ABSTRACT
Coronavirus disease 2019 (COVID-19) is a significant cause for intensive care unit (ICU) admission worldwide. Most COVID-19 infections are associated with lower respiratory abnormalities but it has been increasingly associated with extra-pulmonary manifestations. Guillain–Barre syndrome (GBS) is a rarely diagnosed but severe disease associated with COVID-19 infection. We describe the diagnostic process behind diagnosing GBS in an elderly male who developed acute-onset quadriparesis and respiratory muscle failure associated with severe COVID-19 pneumonia in a military ICU. A 69-year-old male was admitted to the ICU for acute hypoxemic respiratory failure due to COVID-19 pneumonia. He was subsequently intubated and treated with dexamethasone and remdesivir with improvement. On hospital day 32, the patient was extubated. Three days later, he developed acute, symmetric limb quadriparesis and respiratory muscle failure requiring reintubation. Analysis of his cerebrospinal fluid showed a cytoalbuminologic dissociation, and electromyography/nerve conduction study showed slowed nerve conduction velocity. These findings are consistent with GBS. Blood cultures, serum polymerase chain reaction testing, and clinical symptoms were not suggestive of other common pathogens causing his GBS. The patient’s acute GBS in the setting of recent severe COVID-19 infection strongly suggests association between the two entities, as supported by a growing body of case literature. The patient was subjected to intravenous immunoglobulin treatment and was discharged with greatly improved strength in the upper and lower extremities. Our goal in describing this case is to highlight the need for providers to consider, accurately diagnose, and treat GBS as a consequence of severe COVID-19 infection.
CASE
Coronavirus disease-2019 (COVID-19) has rapidly developed from an emerging threat in 2019 to a global disease that has infected over 48 million people and killed 1.2 million people worldwide as of November 2020.1 Coronavirus disease-2019 (COVID-19) has been primarily described as a respiratory syndrome, but has been associated with vascular sequelae such as coagulopathy and stroke, and dysgeusia.2 A rarely diagnosed sequelae of COVID-19 is the denovo development of Guillain–Barre syndrome (GBS). We present a case of an elderly male who developed GBS associated with severe COVID-19 pneumonia.
A 69-year-old male with a history of chronic myelogenous leukemia, hypertension, and coronary artery disease was admitted to the intensive care unit (ICU) for hypoxemic respiratory failure secondary to COVID-19 pneumonia. Upon initial presentation to the emergency department (ED), the patient reported a subjective headache, mild shortness of breath, decreased oral intake, 3 days of watery diarrhea, and subjective fever at home. A review of his medical record did not reveal any prior history of neurologic disease or episodes of GBS. His home medications were significant for metoprolol succinate, folic acid, esomeprazole, and ferrous sulfate. Upon evaluation in the ED, his vital signs were significant for a temperature of 102 °F and an oxygen saturation of 90% on 35 L of high-flow nasal cannula at 45% FiO2. Physical examination did not reveal significant rales or rhonchi in the lung fields bilaterally, and the patient’s strength and sensation were intact in all limbs. Laboratory evaluation was significant for a positive Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) polymerase chain reaction (PCR) nasal swab, as well as an elevated white blood cell count with concomitant lymphopenia, elevated systemic inflammatory markers, and fibrin d-dimer. A portable chest radiograph (Fig. 1) showed evidence of bilateral patchy opacities consistent with early COVID-19 syndrome.3
FIGURE 1.
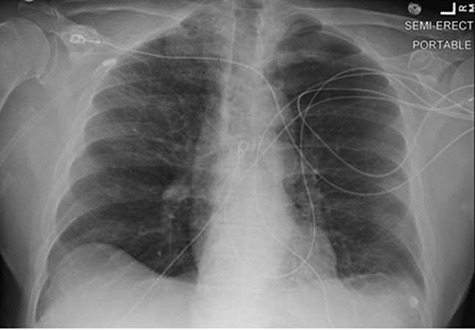
Portable frontal chest radiograph taken on the day of admission to the intensive care unit. There are patchy opacifications with multiple circular lucencies noted in the right upper lobe and peripheral left mid-lung, consistent with findings of early coronavirus disease-2019 pneumonia.
Upon admission, the patient was started on dexamethasone and remdesivir. On hospital day 2, the patient required intubation due to worsening hypoxemic respiratory failure that was refractory to noninvasive positive pressure ventilation. Although intubated for the next 10 days, the patient finished his 10-day course of remdesivir, dexamethasone, and proning as tolerated for at least 12 hours daily with sedation breaks for neurologic examinations. He was also discovered to have a pulmonary embolism and was treated with therapeutic enoxaparin. Throughout this time, his ventilator FiO2 and positive end expiratory pressure requirement was gradually weaned toward physiologic levels.
On hospital day 32, the patient met criteria for and passed a spontaneous breathing trial for ventilator liberation. He was successfully extubated and placed on bilevel positive pressure ventilation. At that time, the patient was able to flex and extend his feet, as well as give a “thumbs up” to providers. The patient was subsequently noted to have progressively decreased volitional limb movement between hospital days 32 and 35. On hospital day 35, the patient complained of “feeling weak” and was found to have symmetric flaccid quadriparesis (1/5) in the upper and lower extremities. Over the next 24 hours, the patient developed worsening hypoxemia and hypercarbia, with an inability to clear airway secretions. He was reintubated due to hypoxemic, hypercapnic respiratory failure. During this time, the patient had no new rashes, oral petechiae, splenomegaly, diarrhea, or abdominal pain. Two sets of blood cultures collected at this time did not show any growth. A serum PCR was negative for all tested bacterial organisms including Haemophilus influenzae and Escherichia coli. Two sets of tracheal cultures were positive only for Candida albicans, which was treated with fluconazole. Multiple differential diagnoses were considered, with critical illness polyneuropathy (CIPN)/myopathy (CIM) initially thought likely, given the lack of clinical and laboratory findings consistent with typical causes of GBS such as Campylobacter jejuni, H. influenzae, and E. coli. However, the timeline of peripheral muscle weakness was inconsistent with CIPN/CIM, as CIPN/CIM often occurs during mechanical ventilation and/or worsening critical disease course between days to 2 weeks into the illness.4 Recent literature described small case reports of COVID-19 associated with GBS, including a case series by Toscano et al. that described five cases of acute-onset GBS with multi-limb paresis in the setting of COVID-19 respiratory symptoms, which led to the reconsideration of GBS associated with COVID-19 in this patient.5
We pursued further examination that revealed continued flaccid tone in the extremities as well as limb areflexia (Table I) with decreased sensation in the lower limbs. An expanded neurologic workup was performed including lumbar puncture with cerebrospinal fluid (CSF) analysis and electromyography/nerve conduction studies (EMG/NCS). Cerebrospinal fluid (CSF) analysis showed an elevated protein count of 92.8 mg/dL with a white blood cell (WBC) count of 9 cells/µL, red blood cell count of 5,000 cells/µL, and a glucose count of 87 mg/dL. The patient’s EMG results were significant for prolonged sensory and motor amplitudes as well as widespread, reduced sensory nerve and compound motor action potential amplitudes. There was uniform but moderate slowing of conduction velocities in the left median and ulnar nerves with severe, demyelinating range slowing of left tibial nerve conduction velocity (29.9 m/s). Needle EMG was significant for reduced recruitment but did not show any signs of ongoing axon loss (no fibrillation or positive sharp waves). There was no clear conduction block evident in any of the motor studies. Upon completion of the EMG/NCS studies for the patient’s left side, he endorsed worsening left upper extremity pain and requested termination of the study, so right extremity EMG/NCS was not performed.
TABLE I.
Physical Examination of Peripheral Limb Reflexes
| Bicep reflex | Tricep reflex | Brachioradialis Reflex |
Patellar reflex | Achilles reflex | Babinski reflex | |
|---|---|---|---|---|---|---|
| Right | Absent | Not tested | Absent | Absent | Absent | Absent |
| Left | Absent | Not tested | Absent | Absent | Absent | Absent |
On examination, bicep, brachioradialis, patellar, Achilles, and Babinski reflex were absent on both left and right.
Given clinical findings, EMG/NCS results, and CSF cytoalbuminologic disassociation, the patient was diagnosed with GBS in the setting of severe COVID-19 infection on hospital day 38 and treated with intravenous (IV) immunoglobulin (IVIg) at 2 g/kg of ideal body weight divided over a 5-day course with slow but significant improvement on weaning of ventilator support. On hospital day 54, the patient was successfully extubated. At the time of extubation, the patient’s upper extremity strength had improved to 2/5 on the left and 4/5 on the right and 3/5 in the lower extremities. His strength continued to improve and he was discharged to home on hospital day 95. On discharge, the patient’s upper extremity strength had improved to 3/5 on the left and 4/5 on the right. His lower extremity strength had also improved to 4/5 bilaterally, and he was able to perform his activities of daily living as evaluated by occupational therapy. When he was evaluated virtually in follow-up approximately 100 days after being diagnosed with GBS, he was fully independent in his activities of daily living and able to walk 3.21 Kilometers independently when allowing for frequent breaks.
DISCUSSION
Guillain–Barre syndrome (GBS) associated with COVID-19 is becoming more frequently described in medical literature.6 The pathogenesis of GBS in COVID-19 is yet unclear, although emerging research suggests immune cross-reactivity with SARS-CoV-2 viral particles anchoring on gangliosides as a cause for immune-mediated demyelination.7 Others have proposed a molecular mimicry effect between SARS-CoV-2 viral proteins and neuronal gangliosides causing autoimmune attack.8 Evidence from murine models support the theory of molecular mimicry, showing SARS-CoV-2 viral spike protein involvement in GBS.8 However, additional studies are needed to elucidate the pathogenesis of GBS in the setting of COVID-19.
The diagnosis of GBS with the Brighton Criteria requires clinical symptoms of progressive limb weakness, decreased deep tendon reflexes or areflexia, and abnormal laboratory and abnormal conduction study findings.9 Case reports of COVID-19-associated GBS have described the onset of weakness from 5 days to 21 days after the start of COVID-19 symptoms.5,6 Guillain–Barre syndrome (GBS) symptom progression is often rapid and last from days to 4 weeks, with relative symmetry. In the natural disease course, strength deteriorates steadily, reaching maximal weakness at 4 weeks. Thereafter, patients experience a plateau phase that may last days to months, followed by a slower recovery phase.10 Patients may also report pain, autonomic dysfunction, or cranial nerve involvement.11 Laboratory analysis of CSF often shows cytoalbuminologic disassociation characterized by a CSF WBC count <10 cells/µL and abnormally elevated CSF protein concentration >58 mg/dL.12,13 Nerve conduction studies may also be performed to support a diagnosis of GBS.14 Guillain–Barre syndrome (GBS) is often caused by preceding viral illness, with over 70% of patients reporting preceding fevers and myalgias. Pathogens known to cause GBS include enteroviruses, Epstein–Barr Virus, and bacteria such as E. coli, C. jejuni, and H. influenzae.12,14,15 Once diagnosed via clinical symptoms, lumbar puncture, and EMG/NCS, the American Academy of Neurology recommends treatment with either IVIg or plasma exchange regimen to hasten recovery in patients with difficulty ambulating.16 Patients are treated with standard dose IVIg of 2 g/kg ideal bodyweight given as five doses of 0.4 g/kg over 5 days. Thereafter, recovery of strength is fluctuating and variable, with full recovery ranging from days in mild cases, to months in severe cases.10
Our patient met the above Brighton Criteria in hospital days 32–35. He had an acute, symmetric quadriparesis, bilateral areflexia, a nadir in symptoms between 12 hours and 28 days, abnormal EMG/NCS findings, and evidence of cytoalbuminologic disassociation with a protein level of 87.8 mg/dL after correction for red blood cells from a traumatic tap in the presence of only 9 WBC/µL.13 Notably, the patient’s clinical course was inconsistent with other common causes of GBS, such as Epstein–Barr Virus and C. jejuni, H. influenzae, and E. coli. He did not have rash, petechiae, splenomegaly, blood cultures, or serum PCR to suggest the common etiologies of GBS. The patient’s acute GBS in the setting of recent severe COVID-19 infection strongly suggests association between the two entities, as supported by a growing body of case literature. In our case as well as in COVID-19-associated GBS cases reported by Toscano et al., the patient had improvement in muscle strength by the 4-week mark after initiation of IVIg therapy.5
The diagnosis of GBS in critically ill COVID patients may not be initially clear and may even arise as part of the sequela of a COVID infection in the absence of a significant pneumonia.5 Furthermore, considerable clinical overlap exists between GBS and CIPN/CIM. Both syndromes are generally marked by loss of deep tendon reflexes, flaccid limb weakness, and possible progression to respiratory muscle failure.4,11 Classically, GBS is known to be cause respiratory weakness resulting in mechanical ventilation, while CIPN/CIM often occurs during prolonged mechanical ventilation.4 Up to 25% of GBS patients may require initiation of mechanical ventilation. Meanwhile, CIM and CIPN are reported from 25% to 83% in intubated patients, respectively.4 However, clinicians must recognize that these classical associations—GBS causing ventilator dependence versus CIPN/CIM during ventilator dependence—are misleading in COVID patients. We would urge providers to consider GBS in patients diagnosed with COVID-19 and with significant objective weakness to optimize therapeutic options.
To differentiate the syndromes, there are many features both subtle and diagnostic that differentiate GBS and CIPN/CIM. In our case, we noticed that the timeline of illness development was atypical for CIPN, which commonly occur by the second week of critical illness, although may occur as early as day 7.4 Critical illness myopathy (CIM) occurs even earlier, often presenting within days of critical illness.17 Our patient developed a rapid-onset quadriparesis with areflexia following a successful extubation, ending 30 days of mechanical ventilation, which is not consistent with CIPN/CIM. In the setting of clinical patient improvement after extubation, our patient’s progressive limb and respiratory weakness is more consistent with GBS. Cerebrospinal fluid (CSF) analysis and EMG/NCS serve to differentiate GBS from CIPN/CIM. The EMG pattern for CIM/CIPN will show a reduction in amplitude of muscle or nerve action potentials with preserved conduction velocity while GBS patients such as ours will have decreased nerve conduction velocity in 80% of cases.11,18 Furthermore, 90% of GBS patients will have CSF cytoalbuminologic dissociation within 2 weeks, whereas CSF protein concentrations are often unchanged in CIM/CIPN.18 These clinical and diagnostic features of CIPN/CIM versus GBS are summed up in Table II.
TABLE II.
Comparison of Clinical Features of Critical Illness Polyneuropathy/Myopathy (CIPN/CIM) Versus Guillain–Barre Syndrome (GBS)
| Clinical feature | CIPN/CIM | GBS |
|---|---|---|
| Time of onset | 1–2 weeks (CIPN), several days (CIM) | 5–21 days |
| Pattern of weakness | Flaccid limb weakness may lead to respiratory muscle failure | Flaccid limb weakness may lead to respiratory muscle failure |
| Reflexes | Decreased bilaterally | Decreased bilaterally |
| Cerebrospinal fluid findings | Usually normal | Cytoalbuminologic dissociation |
| Electromyography/Nerve conduction study findings | Reduction in amplitude of muscle or nerve action potentials with preserved conduction velocity | Decreased conduction velocity |
| Treatment | Treat underlying critical illness, supportive treatment | Intravenous immunoglobulin or plasma exchange with supportive therapy |
A sequela associated with GBS that is most concerning in the ICU is prolonged ventilator dependence due to respiratory muscle weakness. A prolonged or repeat intubation course puts the patient at elevated risk for ventilator-associated pneumonia (VAP).19,20 Although our patient ultimately did not develop a bacterial superinfection, he had multiple risk factors, such as COVID infection and multiple intubations, for development of VAP. Kalanuria et al. estimate that VAP occurs in 9–27% of all mechanically ventilated patients, with risk being described as 3% per day in the first 5 days, 2% per day in the next 5 days, and 1% per day thereafter. Patients who develop VAP have a 9–13% overall risk of death.19 Those who develop VAP later in the hospital course are more likely to be infected by multidrug-resistant bacteria.20 In emerging case data, COVID-19 patients are 2.1 times more likely to develop VAP that non-COVID patients, with observed mortality of 36%.21 Given these data, early detection and treatment of GBS may decrease risk of VAP by decreasing total duration of mechanical ventilation.
For early diagnosis and treatment of GBS to reduce the incidence of VAP, a reliable neurologic examination must be performed regularly on COVID-19 patients to diagnose GBS. Clinical detection of limb weakness with decreased or absent deep tendon reflexes is key. In our case, we were fortunate that the patient was able to endorse weakness close to the onset of his objective quadriparesis between hospital days 33 and 35, thus triggering objective evaluation for COVID-19-associated GBS and start of IVIg treatment. It is possible that an early neurologic examination on day 33 or 34 (versus after the patient’s endorsement of weakness on day 35) could have detected limb quadriparesis to initiate GBS workup and treatment 24–48 hours sooner. Therefore, we propose that all patients with COVID-19 pneumonia, particularly those who are intubated, receive a daily basic neurologic examination consisting of a cranial nerve exam, limb strength, sensation, and reflexes. We acknowledge this may be difficult for COVID-19 patients who are on IV sedation and intubated. Patients under IV sedation are often not cooperative with cranial nerve or strength testing. Furthermore, deep tendon reflexes may be significantly decreased in patients who are sedated. In patients sedated with propofol, 20% may have absent plantar reflex and 66% have decreased response.22 If the patient’s sedation prohibits cooperation with a useful examination, neurologic testing should be performed during the first available window of sedation down-titration or spontaneous breathing trial. Further testing with EMG/NCS as well as lumbar puncture with CSF analysis should be performed in patients who exhibit bilateral and flaccid weakness of the limbs with diminished or absent deep tendon reflexes. Patient with high likelihood of GBS based on the Brighton Criteria should be examined for functional ability. Those with GBS and have difficulty ambulating should promptly receive treatment either with plasma exchange or IVIg to hasten recovery and prevent sequelae.16
CONCLUSION
This is the first described case of GBS associated with COVID-19 pneumonia within the U.S. Military Health System. This case is of additional interest as the patient’s GBS developed de novo during his ICU course, causing hypoxemic, hypercapnic respiratory failure necessitating reintubation. This patient’s case highlights the need for providers to consider GBS as a sequela of COVID-19. Guillain–Barre syndrome (GBS) puts the patient at elevated risk for requiring mechanical ventilation and development of ventilator dependence with significantly increased risk for superimposed VAP and death. To both reduce potential days spent on mechanical ventilation and negative sequela, we urge providers to screen for GBS regularly in ICU patients with COVID-19 and initiate early treatment as indicated. We would additionally encourage providers to consider GBS as another potential sequelae in any patient previously diagnosed with COVID-19 infection, presenting with significant weakness.
ACKNOWLEDGMENT
None declared.
Contributor Information
CPT Alvin C Yiu, Department of Internal Medicine, Tripler Army Medical Center, Honolulu, HI 96859-5001, USA.
CPT Ali Hussain, Department of Internal Medicine, Tripler Army Medical Center, Honolulu, HI 96859-5001, USA.
Uzoagu A Okonkwo, Department of Internal Medicine, Tripler Army Medical Center, Honolulu, HI 96859-5001, USA.
LTC Rachel Villacorta-Lyew, Department of Pulmonary and Critical Care Medicine, Tripler Army Medical Center, Honolulu, HI 96859-5001, USA.
MAJ Michael J McMahon, Department of Pulmonary and Critical Care Medicine, Tripler Army Medical Center, Honolulu, HI 96859-5001, USA.
MAJ Matthew Blattner, Department of Neurology, Tripler Army Medical Center, Honolulu, HI 96859-5001, USA.
FUNDING
None declared.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Khan M, Adil SF, Alkhathlan HZ, et al. : COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules 2020; 26(1): 39.doi: 10.3390/molecules26010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caress JB, Castoro RJ, Simmons Z, et al. : covid‐19–associated Guillain‐Barré syndrome: the early pandemic experience. Muscle Nerve 2020; 62(4): 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong HYF, Lam HYS, Fong AH-T, et al. : Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology 2020; 296(2): E72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shepherd S, Batra A, Lerner DP: Review of critical illness myopathy and neuropathy. Neurohospitalist 2017; 7(1): 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toscano G, Palmerini F, Ravaglia S, et al. : Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med 2020; 382(26): 2574–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paybast S, Gorji R, Mavandadi S: Guillain-Barré syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurologist 2020; 25(4): 101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dalakas MC: Guillain-Barré syndrome: the first documented COVID-19–triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm 2020; 7(5): e781.doi: 10.1212/NXI.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Virani A, Rabold E, Hanson T, et al. : Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases 2020; 20: e00771.doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghazanfar H, Qazi R, Ghazanfar A, Iftekhar S: Significance of Brighton criteria in the early diagnosis and management of Guillain-Barré syndrome. Cureus. 2020; 12(5): e8318.doi: 10.7759/cureus.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Doorn PA, Kuitwaard K, Walgaard C, van Koningsveld R, Ruts L, Jacobs BC: IVIG treatment and prognosis in Guillain–Barré syndrome. J Clin Immunol 2010; 30(S1): 74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walling AD, Dickson G: Guillain-Barré syndrome. Am Fam Physician 2013; 87(3): 191–7. [PubMed] [Google Scholar]
- 12. Dimachkie MM, Barohn RJ: Guillain-Barré syndrome and variants. Neurol Clin 2013; 31(2): 491–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seehusen DA, Reeves MM, Fomin DA: Cerebrospinal fluid analysis. Am Fam Physician 2003; 68(6): 1103–8. [PubMed] [Google Scholar]
- 14. Nguyen TP, Taylor RS: Guillain Barre Syndrome. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 15. Jasti AK, Selmi C, Sarmiento-Monroy JC, Vega DA, Anaya J-M, Gershwin ME: Guillain-Barré syndrome: causes, immunopathogenic mechanisms and treatment. Expert Rev Clin Immunol 2016; 12(11): 1175–89. [DOI] [PubMed] [Google Scholar]
- 16. Hughes RA, Wijdicks EF, Barohn R, et al. : Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2003; 61(6): 736–40. [DOI] [PubMed] [Google Scholar]
- 17. McClafferty B, Umer I, Fye G, et al. : Approach to critical illness myopathy and polyneuropathy in the older SARS-CoV-2 patients. J Clin Neurosci 2020; 79: 241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou C, Wu L, Ni F, Ji W, Wu J, Zhang H: Critical illness polyneuropathy and myopathy: a systematic review. Neural Regen Res 2014; 9(1): 101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalanuria AA, Ziai W, Mirski M: Ventilator-associated pneumonia in the ICU [published correction appears in Crit Care. 2016; 20: 29. Zai, Wendy]. Crit Care 2014; 18(2): 208.doi: 10.1186/cc13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hcc P, Chianca GC, Iorio NLPP: COVID-19: an alert to ventilator-associated bacterial pneumonia. Infect Dis Ther 2020; 9(3): 417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maes M, Higginson E, Pereira-Dias J, et al. : Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care 2021; 25(1): 25.doi: 10.1186/s13054-021-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ordas-Bandera CM, Sanchez-Marcos C, Janeiro-Lumbreras D, et al. : Neurological examination in patients undergoing sedation with propofol: a descriptive study. Rev Neurol 2014; 58(12): 536–40. [PubMed] [Google Scholar]


