Abstract
We prospectively assessed 536 hospitalized patients with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction tests for infectiousness based on symptoms, cycle thresholds, and SARS-CoV-2 history, with repeat testing and serologies in select cases. One hundred forty-eight (28%) patients were deemed noninfectious, most with evidence of prior infection, and managed on standard precautions without evidence of transmission.
Keywords: COVID-19, isolation, polymerase chain reaction, SARS-CoV-2, transmission-based precautions
Many hospitals now test all admitted patients for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using polymerase chain reaction (PCR) assays. Patients with positive tests are placed in private rooms, are cared for using specialized personal protective equipment, may have nonurgent procedures and treatments delayed, and are often unable to receive visitors. PCR positivity, however, does not necessarily indicate infectiousness. SARS-CoV-2 RNA may be detected at low levels for weeks to months following an index illness, but several studies have demonstrated that most immunocompetent patients are no longer contagious after 5–10 days and unlikely to be reinfected within the next 6 months [1–5].
Little is known, however, about the optimal approach to clinically assessing infectiousness in hospitalized patients who test positive by PCR or the proportion of these patients who can be safely cared for using standard precautions. We therefore prospectively assessed all hospitalized patients with positive SARS-CoV-2 tests in a large academic hospital in Massachusetts during the 2020 winter coronavirus disease 2019 (COVID-19) surge for the necessity of isolation using an algorithm that incorporated SARS-CoV-2 history, symptoms, PCR cycle thresholds, and, for some patients, repeat testing and serologies.
METHODS
Study Design, Population, and Hospital Testing Protocol
Brigham and Women’s Hospital is an 803-bed academic hospital in Boston. We prospectively identified all adult hospitalized patients who tested positive for SARS-CoV-2 by PCR (primarily on the Hologic Panther Fusion or Cepheid Xpert platforms) between December 24, 2020, and March 31, 2021, using a daily electronic report. During the study period, all patients were tested on admission via nasopharyngeal swabs regardless of symptoms; patients with respiratory symptoms and an initial negative test were tested again after 12 hours to minimize the chance of a false-negative given the relatively higher pretest probability in this population [6]. Patients who were PCR negative on admission were also tested 72 hours later to identify virus potentially incubating on arrival; testing was continued every 3 days through the first 14 days of hospitalization for patients undergoing aerosol-generating procedures. Testing was also encouraged for hospitalized patients with any new symptoms potentially consistent with COVID-19.
Patients with a known prior SARS-CoV-2 infection within 90 days were flagged as “COVID-Recovered” in our electronic health record system. As per CDC guidance, asymptomatic testing during this 90-day period (including admission testing) was discouraged via electronic best practice alerts, and patients were managed on standard precautions. Testing was encouraged, however, if patients had new symptoms potentially consistent with COVID-19.
Infection Control Assessments of Infectiousness and Isolation Protocol
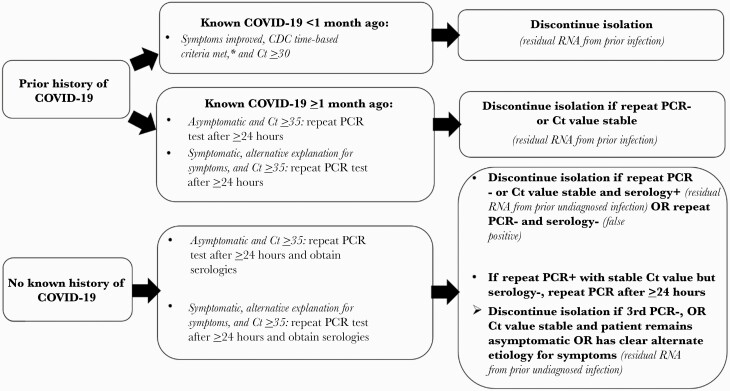
All PCR-positive patients were assessed by Infection Control for likely infectiousness and need for isolation. Patients with documentation of prior infection or cycle threshold (Ct) values ≥35 (on either platform) were assessed for the feasibility of early discontinuation of isolation using an algorithm that incorporated the presence or absence of symptoms potentially consistent with COVID-19, repeat PCR testing ≥24 hours later (to assess viral load kinetics and to ensure that high Ct values did not represent early acute infection [7]), and, if there was no known history of prior infection, SARS-CoV-2 serologies (Figure 1). We used Ct values of ≥35 as a conservative threshold based on studies that have been unable to recover viable virus when Ct values are >24–33 [1–4]. Serologies were performed using the Roche Elecsys Anti-SARS-CoV-2 antinucleocapsid total antibody assay, which has a sensitivity of 99.5% at ≥14 days following a PCR diagnosis and a specificity of 99.8% [8].
Figure 1.
Algorithm for evaluating positive SARS-CoV-2 PCR tests for potential discontinuation of transmission-based precautions. aCDC time/symptom-based criteria required 10 days for asymptomatic or mild–moderate infections or 20 days for severe infections or immunocompromised patients, assuming clinical improvement for symptomatic patients. PCR-positive patients who did not meet criteria for discontinuation of isolation outlined in the algorithm were assumed to be infectious and isolated as per CDC time/symptom-based criteria. Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; Ct, cycle threshold; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Noninfectious cases were classified as follows. Patients with serially high Ct values (or a repeat negative PCR test) were classified as having residual RNA from prior diagnosed (if known history of COVID-19) or undiagnosed (if no known history but reactive serologies) infection. Symptomatic patients were required to have alternate explanations for their symptoms (on case review and on discussion with clinical teams) in addition to high and stable Ct values (or negative repeat tests) in order to have isolation discontinued. Patients with no known COVID-19 history with a repeat negative PCR test and nonreactive serologies were classified as likely false positives. All cases were reviewed by 1 or more physician hospital epidemiologists (C.R., M.B., and M.K.) and discussed with patients’ clinical teams before discontinuing isolation.
Cases deemed noninfectious were managed on standard precautions, which included face masks and eye protection for providers and encouraging patients to wear face masks during provider encounters. PCR-positive patients deemed potentially infectious continued to be managed with N95 respirators (or powered air-purifying respirators) and gowns and gloves in addition to eye protection and patient masking. The duration of isolation was 10 days for asymptomatic/mildly ill patients and at least 20 days plus clear clinical improvement for severely ill or immunocompromised patients, as per CDC guidance. Negative PCR tests were not required for discontinuation of isolation.
Analysis
Descriptive analyses were conducted using Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA) and SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Patient Consent Statement
Written consent was not obtained from patients as the analysis was performed as part of routine hospital Infection Control operations. The study design was approved by the Mass General Brigham Institutional Review Board.
RESULTS
There were 11 583 patients admitted during the study period, of whom 175 were not tested due to being asymptomatic and “COVID-Recovered” (ie, known resolved SARS-CoV-2 infection within the prior 90 days). Of the remaining 11 408 patients, 536 tested positive for SARS-CoV-2 by PCR; 437 (81.5%) tested positive within 1 day of admission. The median age of the PCR-positive patients (interquartile range [IQR]) was 64 (50–74) years, and 283 (52.8%) were male.
Assessments of Infectiousness
Of the 536 PCR-positive patients, 381 (71.1%) were deemed potentially infectious as they were new diagnoses with low Ct values and/or progressive symptoms. In 28 of these cases, isolation was continued despite Ct values ≥35 due to ongoing respiratory symptoms consistent with COVID-19 pneumonia without clear improvement at the time.
One hundred forty-eight of the 536 patients (27.6%) were deemed noninfectious after evaluation: 127 (23.7%) likely had resolved prior infection based on high Ct values on initial testing, negative or stably high Ct values on repeat testing, and a known prior diagnosis (n = 93, 17.4%) or no prior diagnosis but reactive serology (n = 34, 6.3%). Among the 93 patients with known prior infection, the current positive test occurred a median (IQR) of 36 (25–65) days from the index diagnosis. Twenty-one (3.9%) cases were deemed false positives based on absence of symptoms and negative repeat tests and serologies. An additional 7 patients (1.3%) were asymptomatic with Ct values ≥35 but were discharged before further testing could be obtained.
The median Ct value of the first PCR test in patients deemed noninfectious (IQR) was 37.3 (35.6–38.2) vs 24.7 (19.9–31.8) in potentially infectious patients. Among the 144 patients deemed noninfectious, isolation was discontinued a median (IQR) of 2 (1–2) days after the first positive test.
At our hospital, all employees newly diagnosed with SARS-CoV-2 are interviewed by Occupational Health and reviewed daily by Infection Control to identify possible exposures (including any SARS-CoV-2-positive patients they may have cared for in the preceding 2 weeks) and transmissions. We did not identify any health care worker or roommate infections attributable to early discontinuation of isolation. Furthermore, none of the 148 patients in whom isolation was discontinued early by our algorithm were subsequently deemed to have true SARS-CoV-2 infection on the basis of new symptoms and/or repeat positive tests with low Ct values.
Yield of 24-Hour Repeat Testing
Among 114 patients who tested positive with Ct values ≥35 and underwent repeat testing at 24 hours, 3 (2.6%) had decreasing Ct values that triggered a full isolation course. One patient tested positive on 72-hour surveillance testing with a Ct value of 38.0, followed by a Ct value of 33.6 at 24 hours, and was maintained on isolation for 10 days while remaining asymptomatic. The second patient tested positive on hospital day 7 with a Ct value of 36.2 in the context of unit-wide testing for a cluster; the repeat test 24 hours later was positive with a Ct value of 16.7, with subsequent development of mild respiratory symptoms. The third patient tested positive on day 8 (also in the setting of unit-wide cluster screening) with a Ct value of 36.1, then 28.2 on 24-hour repeat testing. None of these 3 patients had evidence of prior infection by history or serology.
DISCUSSION
During the winter COVID-19 second surge in Massachusetts, nearly 1 in 3 hospitalized patients who tested positive for SARS-CoV-2 by PCR were quickly deemed noninfectious and subsequently managed on standard precautions on the basis of clinical history, symptoms, repeat testing, and serologies in select cases. Most noninfectious patients had evidence of remote known or unknown infections. We did not find any evidence of SARS-CoV-2 transmission using this approach.
There is increasing recognition that PCR tests may “overdiagnose” COVID-19 by identifying people with remote infections that are no longer contagious [9]. Kobayashi et al. reported that 10 of 19 patients who tested positive by PCR after admission (mostly as a result of serial asymptomatic screening) at a large Midwest tertiary medical center from July to September 2020 likely had prior infections using an approach similar to ours that incorporated Ct values, repeat tests, and serologies [10]. Our results expand on that analysis by comprehensively assessing all hospitalized patients who tested positive, including patients tested on admission. We frequently identified patients who had a prior undiagnosed infection, a phenomenon likely to become more common over time as only a fraction of SARS-CoV-2 infections are diagnosed and reported [11, 12]. These findings have implications for epidemiologic studies of patients hospitalized with COVID-19, as relying on positive PCR tests alone will result in substantial misclassification rates.
Nearly 4% of PCR-positive patients had likely false-positive tests. Although PCR tests are highly specific, false positives are bound to occur given the high rate of SARS-CoV-2 testing in patients with a relatively low prevalence of infection. False positives can result from technical problems, including reagent contamination and contamination during sampling or sample processing [13]. Some of these patients may also have had prior undiagnosed infections without seroconversion.
Our approach enabled many patients to be quickly released from isolation. This has substantial benefits for patients, as isolation can delay procedures and other medical care, limit visitation and social supports, cause anxiety, and complicate postdischarge planning and home living situations. Our prospective assessments were also able to free up scarce private and airborne infection isolation rooms and avoid unnecessary consumption of personal protective equipment.
Our study has several limitations. First, the proportion of PCR-positive patients who are noninfectious may vary by hospitals’ testing strategies and across labs, assays, and local epidemiology. Second, we were not able to definitively prove lack of infectiousness with viral cell cultures. However, we did not identify any secondary transmissions after early discontinuation of isolation. Third, our results likely underestimate the true number of noninfectious patients, as many had high Ct values in the late stages of COVID-19 yet were continued on isolation due to progressive symptoms that were more likely due to postinfectious inflammatory changes than active SARS-CoV-2 infection. We also used a conservative Ct value threshold of ≥35 for our assessments; other studies suggest that the transmission risk becomes negligible at even lower Ct thresholds [1].
Our strategy of requiring repeat tests after 24 hours for asymptomatic patients with high Ct values also had a relatively low yield, as we only identified 2 patients who were clearly in the early acute phase based on a substantial drop in Ct value; a third patient had a more mild decrease in Ct value and remained asymptomatic. None of these patients had evidence of prior infection. Our findings suggest that it is likely safe in most cases to discontinue precautions in asymptomatic PCR-positive patients with a history of prior infection after a single high Ct value. Given the risks associated with prematurely releasing patients from isolation and the increasing number of reported re-infections [14], however, we deliberately erred on the side of caution.
In conclusion, during the 2020 winter COVID-19 second surge in Massachusetts, nearly 1 in 3 hospitalized patients who tested positive for SARS-CoV-2 by PCR were deemed noninfectious and were safely managed on standard precautions; most cases were consistent with residual RNA from prior known or unknown infections. Active assessments of SARS-CoV-2 PCR tests using clinical data, Ct values, repeat tests, and serologies can safely release many patients from isolation and thereby conserve hospital resources and facilitate patient care.
Acknowledgments
Financial support. This work was funded by the Centers for Disease Control and Prevention (6U54CK000484-04-02).
Disclaimer. The Centers for Disease Control and Prevention had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Potential conflicts of interest. Dr. Rhee and Dr. Klompas report royalties from UpToDate. None of the authors has any conflicts of interest to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rhee C, Kanjilal S, Baker M, Klompas M. Duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity: when is it safe to discontinue isolation? Clin Infect Dis 2021; 72:1467–74. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis 2020; 71: 2663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020; 25:2001483.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim MC, Cui C, Shin KR, et al. Duration of culturable SARS-CoV-2 in hospitalized patients with COVID-19. N Engl J Med. 2021; 384:671–3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lumley SF, O’Donnell D, Stoesser NE, et al. ; Oxford University Hospitals Staff Testing Group. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2021; 384:533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhee C, Baker M, Vaidya V, et al. ; CDC Prevention Epicenters Program. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open 2020; 3:e2020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kissler SM, Fauver JR, Mack C, et al. Viral dynamics of SARS-CoV-2 infection and the predictive value of repeat testing. medRxiv 2020.10.21.20217042 [Preprint]. 1 December 2020. Available at: https://doi.org/10.1101/2020.10.21.20217042. Accessed 4 March 2021. [Google Scholar]
- 8. Roche Diagnostics. Elecsys anti-SARS-CoV-2: product information. 2021. Available at: https://diagnostics.roche.com/us/en/products/params/elecsys-anti-sars-cov-2.html#productInfo. Accessed 4 March 2021.
- 9. Mina MJ, Parker R, Larremore DB. Rethinking COVID-19 test sensitivity - a strategy for containment. N Engl J Med 2020; 383:e120. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi T, Trannel A, Holley SA, et al. COVID-19 serial testing among hospitalized patients in a Midwest tertiary medical center, July-September 2020. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reese H, Iuliano AD, Patel NN, et al. Estimated incidence of COVID-19 illness and hospitalization - United States, February-September, 2020. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Angulo FJ, Finelli L, Swerdlow DL. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open 2021; 4:e2033706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Surkova E, Nikolayevskyy V, Drobniewski F. False-positive COVID-19 results: hidden problems and costs. Lancet Respir Med 2020; 8:1167–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen JI, Burbelo PD. Reinfection with SARS-CoV-2: implications for vaccines. Clin Infect Dis. 2020; doi: 10.1093/cid/ciaa1866 [DOI] [PMC free article] [PubMed] [Google Scholar]