Abstract
Of 4133 persons surveyed at a low-barrier coronavirus disease 2019 (COVID-19) test site with high positivity in an urban Latinx community in January 2021, 86% indicated that they would accept a COVID-19 vaccination. The top reasons for vaccine hesitancy included concerns around side effects and safety and distrust of health care systems.
Keywords: COVID-19, vaccine acceptance, SARS-CoV-2
Coronavirus disease 2019 (COVID-19) vaccine hesitancy may impede effective epidemic control. Up to 45% of US residents and >50% of Black and Hispanic populations have been estimated to be reluctant to receive an approved vaccine [1, 2]. As of March 2021, White persons were roughly 2 times more likely to have received a vaccine than Black and Hispanic persons in the United States [3]. We sought to assess vaccine motivation in a low-income urban Latinx community heavily impacted by COVID-19.
METHODS
Between January 10 and 24, 2021, we conducted a survey about vaccine attitudes and preferences among adults (aged ≥18) seeking free, no-appointment, BinaxNOW rapid COVID-19 testing [4] in San Francisco’s Mission District. Mobilization of the Latinx community in Southeastern San Francisco, a population disparately impacted by the COVID-19 epidemic [5], was conducted through a community-academic–San Francisco Public Health partnership. Before testing, participants completed a short web-based survey (English or Spanish) on demographics, occupation, health-seeking behaviors, and vaccine attitudes, including those of friends and family [2]. We characterized persons as vaccine-hesitant if they indicated that they would “definitely not” or “probably not” get the vaccine. Predictors of vaccine hesitancy were evaluated using multivariate logistic regression.
RESULTS
Over 14 days, 5198 adults were tested, of whom 4133 (79.5%) completed the questionnaire; weather and website outages contributed to noncompletion. Of surveyed participants (2190 [53.0%] men, 3790 [91.7%] aged <65 years, 2968 [71.8%] Latinx/Hispanic, 484 [11.7%] White, 110 [2.7%] Black), 2924 (70.7%) reported household income <$50,000/year, 1813 (43.9%) were first-generation immigrants, and 1924 (46.6%) were frontline essential workers (1420 [73.8%] of whom were Latinx) [6]. BinaxNOW test positivity was 9.1% (n = 376/4133).
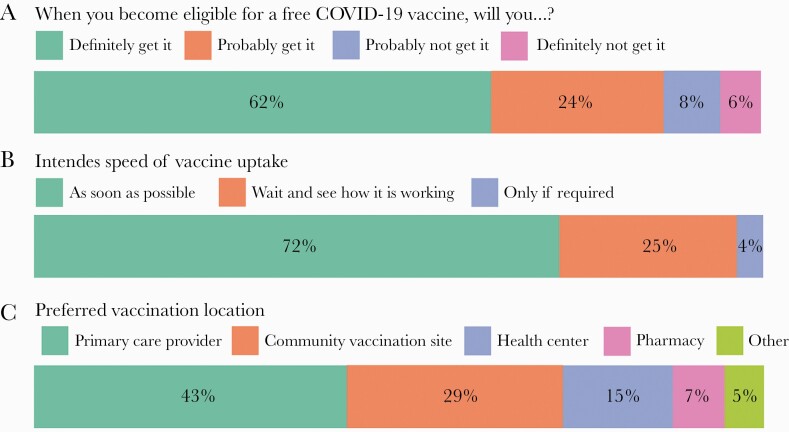
Overall, 86.0% (3555/4133) of persons surveyed were “vaccine-motivated” (reported they would “definitely” or “probably” be vaccinated) (Figure 1). Among frontline essential workers and Latinx persons, vaccine motivation was 87.4% (1682/1924) and 84.7% (2514/2968), respectively. Among vaccine-motivated persons, 45.8% (1627/3555) reported having a primary care provider, and 57.6% (2047/3555) had health insurance. Doctor’s offices (42.8%, 1523/3555) and community vaccination sites (29.4%, 1046/3555) were preferred locations for vaccination.
Figure 1.
A, Vaccine interest among all surveyed. B, Intended speed of vaccine uptake. C, Preferred vaccination location among participants who indicated they would definitely or probably get vaccinated. Abbreviation: COVID-19, coronavirus disease 2019.
In multivariable analysis, predictors of vaccine hesitancy included identifying as female (adjusted odds ratio [aOR], 1.37; 95% CI, 1.06–1.75), identifying as Latinx (aOR, 2.11; 95% CI, 1.29–3.60) or Black (aOR, 2.34; 95% CI, 1.11–4.95; ref. White), not being a frontline worker (aOR, 1.35; 95% CI, 1.06–1.73), having skipped a flu shot in the past year (aOR, 2.31; 95% CI, 1.77–3.03), and reporting less than half of family (aOR, 3.75; 95% CI, 2.74–5.13) or friends (aOR, 4.43; 95% CI, 3.22–6.10) intending to be vaccinated. Being uninsured (aOR, 1.05; 95% CI, 0.78–1.42), having health conditions (diabetes, hypertension, lung/heart disease; aOR, 1.20; 95% CI, 0.87–1.63), or knowing a contact who was hospitalized or died of COVID-19 (aOR, 0.95; 95% CI, 0.70–1.27) were not associated with vaccine hesitancy.
The primary concerns among vaccine-hesitant persons included side effects (40.3%, 233/578) and distrust of vaccine safety (27.3%, 158/578) or health care systems (21.6%, 125/578) (Table 1). However, among vaccine-hesitant respondents, 57.1% (330/578) indicated a great or fair deal of trust in doctors, higher than trust reported in local/state government (27.0%, 156/578) or newspapers/TV/radio (19.9%, 115/578).
Table 1.
Reasons for Reluctance for Those who Indicated That They Would Probably Not or Definitely Not Receive the Vaccine (n = 578)
| Reasons for Vaccine Reluctance | No. of Participants who Indicated Concern (%) |
|---|---|
| Worried about side effects | 233 (40.3) |
| Don’t trust vaccine is safe | 158 (27.3) |
| Don’t trust health care systems in general | 125 (21.6) |
| The vaccine is too new | 122 (21.1) |
| Don’t trust vaccine is effective | 66 (11.4) |
| Don’t trust vaccines in general | 59 (10.2) |
| Might get COVID-19 from vaccine | 25 (4.3) |
| Don’t think I’m at risk | 13 (2.3) |
| I already had COVID so I don’t need it | 7 (1.2) |
Abbreviation: COVID-19, coronavirus disease 2019.
DISCUSSION
Effective vaccination strategies for COVID-19 epidemic control must reach persons at highest risk of infection. In a community-based low-barrier testing setting, we reached high-risk persons, including low-income, Latinx frontline essential workers and their families. In this priority group, we found that 86% of all respondents and 87% of frontline essential workers indicated they would accept vaccination.
Eighty-four percent of Latinx persons surveyed indicated that they would get a vaccine, substantially higher than prior reports [1]. However, opportunities remain to further increase vaccine motivation in this priority population by addressing concerns around safety and side effects. The relatively high trust in doctors reported by vaccine-hesitant persons also suggests an important role for clinicians in increasing vaccine uptake. Leveraging community leaders and trusted influencers in the social networks of intergenerational households may also be an effective strategy to provide education and information on vaccines.
Because we selected for a population seeking COVID-19 testing, our survey may overestimate vaccine motivation in the general population. However, vaccine acceptance in the general population is of less immediate relevance for epidemic control than acceptance among persons at highest risk of infections. Our mobilization strategy, which by design included outreach to the highest-risk persons and was built on longstanding community partnerships, reached exactly this population. The BinaxNOW positivity rate among the population surveyed was 9%, 2.4 times the general test positivity rate in San Francisco during this period; cases identified in this community-based setting comprised 16% of all reported cases in San Francisco during the study period, despite representing only 6% of tests [7]. While our findings may not be generalizable to non-test-seeking persons and at-risk populations in other settings, they demonstrate that high vaccine acceptance in priority populations with demonstrated elevated risk of COVID-19 is achievable in the context of committed community partnerships.
Our survey also provides insights into vaccine distribution strategies. Strategies that rely on current engagement with the health care system may miss a large proportion of the vaccine-seeking population at high risk for infection. Low-barrier community vaccination sites, building on strong community partnerships [8], will continue to play a key role in vaccination strategies to ensure equitable vaccine access for all groups.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the Chan Zuckerberg Initiative, Bevan Dufty and the BART team, Supervisor Hillary Ronen, Mayor London Breed, Dr. Grant Colfax, Dr. Naveena Bobba, Dr. Jonathan Fuchs, Dr. Darpun Sachdev and the San Francisco Department of Public Health, Rob Nakamura from the California Department of Public Health, Salu Ribeiro and Bay Area Phlebotomy and Laboratory services, the PrimaryBio COVID testing platform, and our community ambassadors and volunteers.
Financial support. This study was funded by UCSF, the Chan Zuckerberg Biohub, and the Chan Zuckerberg Initiative.
Potential conflicts of interest. None of the authors have reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Ethical approval. The UCSF Human Research Protection Program Institutional Review Board determined that the study met the criteria for public health surveillance.
Patient consent. We obtained verbal and written consent in the participant’s preferred language before study participation.
References
- 1. Szilagyi PG, Thomas K, Shah MD, et al. National trends in the US public’s likelihood of getting a COVID-19 vaccine—April 1 to December 8, 2020. JAMA 2020; e2026419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamel L, Kirzinger A, Munana C, et al. KFF Covid-19 vaccine monitor: December 2020. Available at: https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/. Accessed 25 January 2021.
- 3. Ndugga N, Pham O, Hill L, et al. Latest data on COVID-19 vaccinations race/ethnicity. Available at: https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/. Accessed 30 March 2021.
- 4. Pilarowski G, Marquez C, Rubio L, et al. Field performance and public health response using the BinaxNOW TM Rapid SARS-CoV-2 antigen detection assay during community-based testing. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chamie G, Marquez C, Crawford E, et al. SARS-CoV-2 community transmission disproportionately affects Latinx population during shelter-in-place in San Francisco. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. When vaccine is limited, who should get vaccinated first? Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations.html. Accessed 25 January 2021.
- 7. San Francisco Department of Public Health. COVID-19 data and reports. Available at: https://data.sfgov.org/stories/s/fjki-2fab. Accessed 29 January 2021.
- 8. Bibbins-Domingo K, Petersen M, Havlir D. Taking vaccine to where the virus is—equity and effectiveness in coronavirus vaccinations. JAMA Health Forum. 2021; 2:e210213.. [DOI] [PubMed] [Google Scholar]