Abstract
While SARS-CoV-2 primarily affects the lungs, the virus may be inflicting detriments to the cardiovascular system, both directly through angiotensin-converting enzyme 2 receptor and initiating systemic inflammation. Persistent systemic inflammation may be provoking vascular dysfunction, an early indication of cardiovascular disease risk. To establish the potential effects of SARS-CoV-2 on the systemic vasculature in the arms and legs, we performed a cross-sectional analysis of young healthy adults (control: 5 M/15 F, 23.0 ± 1.3 y, 167 ± 9 cm, 63.0 ± 7.4 kg) and young adults who, 3–4 wk prior to testing, had tested positive for SARS-CoV-2 (SARS-CoV-2: 4 M/7 F, 20.2 ± 1.1 y, 172 ± 12 cm, 69.5 ± 12.4 kg) (means ± SD). Using Doppler ultrasound, brachial artery flow-mediated dilation (FMD) in the arm and single passive limb movement (sPLM) in the leg were assessed as markers of vascular function. Carotid-femoral pulse wave velocity (PWVcf) was asvsessed as a marker of arterial stiffness. FMD was lower in the SARS-CoV-2 group (2.71 ± 1.21%) compared with the control group (8.81 ± 2.96%) (P < 0.01) and when made relative to the shear stimulus (SARS-CoV-2: 0.04 ± 0.02 AU, control: 0.13 ± 0.06 AU, P < 0.01). The femoral artery blood flow response, as evidenced by the area under the curve, from the sPLM was lower in the SARS-CoV-2 group (−3 ± 91 mL) compared with the control group (118 ± 114 mL) (P < 0.01). PWVcf was higher in the SARS-CoV-2 group (5.83 ± 0.62 m/s) compared with the control group (5.17 ± 0.66 m/s) (P < 0.01). Significantly lower systemic vascular function and higher arterial stiffness are evident weeks after testing positive for SARS-CoV-2 among young adults compared with controls.
NEW & NOTEWORTHY This study was the first to investigate the vascular implications of contracting SARS-CoV-2 among young, otherwise healthy adults. Using a cross-sectional design, this study assessed vascular function 3–4 wk after young adults tested positive for SARS-CoV-2. The main findings from this study were a strikingly lower vascular function and a higher arterial stiffness compared with healthy controls. Together, these results suggest rampant vascular effects seen weeks after contracting SARS-CoV-2 in young adults.
Keywords: COVID-19, flow-mediated dilation, passive limb movement, pulse wave velocity, SARS-CoV-2
INTRODUCTION
Little is known regarding the long-term health consequences of contracting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing the novel coronavirus disease 2019 (COVID-19) (1). The etiology of COVID-19 progression involves interaction between SARS-CoV-2 and the angiotensin-converting enzyme 2 (ACE2) receptor (2), which is present in nearly every human tissue including lung, heart, kidneys, and intestine, suggesting vast consequences for physiological function (3). Further, the systemic cytokine-induced inflammatory response (4, 5) caused by viral detection and propagation can have prolonged, deleterious effects downstream of the initial viral parasitism in the lung (6), quite possibly causing severe physiological impairments to the vasculature (7).
Early investigations have revealed SARS-CoV-2 is able to infect endothelial cells, which are primarily responsible for regulating vascular tone (8). The ACE2 receptor, which the SARS-CoV-2 virus binds to, can be found on these endothelial cells (2), potentially jeopardizing vascular function among COVID-19-positive individuals. Indeed, previous investigations in animal models (9) and humans (10) of viral mimetic activation of Toll-like receptor 3 have reported an increase in inflammation mediated by innate and adaptive immunity, which may provoke vascular dysfunction. Furthermore, the association between SARS-CoV-2 transmission and stroke (11) as well as myocardial infarction (12) risk suggests a link between blood flow delivery impairments and acute cardiovascular risk. While proposals have suggested that endothelial biomarkers and tests of vascular function should be evaluated (13), we have yet to identify any assessments of vascular impairments among those who have recently contracted SARS-CoV-2.
Thus, the purpose of this initial investigation was to determine if contracting SARS-CoV-2 may have prolonged effects on the systemic vasculature among otherwise healthy young adults. Using a cross-sectional comparison with young healthy adults, we hypothesized vascular function, as assessed by flow-mediated dilation (FMD) (14) and reactive hyperemia (RH) (15) in the arm as well as the single passive limb movement (sPLM) (16), would be reduced several weeks after testing positive for SARS-CoV-2. Further, we hypothesized these functional decrements would be accompanied by heightened vascular stiffness, as determined by carotid-femoral pulse wave velocity (PWVcf) (17).
METHODS
Subjects
Subjects were relatively healthy, as evidenced by the lack of chronic cardiovascular, pulmonary, or metabolic diseases as well as based on a subjective physical activity questionnaire; were not pregnant or trying to become pregnant; were premenopausal for female subjects; were nonsmokers; and were not taking any medications known to alter vascular function, including sympathetic adrenergic agonists or antagonists, cholinergic agonists or antagonists, β-blockers, diuretics, statins, or ACE inhibitors. Subjects were included in the SARS-CoV-2 group if they tested positive for SARS-CoV-2 using nasopharyngeal swab polymerase chain reaction assay 3–4 wk before study testing. Control subjects were studied February 4–6, 2020, before the first confirmed case of COVID-19 in North Carolina, United States, on March 3, 2020 (18), and before the World Health Organization declaring COVID-19 a pandemic on March 11, 2020. Control subjects had not experienced flu-like symptoms. All procedures were approved by the Appalachian State University Institutional Review Board, and the measurements were performed in a thermoneutral environment. The subjects provided written informed consent in accordance with the standards outlined by the Declaration of Helsinki.
Study Procedures
Subjects were tested in a fasted state, having abstained from food and caffeine for at least 12 h and alcohol or exercise for at least 24 h before testing procedures. All subjects had their health history recorded, including physical activity and any current medications. Subjects were tested in a quiet, thermoneutral environment (barometric pressure: 692–736 mmHg, temperature: 22–23°C, relative humidity: 33–50%). All procedures were performed with the subjects lying supine for at least 20 min before testing and following supine brachial artery blood pressure (BP) measurements. Study procedures were ordered similarly between subjects to maintain standardization.
Experimental Measurements
COVID-19 symptom severity survey.
Subjects who tested positive for SARS-CoV-2 were asked to rank their COVID-19 symptoms on the day of study testing. On a scale of 0–100 of increasing severity, subjects subjectively ranked their symptoms of chest pain, chills, diarrhea, dizziness or vertigo, dry cough, dry eyes, dry mouth, fatigue, fever over 37.9°C, headache, lack of appetite, loss of smell or taste (anosmia), muscle or body aches, nasal congestion or runny nose, nausea or vomiting, shortness of breath, difficulty breathing, dyspnea, sore joints, or sore throat. The values for each symptom were totaled and averaged for each symptom severity.
Brachial artery flow-mediated dilated and reactive hyperemia.
Brachial artery FMD measurements were obtained from the right brachial artery using current guidelines as a functional, upper limb marker of vascular function and cardiovascular risk (14). Baseline measurements of the right brachial artery diameter and blood velocity were taken for 1 min using a Doppler ultrasound system (GE Logiq eR7 and L4-12T-RS transducer, GE Medical Systems, Milwaukee, WI). Sample volume was optimized in relation to vessel diameter and centered within the vessel for each subject. Measurements of brachial artery diameter and velocity were obtained with the Doppler ultrasound in duplex mode with B-mode imaging frequency of 12 MHz and Doppler frequency of 4 MHz. An angle of insonation of ≤60° (19) was achieved for all measurements. Immediately after baseline measurements, a blood pressure cuff, placed distal to the elbow, was rapidly inflated to 250 mmHg for 5 min. The blood pressure cuff was rapidly deflated, and brachial artery diameter and velocity were recorded for 2 min. Brachial artery diameter, blood velocity, blood flow, shear rate from cuff deflation to peak diameter, and 2-min RH were analyzed offline for continuous second-by-second measurements (Cardiovascular Suite v. 4.0, Quipu, Pisa, Italy). Blood flow was determined as: , where blood velocity was obtained as the time average mean on the Doppler ultrasound. RH was determined as the area under the curve for the blood flow response following cuff occlusion, providing an index of microvascular function, which is inversely related to cardiovascular disease risk (20) and is predictive of future cardiovascular events in healthy and diseased populations (15).
Femoral artery single passive leg movement.
Femoral artery sPLM measurements were obtained from the right femoral artery using current guidelines as a functional, lower-limb marker of vascular function (16). While in the supine position with the subject’s left leg supported on a stool and right leg supported by a research team member at heart level, baseline measurements of the common femoral artery diameter and blood velocity, at least 3 cm proximal the femoral artery bifurcation, were recorded for 1 min before passive limb movement using similar Doppler ultrasound system settings used for the brachial artery FMD procedure. Immediately following baseline measurements, the research team member supporting the right thigh and ankle manually moved the knee joint one time through 90° range of motion, flexion-extension, at 1 Hz while common femoral artery diameter and blood velocity were recorded for 1 min after the movement.
Carotid-femoral pulse wave velocity.
Ultrasound Doppler measurements were taken at the carotid and femoral arteries to assess peripheral arterial stiffness. Brachial artery blood pressure was determined before pulse wave velocity (PWV) measurements. PWVcf was calculated using the foot-to-foot ECG-gated method using current guidelines (17) and expressed as meters per second (m/s).
Statistical Analysis
Statistics were performed using commercially available software (IBM SPSS Statistics v. 26, Armonk, NY). Two-tailed Student’s t tests for two samples of equal variance were performed between groups. As an exploratory investigation, additional comparisons were made between groups within sexes while controlling for multiple comparisons. Data were checked for normality using the Shapiro–Wilk test and boxplots. Significant differences for FMD% and PWVcf were determined using analysis of covariance and general linear model, with baseline diameter and resting mean arterial pressure as covariates, respectively. Statistical significance was specified at P < 0.01. Subject characteristics and outcome measures are expressed as means ± SD.
RESULTS
Subject Characteristics
Subject characteristics of 15 female and five male subjects who did not test positive for SARS-CoV-2 (control group) as well as four male and seven female subjects who tested positive for SARS-CoV-2 (SARS-CoV-2 group) are presented in Table 1. Subjects who tested positive for SARS-CoV-2 were studied 25 ± 5 days since symptom onset (n = 10) and 24 ± 6 days after their positive testing date (n = 11). One female subject who tested positive for SARS-CoV-2 was asymptomatic, although most had mild, lingering symptoms. Subjects were devoid of any medication usage other than oral contraceptives for most of the female subjects in each group.
Table 1.
Subject characteristics
| Control (n = 5 M/15 F) | SARS-CoV-2 (n = 4 M/7 F) | Control (n = 5 M) | SARS-CoV-2 (n = 4 M) | Control (n = 15 F) | SARS-CoV-2 (n = 7 F) | |
|---|---|---|---|---|---|---|
| Age, yr | 23.0 ± 1.3 | 20.1 ± 1.1* | 22.6 ± 1.1 | 20.8 ± 0.5 | 23.2 ± 1.3 | 19.9 ± 1.2 |
| Height, cm | 167.4 ± 9.3 | 171.5 ± 11.9 | 179.3 ± 7.4 | 182.2 ± 7.0 | 163.4 ± 7.3 | 165.4 ± 9.4 |
| Weight, kg | 63.0 ± 7.4 | 69.5 ± 12.4 | 66.8 ± 7.0 | 75.1 ± 8.1 | 61.8 ± 7.3 | 66.3 ± 13.8 |
| BMI, kg/m2 | 22.5 ± 2.2 | 23.5 ± 2.9 | 20.8 ± 1.7 | 22.5 ± 1.1 | 23.1 ± 2.1 | 24.1 ± 3.5 |
| Supine systolic arterial pressure, mmHg | 111.8 ± 13.4 | 121.3 ± 12.3 | 114.2 ± 11.2 | 122.0 ± 13.5 | 110.9 ± 14.5 | 120.9 ± 12.7 |
| Supine diastolic arterial pressure, mmHg | 77.7 ± 7.7 | 71.8 ± 7.1 | 79.0 ± 12.0 | 68.6 ± 6.5 | 77.2 ± 6.0 | 73.6 ± 7.3 |
| Supine mean arterial pressure, mmHg | 89.9 ± 7.5 | 88.3 ± 8.2 | 90.8 ± 9.6 | 86.4 ± 8.4 | 91.4 ± 9.5 | 89.4 ± 8.6 |
| Physical activity frequency, day/wk | 4.1 ± 1.5 | 3.6 ± 1.2 | 5.4 ± 1.8 | 3.0 ± 0.8 | 3.7 ± 1.1 | 3.9 ± 1.4 |
| Physical activity duration, min/day | 44.3 ± 14.4 | 38.9 ± 12.5 | 51.0 ± 12.3 | 37.5 ± 12.3 | 42.0 ± 14.7 | 39.6 ± 13.5 |
| Oral contractive use, % females | 75 | 71 | ||||
| Number of symptoms | 2.9 ± 2.3 | 2.8 ± 1.0 | 3.0 ± 2.9 | |||
| Average symptom severity, 0–100 | 15.0 ± 12.2 | 13.0 ± 6.9 | 16.1 ± 14.9 |
Values are means ± SD; two-tailed Student’s t tests for two samples of equal variance were performed between control (n = 5 M/15 F) and SARS-CoV-2 (n = 4 M/7 F) groups. BMI: body mass index.
*P < 0.01, between groups.
Brachial Artery Flow-Mediated Dilation
Measurements of brachial artery FMD are presented in Fig. 1. Baseline brachial artery diameters were similar between groups (total control: 3.69 ± 0.49 mm, total SARS-CoV-2: 3.85 ± 0.38 mm; male control: 4.23 ± 0.19 mm, male SARS-CoV-2: 4.11 ± 0.34 mm; female control: 3.49 ± 0.41 mm, female SARS-CoV-2: 3.71 ± 0.34 mm). Time to peak vasodilation was similar between groups (total control: 58 ± 23 s, total SARS-CoV-2: 59 ± 26 s; male control: 64 ± 28 s, male SARS-CoV-2: 55 ± 19 s; female control: 55 ± 22 s, female SARS-CoV-2: 61 ± 31 s). Absolute change in brachial artery diameter from baseline to peak vasodilation was different between groups (total control: 0.30 ± 0.12 mm, total SARS-CoV-2: 0.12 ± 0.07 mm, P < 0.01; male control: 0.37 ± 0.08 mm, male SARS-CoV-2: 0.14 ± 0.12 mm, P < 0.01; female control: 0.28 ± 0.12 mm, female SARS-CoV-2: 0.12 ± 0.03 mm, P < 0.01). Sum of shear at peak vasodilation was not different between groups (total control: 79,077 ± 29,859 AU, total SARS-CoV-2: 78,279 ± 28,528 AU; male control: 65,262 ± 24,192 AU, male SARS-CoV-2: 70,987 ± 18,613 AU; female control: 83,682 ± 30,852 AU, female SARS-CoV-2: 82,445 ± 33,579 AU). The FMD response was different between groups when expressed as a percentage (total control: 8.81 ± 2.96%, total SARS-CoV-2: 2.71 ± 1.21%, P < 0.01; male control: 8.70 ± 1.75%, male SARS-CoV-2: 1.87 ± 1.45%, P < 0.01; female control: 8.85 ± 3.31%, female SARS-CoV-2: 3.20 ± 0.81%, P < 0.01) and when made relative to the shear stimulus (total control: 0.13 ± 0.06 AU, total SARS-CoV-2: 0.04 ± 0.02 AU, P < 0.01; male control: 0.14 ± 0.05 AU, male SARS-CoV-2: 0.03 ± 0.02 AU, P < 0.01; female control: 0.12 ± 0.06 AU, female SARS-CoV-2: 0.04 ± 0.01 AU, P < 0.01).
Figure 1.
Brachial artery flow-mediated dilation (FMD) expressed as percentage change (A) and normalized to shear (B). Two-tailed Student’s t tests for two samples of equal variance were performed between control (n = 5 M/15 F) and SARS-CoV-2 (n = 4 M/7 F) groups. *P < 0.01, between groups. Data are means ± SD.
Reactive Hyperemia
Baseline brachial artery blood flow was similar between groups (total control: 125 ± 63 mL/min, total SARS-CoV-2: 150 ± 60 mL/min; male control: 157 ± 64 mL/min, male SARS-CoV-2: 186 ± 48 mL/min; female control: 115 ± 60 mL/min, female SARS-CoV-2: 130 ± 59 mL/min). Likewise, the blood flow response to the 5-min cuff occlusion, as assessed by area under the curve (AUC), between groups was similar (total control: 570 ± 210 mL/min, total SARS-CoV-2: 613 ± 175 mL/min; male control: 730 ± 265 mL/min, male SARS-CoV-2: 711 ± 200 mL/min; female control: 559 ± 277 mL/min, female SARS-CoV-2: 557 ± 145 mL/min).
Femoral Artery Single Passive Limb Movement
Measurements of femoral artery sPLM are presented in Fig. 2. Baseline femoral artery blood flow was similar between groups (total control: 563 ± 159 mL/min, total SARS-CoV-2: 743 ± 358 mL/min; male control: 592 ± 221 mL/min, male SARS-CoV-2: 673 ± 118 mL/min; female control: 553 ± 141 mL/min, female SARS-CoV-2: 784 ± 448 mL/min). Peak blood flow following the sPLM was similar between groups (total control: 914 ± 298 mL/min, total SARS-CoV-2: 1,053 ± 474 mL/min; male control: 908 ± 535 mL/min, male SARS-CoV-2: 975 ± 179 mL/min; female control: 916 ± 199 mL/min, female SARS-CoV-2: 1,098 ± 592 mL/min). Area under the curve, as an indication of microvascular hyperemic response to the sPLM, was different between groups (total control: 118 ± 114 mL, total SARS-CoV-2: −3 ± 91 mL, P < 0.01) but not when separated by sex (male control: 85 ± 113 mL, male SARS-CoV-2: 39 ± 24 mL, P > 0.01; female control: 129 ± 117 mL, female SARS-CoV-2: 25 ± 71 mL, P > 0.01).
Figure 2.

Single passive limb movement. Common femoral artery blood flow change from baseline following a single passive limb movement (A) with the 60-s area under the curve (B). Two-tailed Student’s t tests for two samples of equal variance were performed between control (n = 5 M/15 F) and SARS-CoV-2 (n = 4 M/7 F) groups. *P < 0.01, between groups. Data are means ± SD.
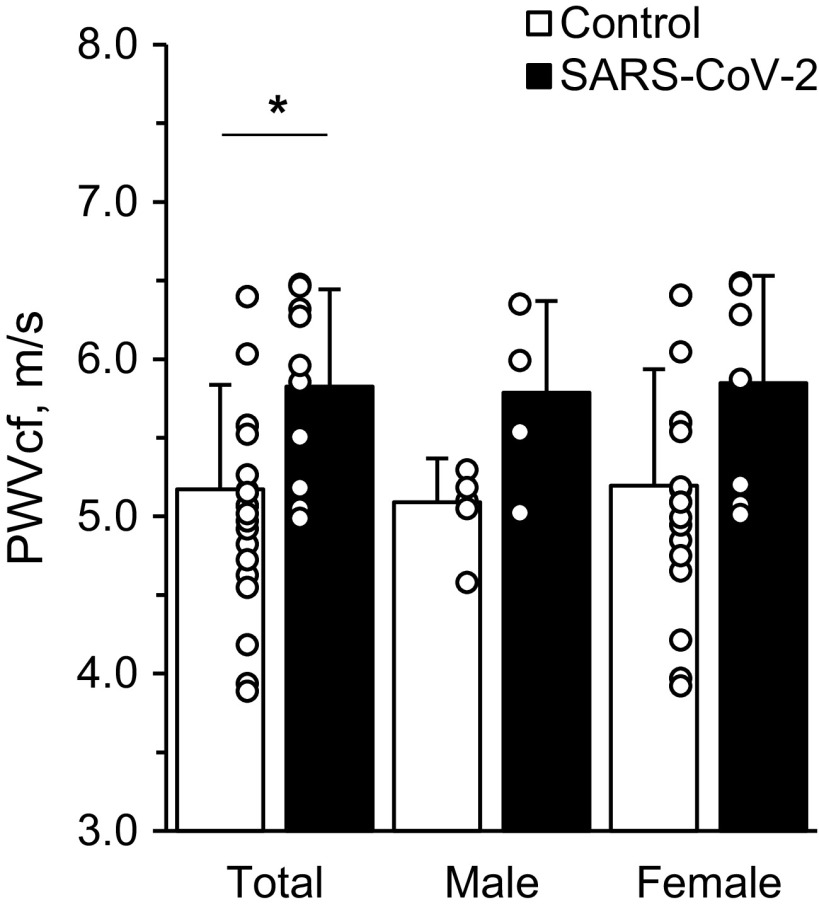
Carotid-Femoral Pulse Wave Velocity
Measurements of PWVcf are presented in Fig. 3. PWVcf was different between groups (total control: 5.17 ± 0.66 m/s, total SARS-CoV-2: 5.83 ± 0.62 m/s, P < 0.01) but not when separated by sex (male control: 5.09 ± 0.28 m/s, male SARS-CoV-2: 5.79 ± 0.58 m/s, P > 0.01; female control: 5.20 ± 0.74 m/s, female SARS-CoV-2: 5.85 ± 0.68 m/s, P > 0.01).
Figure 3.
Carotid-femoral pulse wave velocity. Two-tailed Student’s t tests for two samples of equal variance were performed between control (n = 5 M/15 F) and SARS-CoV-2 (n = 4 M/7 F) groups. *P < 0.01, between groups. Data are means ± SD.
DISCUSSION
Our data indicate SARS-CoV-2 may have detrimental effects on the systemic vasculature in young adults. In support of our hypothesis, we observed a significantly lower brachial artery FMD among subjects who, 3–4 wk before study testing, tested positive for SARS-CoV-2. Contrary to our hypothesis, RH was similar between groups, suggesting microvascular function, at least in the arm, may be intact. Further, as a lower-extremity vascular function assessment, the SARS-CoV-2 group had a lower femoral artery hyperemic response to the sPLM test, as evidenced by a smaller area under the curve. The results from the FMD and sPLM provide evidence for impaired NO bioavailability and lower vascular function. Finally, we observed a greater PWVcf among subjects who tested positive for SARS-CoV-2, providing evidence for an additional biomarker of arterial stiffness and cardiovascular disease risk. Although exploratory, we observed similar findings between groups within male and female subjects, suggesting there may not be sex differences in vascular function 3–4 wk following SARS-CoV-2 infection. Findings from this cross-sectional comparison provide evidence for the damaging effects of contracting SARS-CoV-2 among young adults.
Flow-Mediated Dilation and SARS-CoV-2
The brachial artery FMD technique is a noninvasive assessment of systemic vascular function (21) that strongly correlates with coronary vascular function (22) and is predictive of future cardiovascular events (23). In the current investigation, the 6% lower brachial artery FMD is clinically meaningful, as every 1% lower brachial artery FMD% is associated with an ∼13% higher risk of cardiovascular events such as heart attack, stroke, or death (24).
Reactive Hyperemia and SARS-CoV-2
Whereas brachial artery FMD is arguably a functional bioassay for endothelial-derived NO (25), the RH response to forearm cuff occlusion is only minimally influenced by NO (26) and rather alternative pathways such as inwardly rectifying K+ channels and Na+/K+ ATPase (27). Therefore, the discrepancy in the observed reduction in FMD and lack of a change in RH should not be too surprising if NO is to be primarily affected by SARS-CoV-2 directly or by a subsequent cytokine storm, oxidative stress, or inflammation (4, 5). While SARS-CoV-2 may induce systemic inflammatory response, previous investigations have provided evidence of direct inflammatory response in endothelial cells, which may provoke vascular dysfunction (9). Several mechanisms, including cytokines, Toll-like receptors, immune cell activation, and NADPH oxidase 2, may underlie endothelial and vascular dysfunction in SARS-CoV-2 (28). However, more work should surely discern whether the observed functional decrements are caused by an oxidative stress-induced decrease in NO or other vascular regulators.
Passive Limb Movement and SARS-CoV-2
The sPLM test is a lower-limb assessment of microvascular function, as the movement provokes NO-dependent microvascular vasodilation (14). The low hyperemic response to the sPLM test in the current investigation may indicate a diminished ability of the small arterioles to dilate when necessary. Most notably, the quick restoration of femoral artery blood flow following the movement may be an indication of diminished NO bioavailability, as previously observed when NO synthesis is blocked using Nw-monomethyl-l-arginine acetate (l-NMMA) (29). Ultimately, the results from the FMD and sPLM tests provide evidence for lower vascular function, which warrants further review to determine if NO or other vascular regulators are responsible for these observed functional decrements.
Pulse Wave Velocity and SARS-CoV-2
An ∼1-m/s elevation in PWV is associated with a 15% higher risk of cardiovascular events, mortality, and all-cause death (30). While we observed a 0.75-m/s higher PWVcf with the SARS-CoV-2 group, which may suggest higher arterial stiffness and cardiovascular disease risk, this elevated level is still within the expected range for this age-group and may not be clinically relevant.
Limitations
We recognize SARS-CoV-2 was in the United States before the COVID-19 pandemic declaration in March 2020. However, all control subjects were healthy at the time of testing in a US region lacking any positive SARS-CoV-2 cases (18). While the current investigation utilized noninvasive, functional biomarkers of vascular function, we recognize the current assessments cannot determine endothelial-dependent vasodilation. Certainly, future experiments could provoke endothelial-independent vasodilation using sublingual nitroglycerine or examining circulating or urinary nitrate/nitrite levels to determine NO bioavailability among individuals with SARS-CoV-2. While sPLM repeated measures may improve the precision of this measure, we believe these results corroborate the observed vascular function decrements regardless.
Conclusion
These results suggest numerous systemic vascular consequences among young adults that should not be overlooked, especially among those at higher risk of cardiovascular complications from contracting SARS-CoV-2. Remarkably, these decrements occurred in young, relatively healthy individuals devoid of any chronic diseases. Certainly, more work is needed to discern if these observations persist beyond the first 3–4 wk of contracting SARS-CoV-2 in male and female, symptomatic and asymptomatic individuals. We recognize the limitations of a cross-sectional comparison such as this and encourage future investigations to track individuals with SARS-CoV-2 longitudinally to determine the vascular recovery period. However, compared with healthy individuals, the effects of SARS-CoV-2 on the vasculature appear striking and imperative for scientific and medical community to understand the impact of this disease on human health.
GRANTS
This study was partially supported by an internal COVID-19 Research Cluster Award at Appalachian State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.M.R., J.L.S., and A.S.L.S. conceived and designed research; S.M.R., J.L.S., V.M.P., M.A.A., L.K.K., L.K.B., and A.S.L.S. performed experiments; S.M.R., J.L.S., V.M.P., M.A.A., L.K.K., L.K.B., and A.S.L.S. analyzed data; S.M.R., J.L.S., V.M.P., M.A.A., L.K.K., L.K.B., and A.S.L.S. interpreted results of experiments; S.M.R. and V.M.P. prepared figures; S.M.R., J.L.S., V.M.P., and A.S.L.S. drafted manuscript; S.M.R., J.L.S., V.M.P., M.A.A., L.K.K., L.K.B., and A.S.L.S. edited and revised manuscript; S.M.R., J.L.S., V.M.P., M.A.A., L.K.K., L.K.B., and A.S.L.S. approved final version of manuscript.
REFERENCES
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 3.Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181: 905–913.e7, 2020. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 39: 529–539, 2017. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 53: 25–32, 2020. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li K, Hao Z, Zhao X, Du J, Zhou Y. SARS-CoV-2 infection-induced immune responses: friends or foes? Scand J Immunol, 92: e12895, 2020. doi: 10.1111/sji.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, Schwartz A, Uriel N. COVID-19 and cardiovascular disease. Circulation 141: 1648–1655, 2020. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 8.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417–1418, 2020. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer S, Steinmetz M, Asdonk T, Motz I, Coch C, Hartmann E, Barchet W, Wassmann S, Hartmann G, Nickenig G. Activation of endothelial toll-like receptor 3 impairs endothelial function. Circ Res 108: 1358–1366, 2011. doi: 10.1161/CIRCRESAHA.111.243246. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol 5: 253, 2014. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 382: e60, 2020. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmud E, Dauerman HL, Welt FG, Messenger JC, Rao SV, Grines C, Mattu A, Kirtane AJ, Jauhar R, Meraj P, Rokos IC, Rumsfeld JS, Henry TD. Management of acute myocardial infarction during the COVID-19 pandemic. J Am Coll Cardiol 96: 336–345, 2020.doi: 10.1002/ccd.28946. [DOI] [PubMed] [Google Scholar]
- 13.Evans PC, Ed Rainger G, Mason JC, Guzik TJ, Osto E, Stamataki Z, Neil D, Hoefer IE, Fragiadaki M, Waltenberger J, Weber C, Bochaton-Piallat ML, Back M. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for atherosclerosis and vascular biology, and the ESC Council of basic cardiovascular science. Cardiovasc Res 116: 2177–2184, 2020. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr., Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol 27: 2113–2119, 2007. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gifford JR, Richardson RS. CORP: ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol (1985) 123: 1708–1720, 2017. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-Invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. [PMC doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 18.NC Department of Health and Human Services. North Carolina identifies first case of COVID-19 (Online). https://web.archive.org/web/20200407230739/https://www.ncdhhs.gov/news/press-releases/north-carolina-identifies-first-case-covid-19 [2020. Nov 22].
- 19.Rizzo RJ, Sandager G, Astleford P, Payne K, Peterson-Kennedy L, Flinn WR, Yao JS. Mesenteric flow velocity variations as a function of angle of insonation. J Vasc Surg 11: 688–694, 1990. doi: 10.1067/mva.1990.19707. [DOI] [PubMed] [Google Scholar]
- 20.Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and agonist-induced dilations. Circ Res 83: 960–965, 1998. doi: 10.1161/01.RES.83.9.960. [DOI] [PubMed] [Google Scholar]
- 21.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 22.Broxterman RM, Witman MA, Trinity JD, Groot HJ, Rossman MJ, Park SY, Malenfant S, Gifford JR, Kwon OS, Park SH, Jarrett CL, Shields KL, Hydren JR, Bisconti AV, Owan T, Abraham A, Tandar A, Lui CY, Smith BR, Richardson RS. Strong relationship between vascular function in the coronary and brachial arteries. Hypertension 74: 208–215, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010.doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 25.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995. doi: 10.1161/01.CIR.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 26.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol (1985) 81: 1807–1814, 1996. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- 27.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res 113: 1023–1032, 2013. doi: 10.1161/CIRCRESAHA.113.301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqi HK, Libby P, Ridker PM. COVID-19 - a vascular disease. Trends Cardiovasc Med 31: 1–5, 2020. doi: 10.1016/j.tcm.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]