than 20 ng/mL in 95 (35.2%) patients. During admission, 72 patients (26.7%) died, 59 (21.9%) needed mechanical ventilation, and 87 (32.2%) required ICU. Vitamin D levels showed no significant association with mortality (OR = 0.69; 95% CI, 0.39–1.24; P = 0.21), need for mechanical ventilation (OR = 1.23; 95% CI, 0.68–2.24; P = 0.49), new DVT or PE(OR= 0.92; 95% CI, 0.16–5.11; P = 1.00) or ICU admission (OR = 1.38; 95% CI, 0.81–2.34; P = 0.23). We did not find any significant association of vitamin D levels with mortality, the need for mechanical ventilation, ICU admission and the development of thromboembolism in COVID-19 patients.
NEW & NOTEWORTHY Low vitamin D has been associated with increased frequency and severity of respiratory tract infections in the past. Current literature linking clinical outcomes in COVID-19 with low vitamin D is debatable. This study evaluated the role of vitamin D in severe disease outcomes among COVID-19 patients and found no association of vitamin D levels with mortality, the need for mechanical ventilation, ICU admission, and thromboembolism in COVID-19.
INTRODUCTION
Coronavirus disease (COVID-19) originated in Wuhan, China, and has now become a pandemic resulting in 926,000 deaths worldwide as of mid-September 2020. The lack of evidence-based information and highly variable clinical presentation of individuals infected with this novel virus has perplexed clinicians worldwide. Elderly patients, especially those with underlying comorbidities, are at a higher risk for severe infection and worse clinical outcomes (1, 2). Prior studies point that 25-hydroxyvitamin D plays a role in immune regulation and induction of antimicrobial peptides to both viral and bacterial infections (3–6). Review of literature points towards the association of low levels of vitamin D and increased frequency and susceptibility to acute respiratory tract infections including COPD exacerbation (7–10). Many reports also suggest that vitamin D supplementation reduces the risk of respiratory tract infections, decreases symptom duration, and length of stay in hospitalized patients (11–14).
Vitamin D has immunomodulating properties (3) and acts at various levels, i.e., maintains cellular junctions (15), enhances innate immunity (16), induces antimicrobial peptides (cathelicidins and defensins) (5, 17), which lowers viral replication, decreases proinflammatory Th1 cytokines (18–20), increases anti-inflammatory cytokines (21), and modulates adaptive immunity (22). Cytokine release syndrome has been reported in some of the critically ill COVID-19 patients (23, 24), and given its immunomodulating properties, one can hypothesize that vitamin D levels might have a role in this syndrome. Vitamin D has been documented to have antioxidant (25) and antifibrotic properties (26), and modulate renin-angiotensin-aldosterone-system (RAAS) and angiotensin converting enzyme-2 (ACE2) expression (27). A leading cause of mortality in COVID-19 patients has been reported to be acute respiratory distress syndrome (ARDS) (28). Vitamin D has been shown to reduce lung permeability in ARDS and regenerate lung lining (29). This led us to explore a possible correlation between vitamin D levels and clinical outcomes in COVID-19 patients. Interestingly, vitamin D deficiency is very prevalent in the United States and ∼41% of the adult US population has inadequate vitamin D levels (30).
A high incidence of thrombotic complications has been reported in COVID-19 patients who need intensive care (31). There have been conflicting results reported by studies on the role of vitamin D and venous thromboembolism. Some studies have documented that 25-hydroxyvitamin D plays a critical role in the pathogenesis of deep vein thrombosis (DVT) (32) and reported an association between decreased vitamin D levels and increased risk of venous thromboembolism (33). However, other studies have refuted any such correlation (34).
The immunomodulating role of vitamin D may play a role in COVID-19 disease progression and there is a paucity of literature on the role of vitamin D in COVID-19. The main objective of this study is to understand the association between vitamin D levels and mortality among COVID-19 patients. Our study also explores if vitamin D levels have any association with other clinical outcomes such as the need for mechanical ventilation, development of new DVT or pulmonary embolism (PE), and intensive care unit (ICU) requirement in COVID-19 patients.
METHODS
Study Design
We conducted a retrospective cohort study on 2001 adult patients with a confirmed COVID-19 diagnosis. The study was exempt by the Detroit Medical Center (DMC) and Wayne State University Institutional Review Board (IRB application #20-06-2422). No external funding was received for conducting the study.
Study Site and Patient Population
Adult patients (≥18 years of age) with a confirmed COVID-19 diagnosis (either via nasopharyngeal or oropharyngeal swab) were included. Testing for COVID-19 was done at DMC, one of the largest academic medical centers and healthcare providers in southeast Michigan. DMC comprises four distinct hospitals in Michigan and all four hospital locations were included in the study. These hospitals primarily serve the Detroit metropolitan area.
Data Collection
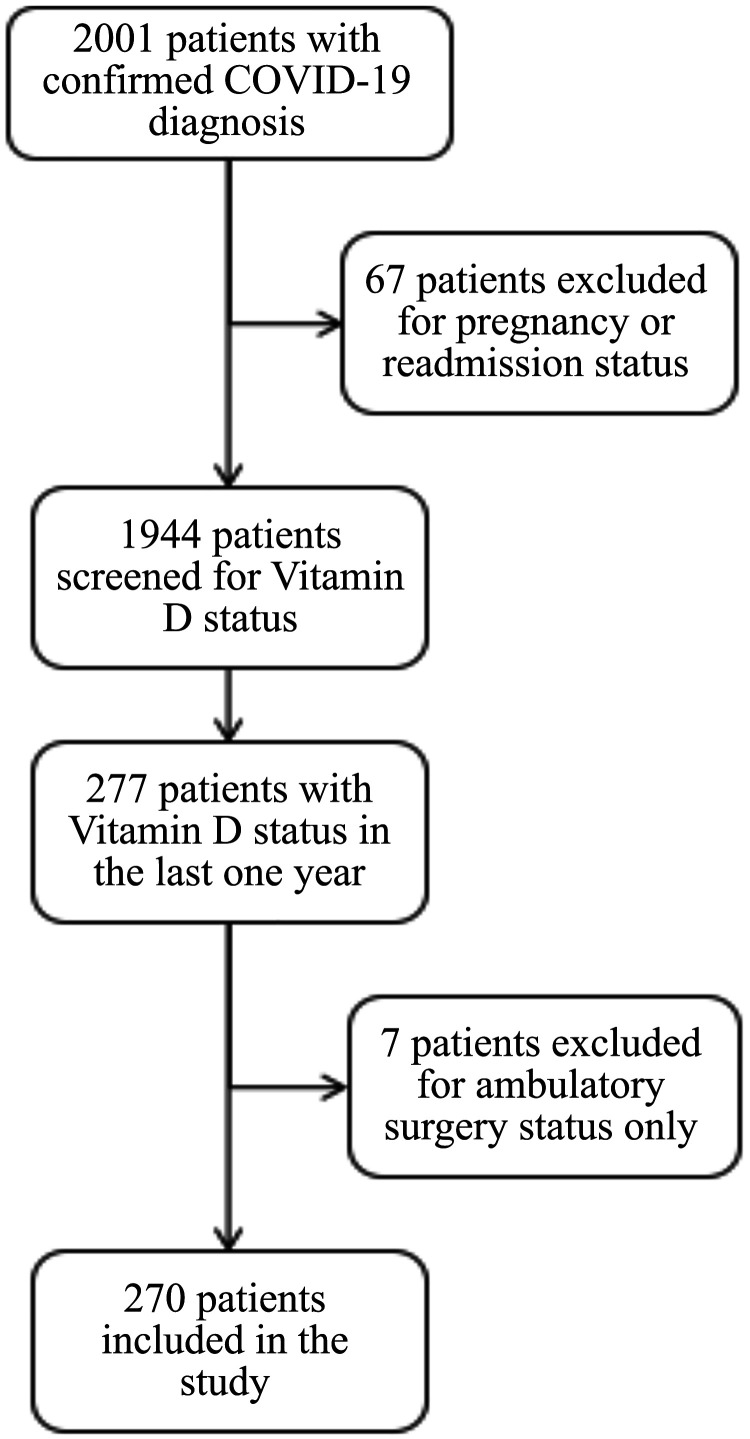
A list of 2001 patients who visited DMC between March 10, 2020, and June 30, 2020, with a laboratory-confirmed COVID-19 PCR diagnosis was collected in collaboration with institutional information technology services. Patients under the age of 18, any readmission during the time frame, and pregnant patients were excluded from the study. A total of 67 patients were excluded initially as they met the above criteria. We reviewed 1,944 electronic medical records to screen if these patients had a previously documented vitamin D, 25-OH level within the past 12 months. After the initial screen, there were a total of 277 patients with a documented vitamin D level within the past 12 months. However, 7 of these patients were excluded from the study as they presented only for ambulatory surgery leaving a total of 270 patients, who were included in the study (Fig. 1). We then classified the patients based on their vitamin D levels into 2 groups as ≥ 20 ng/mL (patients with normal vitamin D levels) and < 20 ng/mL (patients with low vitamin D levels). Data points were manually collected and coded for each patient. Data regarding the prescription of vitamin D supplements (weekly/daily) were also collected. For additional analysis based upon stratified vitamin D levels, patients with normal vitamin D levels were further divided into two subgroups, patients with vitamin D level 20–30 ng/mL and patients with vitamin D level > 30 ng/mL.
Figure 1.
Flowchart depicting patient inclusion criteria. Adult patients (≥ 18 years of age) with a confirmed COVID-19 diagnosis and a documented vitamin D level in the past 12 months were included. Patients under the age of 18, any readmission during the time frame, ambulatory surgery and pregnant patients were excluded from the study.
Outcomes
The main outcomes for this study were mortality, the need for mechanical ventilation, new DVT or PE during hospitalization, and ICU admission among COVID-19 patients. All of the patients included in the study had a documented outcome (mortality/discharged status) at the time of data collection. Additionally, the number of prior comorbidities, BMI, disposition upon emergency department (ED) visit (discharge home, inpatient admission, and direct ICU admission), and maximum oxygen requirement during admission were collected. Charts were screened to determine if the patient required transfer to ICU from inpatient floors. Demographic data collected included age, sex, and race.
Statistical Analysis
Categorical variables have been described as frequency and percentages. We categorized age into two groups (18–64 years, and 65 and older). A crude relative association measure (odds ratio, OR) was calculated for each correlation using the Pearson chi-square and Fisher test. An adjusted odds ratio was calculated using binary logistic regression. We adjusted for age, sex, BMI, and presence of comorbidities. Age and BMI were taken as continuous variables and the presence of comorbidities as a categorical variable for the adjusted model. Subgroup analyses were done based on sex and age groups as defined earlier. Subgroup analysis based on race was limited to Blacks and Whites due to the limited sample size of other races. The 95% confidence intervals (CI) were estimated using a binomial distribution. A P value of less than 0.05 was determined to be significant. Bonferroni correction was used to protect from the inflated type 1 error while performing multiple analyses. Additional analyses were performed on the stratified vitamin D levels (< 20 ng/mL, 20–30 ng/mL and > 30 ng/mL). Among the patients with low vitamin D levels, further comparison was made between the patients who were prescribed vitamin D supplements and those who were not prescribed any vitamin D supplements. Statistical analyses were completed using IBM SPSS Statistics software (version 26).
RESULTS
Baseline Characteristics
There were 2001 patient records with positive COVID-19 test at the 4 DMC hospitals with a nasopharyngeal/oropharyngeal PCR swab between March 10, 2020, and June 30, 2020. Based on the exclusion criteria, only 270 patients were included in the study. In the cohort analysis, there were 117 males (43.3%) and 153 females (56.7%). The mean age of patients was 63.81 years (mean ± SD, 14.69). More than half of the patients (n = 139, 51.5%) were in the 65 and older age group, with Blacks being the predominant race (n = 216, 80%). Distribution of vitamin D levels showed that more than one-third of the patients had levels less than 20 ng/mL (n = 95, 35.2%). Among the patients with low vitamin D levels, only 27.4% (n = 26) were prescribed vitamin D supplements. About 70% of patients had three or more comorbid diseases (n = 187, 69.3%). The mean BMI of patients was 32.09 (mean ± SD, 9.12), and more than 50% of patients (n = 139) were in the obese category as per the World Health Organization criteria. The baseline characteristics of the population included are detailed in Table 1.
Table 1.
Baseline characteristic of patients
| Characteristic | Cohort (n = 270) |
|---|---|
| Age group, n (%) | |
| 18-30 years | 5 (1.9) |
| 31-45 years | 26 (9.6) |
| 46-64 years | 100 (37) |
| 65+ years | 139 (51.5) |
| Sex, n (%) | |
| Male | 117 (43.3) |
| Female | 153 (56.7) |
| Race/ethnicity, n (%) | |
| Blacks | 216 (80) |
| Whites | 48 (17.8) |
| Asian | 3 (1.1) |
| Middle Eastern | 3 (1.1) |
| Number of comorbidities, n (%) | |
| 0 | 14 (5.2) |
| 1 | 30 (11.1) |
| 2 | 39 (14.4) |
| 3 or 3+ | 187 (69.3) |
| Vitamin D levels, n (%) | |
| ≥20 ng/mL | 175 (64.8) |
| <20 ng/mL | 95 (35.2) |
| BMI categories, n (%) | |
| Underweight (BMI < 18.5) | 5 (1.9) |
| Normal (18.5 to < 25) | 52 (19.3) |
| Overweight (25 to < 30) | 74 (27.4) |
| Obese (> 30) | 139 (51.5) |
| Vitamin D supplementation, n (%) | |
| ≥20 ng/mL | 58 (33.1) |
| <20 ng/mL | 26 (27.4) |
n = Number of patients. BMI, body mass index.
Clinical Course
The total mortality in this cohort was 26.7% (n = 72). About 14.8% (n = 40) of the patients were admitted straight to ICU from the ED. An additional 47 patients were later transferred to ICU from the inpatient service. Approximately one in every three patients in this study (n = 87, 32.2%) who came to ED ended up requiring ICU. Around 3.7% of the total patients were sent home from ED (n = 10), whereas 81.5% (n = 220) were admitted to the inpatient service. Close to 81% (n = 219) of patients required supplemental oxygen during their admission stay and 21.9% (n = 59) required mechanical ventilation. About 2.2% of the patients (n = 6) developed new DVT or PE during their hospitalization. The clinical course of the patient population is further detailed in Table 2.
Table 2.
Admission characteristics of patients, n (%)
| Mortality | 72 (26.7) |
| Mechanical ventilation | 59 (21.9) |
| ICU admission | 87 (32.2) |
| Admission disposition | |
| ER visit only (discharged from ER) | 10 (3.7) |
| Inpatient admission | 220 (81.5) |
| Direct ER to ICU admission | 40 (14.8) |
| Maximum supplemental oxygen during admission | |
| Room air only | 51 (18.9) |
| Nasal canula | 100 (37) |
| Venti-mask | 15 (5.6) |
| Non-rebreather | 37 (13.7) |
| High flow oxygen | 7 (2.6) |
| BPAP/CPAP | 1 (0.4) |
| Mechanical ventilation | 59 (21.9) |
| New DVT or PE | 6 (2.2) |
n = Number of patients. BPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure.
Vitamin D and Mortality
In the cohort analysis vitamin D levels showed no significant association with mortality (OR = 0.69; 95% CI, 0.39–1.24; P = 0.21). No correlation between mortality and vitamin D levels was seen in either males (OR = 1.10; 95% CI, 0.46–2.63; P = 0.83) or females (OR = 0.49; 95% CI, 0.22–1.09; P = 0.08). With subgroup analysis based on age groups and race, no significant association was found between vitamin D levels and mortality in patients less than 65 years old (OR = 0.90; 95% CI, 0.37–2.18; P = 0.81), or in patients 65 years and older (OR = 0.83; 95% CI, 0.35–1.92; P = 0.66), Blacks (OR = 0.78; 95% CI, 0.41–1.48; P = 0.44) or Whites (OR = 0.51; 95% CI, 0.12–2.19; P = 0.36). Similarly, no correlation between vitamin D and mortality was noted in the total cohort when adjustment was made for age, sex, BMI, and presence of comorbidities (adjusted OR = 1.04; 95% CI, 0.55–1.97; P = 0.90).
Vitamin D and Mechanical Ventilation/ICU Admission
We found no significant association between vitamin D levels and need for mechanical ventilation (OR = 1.23; 95% CI, 0.68–2.24; P = 0.49) or ICU admission (OR = 1.38; 95% CI, 0.81–2.34; P = 0.23). No correlation between the need for mechanical ventilation and vitamin D levels was seen in either males (OR = 1.24; 95% CI, 0.52–2.91; P = 0.63), females (OR = 1.20; 95% CI, 0.52–2.76; P = 0.67), Blacks (OR = 1.36; 95% CI, 0.71–2.62; P = 0.34) or Whites (OR= 0.77; 95% CI, 0.13–4.49; P = 0.77). The need for ICU admission was higher among males with low vitamin D levels compared to the males with normal vitamin D levels (OR = 2.32; 95% CI, 1.07–5.03; P = 0.03) in the unadjusted models, and in the models adjusted for age, BMI, and comorbidities (adjusted OR = 2.60; 95% CI, 1.07–6.28; P = 0.03). However, after Bonferroni correction was made to protect from the inflated type 1 error due to multiple comparisons, these results were noted to be statistically non-significant. No association between vitamin D levels and need for ICU admission was seen in females (OR = 0.82; 95% CI, 0.38–1.76; P = 0.61). Similarly, no significant association was noted between vitamin D levels and the need for ICU admission or mechanical ventilation among patients less than 65 years, or 65 years and older for unadjusted models, as well as when the models were fully adjusted for age, sex, BMI, and presence of comorbidities. Further details on the results, unadjusted and after adjusting for age, sex, BMI, and comorbidities, are summarized in Tables 3 and 4.
Table 3.
Association between vitamin D levels and mortality, mechanical ventilation and ICU admission, unadjusted odds ratio
| Mortality |
ICU Admission |
Mechanical Ventilation |
||||
|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| Total cohort | 0.69 (0.39–1.24) | 0.21 | 1.38 (0.81–2.34) | 0.23 | 1.23 (0.68–2.24) | 0.49 |
| Males | 1.10 (0.46–2.63) | 0.83 | 2.32 (1.07–5.03) | 0.03 | 1.24 (0.52–2.91) | 0.63 |
| Females | 0.49 (0.22–1.09) | 0.08 | 0.82 (0.38–1.76) | 0.61 | 1.20 (0.52–2.76) | 0.67 |
| Less than 65 years | 0.90 (0.37–2.18) | 0.81 | 1.39 (0.66–2.94) | 0.39 | 1.81 (0.75–4.40) | 0.19 |
| 65+ years | 0.83 (0.35–1.92) | 0.66 | 1.67 (0.74–3.75) | 0.21 | 1.04 (0.42–2.59) | 0.94 |
| Blacks | 0.78 (0.41–1.48) | 0.44 | 1.33 (0.73–2.41) | 0.35 | 1.36 (0.71–2.62) | 0.34 |
| Whites | 0.51 (0.12–2.19) | 0.36 | 2.56 (0.74–8.89) | 0.14 | 0.77 (0.13–4.49) | 0.77 |
Table 4.
Association between vitamin D levels and mortality, mechanical ventilation, and ICU admission, adjusted model
| Mortality |
ICU Admission |
Mechanical Ventilation |
||||
|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| Total cohort adjusted* | 1.04 (0.55–1.97) | 0.9 | 1.51 (0.85–2.69) | 0.16 | 1.36 (0.71–2.60) | 0.35 |
| Males** | 1.94 (0.72–5.25) | 0.19 | 2.60 (1.07–6.28) | 0.03 | 1.32 (0.51–3.43) | 0.56 |
| Females** | 0.62 (0.26–1.49) | 0.28 | 0.91 (0.40–2.05) | 0.82 | 1.29 (0.52–3.20) | 0.58 |
| Less than 65 years* | 1.14 (0.43–2.99) | 0.79 | 1.31 (0.58–2.99) | 0.51 | 2.00 (0.75–5.34) | 0.16 |
| 65+ years* | 0.96 (0.40–2.31) | 0.93 | 1.66 (0.73–3.79) | 0.23 | 0.99 (0.39–2.53) | 0.99 |
| Blacks* | 1.17 (0.57–2.39) | 0.66 | 1.36 (0.71–2.60) | 0.36 | 1.48 (0.72–3.02) | 0.29 |
| Whites* | 0.86 (0.16–4.53) | 0.86 | 3.71 (0.81–16.91) | 0.09 | 1.08 (0.15–7.66) | 0.94 |
*Adjusted for age, sex, body mass index (BMI), and comorbidities; **adjusted for age, BMI, and comorbidities.
Vitamin D and New DVT/PE
Vitamin D levels showed no significant association with development of new DVT or PE among COVID-19 patients (OR= 0.92; 95% CI, 0.16–5.11; P = 1.00). Further subgroup analysis was not done due to a limited number of patients developing thromboembolic episodes during the course of their admission.
Stratified Vitamin D Levels and Clinical Outcomes
The analyses performed for stratified vitamin D levels (< 20 ng/mL, 20-30 ng/mL, and > 30 ng/mL) showed no statistically significant association of these vitamin D levels with mortality, the need for mechanical ventilation and ICU admission in our cohort. Additionally, among patients with low vitamin D levels (< 20 ng/mL), no significant association was noted between vitamin D supplementation and clinical outcomes in COVID-19. More details on these results have been summarized in Table 5.
Table 5.
Association of stratified vitamin D levels/vitamin D supplementation with mortality, mechanical ventilation, and ICU admission, adjusted odds ratio for age, sex, BMI and comorbidities
| Mortality |
ICU Admission |
Mechanical Ventilation |
||||
|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| Vitamin D supplements (among patients with low vitamin D levels) | ||||||
| Supplements (Yes vs no) | 0.86 (0.26–2.80) | 0.8 | 0.96 (0.35–2.59) | 0.93 | 0.68 (0.22–2.13) | 0.51 |
| Stratified vitamin D levels | ||||||
| <20 ng/ml | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||
| 20-30 ng/mL | 1.20 (0.57–2.54) | 0.63 | 0.70 (0.35–1.40) | 0.31 | 0.69 (0.31–1.53) | 0.36 |
| >30 ng/mL | 0.81 (0.39–1.66) | 0.56 | 0.63 (0.33–1.22) | 0.17 | 0.77 (0.37–1.60) | 0.48 |
DISCUSSION
This retrospective cohort study demonstrated no significant association between vitamin D levels and mortality among COVID-19 patients. A review of literature points toward equivocal evidence linking clinical outcomes in COVID-19 patients with low vitamin D levels. Risk factors for low vitamin D levels include elderly, obesity, and males (35), and higher mortality in COVID-19 patients is also noticed among these patient populations (36–38). Another study suggests higher mortality seen in Italy and Spain could be attributed to a higher prevalence of low vitamin D seen in these countries (39). The study by llie et al. (40) also reports an association between vitamin D levels and COVID-19 mortality in European countries. However, these studies only provide inferred evidence based on the high prevalence of vitamin D deficiency in these countries and do not account for potential confounders like other underlying comorbidities and BMI. Also, the study by Laird et al. (39) has relied on literature and data as old as 20 years ago to derive the mean vitamin D levels in the patient population and correlated it with the current data of mortality among COVID-19 patients. Recent studies have also tried to elucidate if low vitamin D levels are associated with increased risk of testing positive for COVID-19, but the results have been conflicting (41–43).
Some of the literature suggests increased morbidity and mortality among Blacks with COVID-19 (44–46) and a plausible explanation is low levels of vitamin D seen in Blacks (47). However, our study demonstrated no such correlation between low vitamin D levels and mortality among COVID-19 patients who were Blacks. Hence further studies are needed before any such link between mortality and low vitamin D levels can be established.
High incidence of vitamin D deficiency has been reported among critically ill patients admitted to intensive care, resulting in increased length of stay and mortality (48–54). Literature review also suggests that low vitamin D levels may also be associated with worse disease outcomes especially in pneumonia (55, 56) and with the development of ARDS and acute lung injury (29, 57). A recent metanalysis by Munshi et al. (58) reported poor outcomes in COVID-19 patients with low vitamin D levels, however, in their study, poor outcomes were clubbed together as the development of ARDS, mortality, need for ICU admission, and mechanical ventilation. Our study looked at each of these severe disease clinical outcomes separately to identify individual correlations. We found that the need for ICU admission was higher among males with low vitamin D levels, however, after Bonferroni correction was applied, these results failed to reach the level of statistical significance. A study conducted by Carpagnano et al. (59) noticed a high prevalence of low vitamin D among patients admitted to ICU, and the majority of patients in the vitamin D deficiency group of their study were males.
We did not find any significant correlation between low vitamin D levels and the development of new DVT or PE in COVID-19 patients. This could be, in part, due to the low number of patients in this study who developed new DVT or PE. The role of vitamin D and thromboembolism is debatable in the literature. In the past, some large population studies have shown no correlation between vitamin D levels or vitamin D supplementation, on the risk of development of thromboembolism (60–62).
Some of the literature on vitamin D levels recommends maintaining vitamin D levels at a minimum of at least 30 ng/mL to achieve optimal health benefits (63). In our study, we did not see any significant association between vitamin D levels < 20 ng/mL, 20-30 ng/mL, and > 30 ng/mL with mortality, the need for ICU admission, and the need for mechanical ventilation in COVID-19 patients.
We acknowledge that our study has several limitations that need to be addressed. Although we had a large database of over 2000 patients, a large number of patients did not have recorded vitamin D levels within in the last year. This significantly reduced the number of patients who could be included in this study. Also, we relied on electronic medical records and clinical notes to gather data including the presence of comorbidities and documentation of vitamin D levels. Hence there is a possibility of both selection and information bias. The data for this study were collected from 4 hospitals in southeast Michigan, predominantly serving the underserved population having multiple comorbidities. Our sample size consisted of very few patients with other races besides Blacks and Whites, thereby limiting analysis in this population group. Also, very few patients in our cohort developed new DVT or PE during their hospital stay, hence more studies with a large sample size are needed before any conclusive inference can be made in this regard. Although the use of vitamin D levels before the patient developed illness helped avoid the negative acute phase impact of the illness on vitamin D levels, it would have been ideal if we had the measurements immediately preceding the infection from COVID-19. But it was not a possibility given the nature and design of the current study. We believe that further community-based studies will provide a better understanding of the possible role of vitamin D in the disease progression and severity of symptoms in COVID 19 patients.
Conclusions
This study did not find any significant association of vitamin D levels with mortality, the need for mechanical ventilation, ICU admission, and the development of thromboembolism in patients with COVID-19. Further studies are warranted before any conclusive association can be made between vitamin D levels and the clinical course of COVID 19 patients.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.L., P.N., and N.P. conceived and designed research; P.L. and S.K. analyzed data; P.L. and S.K. interpreted results of experiments; P.L., P.N., N.P., and S.K. prepared figures; P.L., P.N., and S.K. drafted manuscript; P.L., P.N., N.P., and S.K. edited and revised manuscript; P.L., P.N., N.P., and S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We extend our gratitude to the Research Design and Analysis Unit at Wayne State University for their assistance with the analyses of the project.
REFERENCES
- 1.Hwang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 94: 91–95, 2020. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 7: 4240–4270, 2015. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 181: 7090–7099, 2008. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Olliver M, Spelmink L, Hiew J, Meyer-Hoffert U, Henriques-Normark B, Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J Infect Dis 208: 1474–1481, 2013. doi: 10.1093/infdis/jit355. [DOI] [PubMed] [Google Scholar]
- 7.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect 134: 1129–1140, 2006. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginde AA, Mansbach JM, Camargo CA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 169: 384–390, 2009. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 65: 215–220, 2010. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 10.Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol 136: 321–329, 2013. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Charan J, Goyal JP, Saxena D, Yadav P. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J Pharmacol Pharmacother 3: 300–303, 2012. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Stelmach I, Kumar GT, Urashima M, Camargo CA. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 356: i6583, 2017. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 91: 1255–1260, 2010. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Du J, Huang L, Wang Y, Shi Y, Lin H. Preventive effects of vitamin D on seasonal influenza A in infants: a multicenter, randomized, open, controlled clinical trial. Pediatr Infect Dis J 37: 749–754, 2018. doi: 10.1097/INF.0000000000001890. [DOI] [PubMed] [Google Scholar]
- 15.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res 55: 96–108, 2011. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 16.Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol 27, 2017.doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 17.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 182: 4289–4295, 2009. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 7: 3011–3021, 2015. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemire JM, Adams JS, Kermani-Arab V, Bakke AC, Sakai R, Jordan SC. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol 134: 3032–3035, 1985. [PubMed] [Google Scholar]
- 20.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188: 2127–2135, 2012. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol 183: 5458–5467, 2009. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 7: 8251–8260, 2015. doi: 10.3390/nu7105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 368: 473–474, 2020. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Wu Z, Li JW, Zhao H, Gq W. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents 55: 105954, 2020. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei GS, Zhang C, Cheng BH, Lee CH. Mechanisms of action of vitamin D as supplemental therapy for pneumocystis pneumonia. Antimicrob Agents Chemother 61, 2017. doi: 10.1128/AAC.01226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y, Liu T, Yao L, Xing Y, Zhao X, Fu J, Xue X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci Rep 7: 3312, 2017. doi: 10.1038/s41598-017-03474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep 16: 7432–7438, 2017. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. and HLH Across Speciality Collaboration UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033–1034, 2020. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dancer RC, Parekh D, Lax S, D'Souza V, Zheng S, Bassford CR, Park D, Bartis DG, Mahida R, Turner AM, Sapey E, Wei W, Naidu B, Stewart PM, Fraser WD, Christopher KB, Cooper MS, Gao F, Sansom DM, Martineau AR, Perkins GD, Thickett DR. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 70: 617–624, 2015. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res 31: 48–54, 2011. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMP, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191: 145–147, 2020. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu WX, He DR. Low vitamin D levels are associated with the development of deep venous thromboembolic events in patients with ischemic stroke. Clin Appl Thromb Hemost 24: 69S–75S, 2018. doi: 10.1177/1076029618786574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brøndum-Jacobsen P, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. 25-Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18,791 participants. J Thromb Haemost 11: 423–431, 2013. doi: 10.1111/jth.12118. [DOI] [PubMed] [Google Scholar]
- 34.Brodin E, Lerstad G, Grimnes G, Brækkan SK, Vik A, Brox J, Svartberg J, Jorde R, Hansen JB. Serum levels of vitamin D are not associated with future risk of venous thromboembolism: the Tromsø study. Thromb Haemost 109: 885–890, 2013. doi: 10.1160/TH12-10-0728. [DOI] [PubMed] [Google Scholar]
- 35.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ 340: b5664, 2010. doi: 10.1136/bmj.b5664. [DOI] [PubMed] [Google Scholar]
- 36.Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, Small-Saunders J, Rajagopalan KN, Greendyk R, Chae SR, Natarajan K, Roh D, Edwin E, Gallagher D, Podolanczuk A, Barr RG, Ferrante AW, Baldwin MR. Body mass index and risk for intubation or death in SARS-CoV-2 infection: a retrospective cohort study. Ann Intern Med 173: 782–790, 2020. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, for the COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 323: 1574–1581, 2020. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tartof SY, Qian L, Hong V, Wei R, Nadjafi RF, Fischer H, Li Z, Shaw SF, Caparosa SL, Nau CL, Saxena T, Rieg GK, Ackerson BK, Sharp AL, Skarbinski J, Naik TK, Sb M. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med 173: 773–781, 2020. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laird E, Rhodes J, Kenny RA. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J 113: 81, 2020. [PubMed] [Google Scholar]
- 40.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 32: 1195–1198, 2020. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 12: 1359, 2020. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, Jani BD, Welsh P, Mair FS, Gray SR, O'Donnell CA, Gill JM, Sattar N, Pell JP. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr 14: 561–565, 2020. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open 3: e2019722, 2020. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khunti K, Singh AK, Pareek M, Hanif W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ 369: m1548, 2020. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 45.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus Disease 2019 Case Surveillance: United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 69: 759–765, 2020. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 323: 2466–2467, 2020. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohlmeier M. Avoidance of vitamin D deficiency to slow the COVID-19 pandemic. BMJ Nutr Prev Health 3: 67–73, 2020. doi: 10.1136/bmjnph-2020-000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, Kb C. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med 39: 671–677, 2011. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Haan K, Groeneveld AB, de Geus HR, Egal M, Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care 18: 660 2014. doi: 10.1186/s13054-014-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med 360: 1912–1914, 2009. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 51.Lucidarme O, Messai E, Mazzoni T, Arcade M, Du Cheyron D. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med 36: 1609–1611, 2010. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]
- 52.McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc 12: 208–211, 2011. doi: 10.1016/j.jamda.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Venkatram S, Chilimuri S, Adrish M, Salako A, Patel M, Diaz-Fuentes G. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care 15: R292, 2011. doi: 10.1186/cc10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang YP, Wan YD, Sun TW, Kan QC, Wang LX. Association between vitamin D deficiency and mortality in critically ill adult patients: a meta-analysis of cohort studies. Crit Care 18: 684, 2014. doi: 10.1186/s13054-014-0684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leow L, Simpson T, Cursons R, Karalus N, Hancox RJ. Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology 16: 611–616, 2011. doi: 10.1111/j.1440-1843.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 56.Remmelts HH, van de Garde EM, Meijvis SC, Peelen EL, Damoiseaux JG, Grutters JC, Biesma DH, Bos WJ, Rijkers GT. Addition of vitamin D status to prognostic scores improves the prediction of outcome in community-acquired pneumonia. Clin Infect Dis 55: 1488–1494, 2012. doi: 10.1093/cid/cis751. [DOI] [PubMed] [Google Scholar]
- 57.Parekh D, Thickett DR, Turner AM. Vitamin D deficiency and acute lung injury. Inflamm Allergy Drug Targets 12: 253–261, 2013. doi: 10.2174/18715281113129990049. [DOI] [PubMed] [Google Scholar]
- 58.Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, Youssef MR, Omar M, Attia AS, Fawzy MS, Killackey M, Kandil E, Duchesne J. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol 93: 733–740, 2021. doi: 10.1002/jmv.26360. [DOI] [PubMed] [Google Scholar]
- 59.Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, Palumbo A, Di Gioia G, Valerio VN, Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest 9: 1–7, 2020. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blondon M, Rodabough RJ, Budrys N, Johnson KC, Berger JS, Shikany JM, Raiesdana A, Heckbert SR, Manson JE, LaCroix AZ, Siscovick D, Kestenbaum B, Smith NL, de Boer IH. The effect of calcium plus vitamin D supplementation on the risk of venous thromboembolism: from the Women's Health Initiative Randomized Controlled Trial. Thromb Haemost 113: 999–1009, 2015. doi: 10.1160/TH14-05-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw KT, Camargo CA, Jr.. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol 2: 608–616, 2017. doi: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vučković BA, van Rein N, Cannegieter SC, Rosendaal FR, Lijfering WM. Vitamin supplementation on the risk of venous thrombosis: results from the MEGA case-control study. Am J Clin Nutr 101: 606–612, 2015. doi: 10.3945/ajcn.114.095398. [DOI] [PubMed] [Google Scholar]
- 63.Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients 12: 2097, 2020. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]