Abstract
Background:
The aryl hydrocarbon receptor repressor (AHRR), a member of the growing superfamily, is a basic helix-loop-helix/PerAHR nuclear translocator (ARNT)-Sim (bHLH-PAS) protein. AHRR has been proposed to function as a putative new tumor suppressor gene based on studies in multiple types of human cancers. This current study aims to investigate AHHR expression and its prognostic significance in gallbladder cancer.
Methods:
The study includes 48 gallbladder cancer and 34 chronic cholecystitis cases as controls. The expression level of AHRR was analyzed by using semi-quantitative PCR and immunohistochemical staining. The results were correlated with different clinical parameters.
Results:
We demonstrate that the expression of AHRR is significantly down-regulated in gallbladder cancer tissue samples as compared to that in chronic cholecystitis tissue samples by reverse transcriptase PCR (RT-PCR) (P = 0.017) and immunohistochemistry analysis (P = 0.002). Interestingly, our RT-PCR data revealed that AHRR mRNA expression is frequently down-regulated (45.8%; 22/48) in cases as compared to 14.7% (5/34) in controls. Similarly, immunohistochemical analysis data show significant down-regulation of AHRR expression in 77.1% (37/48) of gallbladder cancer cases than 44.1% (15/34) in controls (P < 0.017). Reduced mRNA and protein expression is significantly associated with advanced T-stage (P = 0.001), histological differentiation (P = 0.001), and tumors with nodal metastasis (P = 0.001). Decreased expression of AHRR is significantly associated with poor prognosis in gallbladder cancer patients.
Conclusion:
In conclusion, the present study suggests that low AHRR expression may be critical in gallbladder cancer development. Our data suggests that AHRR may act as a tumor suppressor gene and its expression profile may be useful as a diagnostic marker in gallbladder cancer.
Keywords: AHRR, ARNT, chronic cholecystitis, gallbladder cancer, tumor suppressor gene
INTRODUCTION
Gallbladder cancer (GBC) is the sixth common cancer among gastrointestinal cancers and is one of the most common cancers of the biliary tract with a very high global mortality rate.[1,2] It presents at an advanced stage with a poor prognosis. The average survival of GBC patients is 6 months and 5-year survival rate is less than 5%.[3,4] The incidence of GBC varies geographically and has a particularly high incidence in Chile, Japan, and northern India.[5] The etiological factors of GBC include age, gender, cholelithiasis, heavy metals, environmental, and genetic factors.[4] There are several reports that demonstrate critical molecular genetic events for the initiation and progression of gallbladder carcinogenesis; however, the genetic basis is still unclear. Elucidation of underlying genetic events offers the promise of a breakthrough in the discovery of biomarkers and novel therapeutic targets.
The AHRR is a newly discovered member of the growing superfamily of basic helix-loop- helix/Per-ARNT-Sim (bHLH/Per-ARNT-Sim) transcription factors, which includes the aryl hydrocarbon receptor (AHR) and hypoxia inducible-factor 1 (HIF1). AHRR has been identified as an AHR-regulated gene.[6] AHRR maps the chromosome at 5 (5p15) in humans. This chromosomal region has been shown to be frequently deleted in various tumors, including cervical cancer, testicular germ cell tumors, colorectal cancer, early-stage ovarian tumors, bladder cancer, esophageal cancer, and lung tumors.[7,8,9,10,11,12] AHRR is potentially involved in a vast array of normal and pathological processes ranging from xenobiotic response to tumor progression. Various in vitro studies conducted on different cancer cell lines have demonstrated AHRR as a potential tumor suppressor gene.[13,14] It has been shown that AHRR expression status has the potential to be an independent prognostic factor for primary gastric adenocarcinoma.[15,16]
No previous reports exist concerning the expression status of AHRR, and the prognostic value of this protein in gallbladder cancer have been studied so far, to the best of our knowledge. In this study, the expression profile of AHRR in GBC was evaluated using reverse transcriptase PCR (RT-PCR) and immunohistochemistry. Additionally, we have shown the correlation of AHRR expression profile with clinicopathological features of carcinoma gallbladder.
METHODS
Study design
The study was conducted to evaluate the expression profile of AHRR in GBC patients by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) and Immunohistochemistry (IHC).
Setting
Tissue samples and clinical data were collected from the Department of General Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi. The histological and IHC work was performed in the Department of Pathology, Institute of Medical Sciences, and the molecular work was carried out in the Department of Molecular and Human Genetics, Institute of Science, Banaras Hindu University, Varanasi, and was undertaken from the period of July 2017 to July 2019. The study was approved by the institutional ethical committee of the Institute of Medical Sciences, Banaras Hindu University.
Subjects
Newly diagnosed GBC patients were included in the study as cases and chronic cholecystitis (CC) as control. Biopsies of forty-eight and thirty-four cases of GBC and controls were collected, respectively, after obtaining written informed consent. Samples were collected in formalin and RNA later (Ambion, USA) processed for IHC and RNA extraction. Samples collected in RNA later were immediately snap-frozen in liquid nitrogen and then stored at -86°C in a deep freezer. Histopathologically proven cases of GBC were included in the study. The patients who received chemotherapy or radiotherapy preoperatively were excluded.
RNA isolation, cDNA preparation & RT-PCR
RNA isolation was carried out by using TRI Reagent® (Ambion®, Life Technologies) and quantified by Nanodrop spectrophotometer. Complementary DNA (cDNA) from mRNA was synthesized by using a high capacity cDNA reverse transcription kit (Applied Biosystems, USA).
cDNA was PCR amplified using gene-specific primers for AHRR (common for both the isoforms) and β-actin as the internal control. The primers for AHRR and β-actin were designed using the “primer3 input” program. Primer sequences are as follows: AHRR F 5' CAGTTACCTCCGGGTGAAGA 3' R 5' TGGAAGCCCAGATAGTCCAC 3' and β-actin F 5' AAATCTGGCACCACACCTTC 3' R 5' AGCACAGCCTGGATAGCAAC 3'. RT-PCR was carried out using 7.5 μL of 2X PCR (Cat. no# K 0172, Fermentas, USA) and 20 pmol of each forward and reverse primer in 15μL of reaction volume containing cDNA sample in a Veriti 96-well thermal cycler (Applied Biosystems, USA). The following PCR cycle conditions were used: 94°C initial denaturation for 5 min, denaturation at 94°C for 30 sec, annealing at 58°C (β-Actin and AHRR) for 30 sec, and extension at 72°C for 35 cycles, followed by 7 min final extension at 72°C.
Gene-specific expression levels were determined with relative band intensities calculated by Spot Denso densitometry software (Alpha Imager, USA) normalized against the expression level of β-Actin for each control and tumor sample. The relative intensity of each band was normalized against β-Actin for each sample by calculating the ratio of the band intensity of AHRR to β- Actin. The differential expression in GBC was determined by comparing the average value ± 2 X SD of the normal to the tumor samples.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on tissue sections (4μm thickness) of formalin--fixed paraffin-embedded (FFPE) primary tumor and control biopsies. The sections were mounted onto slides and dried 12–-24 h at 37°C followed by deparaffinization with xylene and hydration in a graded series of alcohols. Following a brief proteolytic digestion and a peroxidase blocking of tissue slides, the slides were incubated with primary antibody against AHRR (1:100, rabbit polyclonal antibody, cat #40558 from SAB signal-way antibody, USA) overnight at 40°C. After washing, the slides were incubated with an anti-rabbit secondary antibody labeled by horseradish peroxidise (HRP). Visualization was performed by 3,30-diaminobenzidine tetrahydrochloride (DAB) and counterstained by hematoxylin. Negative controls were carried out by omitting the primary antibody in each IHC run.
Evaluation of immunostaining results
A semi-quantitative scoring method was used to assess each sample based on the staining, intensity, and percentage. The staining intensity was visually scored and stratified according to the following criteria: no staining (0), mid staining (+1), moderate staining (+2), strong staining (+3). The percentage scoring of immunoreactive tumor cells was defined as <5% (0 point), 6%–25% (1 point), 26%–50% (2 points), 51%–-75% (3 points), and >75% (4 points). The final immunoreactivity scores (IRS) of each case were calculated by adding the two scores for the immunostaining percentage together. A score of more than 4 was recorded as high expression while others were recorded as low expression.
Statistical analysis
The statistical analysis was performed using SPSS statistics version 17.0 for windows (US). A chi-square test was applied for nonparametric variables. A Student's t-test was performed for comparing two groups. A P value of less than 0.05 was considered significant.
RESULTS
Demographic details
This study included 48 cases of GBC and 34 CC. The mean ages of case and control groups were 51.09 ± 11.7 and 46.44 ± 12.02 years, respectively. 87.5% of cases of GBC and 85.3% of CC were females, which suggests their prevalence. Early T stage (T1/T2) was present in 12 (25%) and late T stage (T3/T4) present in 36 (75%) of GBC cases. In nodal status, 15 (31%) patients had N0 and 33 (69.5%) patients had N1. Metastatic disease was present in only 2 (4.2%) patients. Of the 48 cases of histopathologically proven adenocarcinoma of gallbladder, 14 (29%), 25 (52%), and 9 (18.75%) had well, moderately, and poorly differentiated adenocarcinoma, respectively.
AHRR Expression in case and control
The integrated band density value of AHRR mRNA expression to β-actin in GBC was calculated by comparing the average value ± 2 X SD of the normal to the tumor samples. The mean level of AHRR/β-actin mRNA in the GBC tissues (0.54 ± 0.298) was significantly lower than in the chronic cholecystitis tissues (0.67 ± 0.120). RT--PCR showed significantly and frequently down-regulated AHRR expression in 77.1% (P = 0.002) of GBC cases compared with control group [Figure 1; Table 1].
Figure 1.
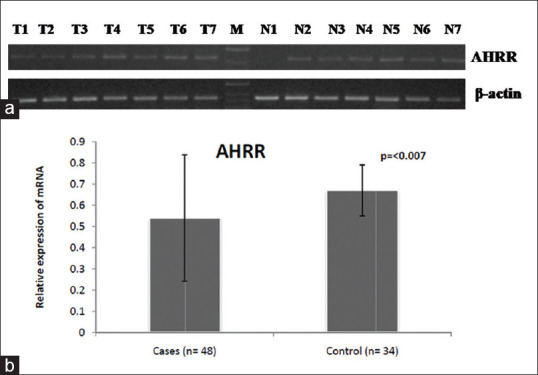
(a) mRNA expression pattern of AHRR gene in gallbladder cancer and control group. The expression of beta-actin is shown as an internal control (b) Normalized expression of AHRR at mRNA level
Table 1.
AHRR expression in Gallbladder cancer and Chronic Cholecystitis
| AHRR Expression | GBC (n=48) | CC (n=34) | P |
|---|---|---|---|
| RT-PCR | |||
| Low | 37 (77.1%) | 15 (44.1%) | 0.002 |
| High | 11 (22.9%) | 19 (55.9%) | |
| Total | 48 (100%) | 34 (100%) | |
| Immunohistochemistry | |||
| No Expression | 18 (37.5%) | 2 (4.2%) | 0.009 |
| Weak | 6 (12.5%) | 4 (8.3%) | |
| Moderate | 20 (41.6%) | 24 (50%) | |
| Strong | 4 (8.4%) | 4 (8.3%) | |
| Total | 48 (100%) | 34 (100%) | |
Statistical significance at P<0.05
Immunohistochemistry analysis also showed significant down-regulation of AHRR expression in GBC cases than in controls (P = 0.009) [Table 1]. AHRR showed moderate immunohistochemical staining intensity in 41.6% and no staining in 37.5% of GBC cases [Figure 2]. We found a significant correlation of AHRR expression with tumor, node, metastasis (T, N, M) status, and histology of GBC cases. AHRR expression is down-regulated in 97.3% of GBC cases having T3/T4 status (P = 0.001). There is low AHRR expression in 59.6% and 94.6% of N0 (P = 0.001) and M0 (P = 0.001) status of GBC group of patients. There is decreased AHRR expression in 67.6% of GBC with moderately differentiated histology (P = 0.001). However, there is no significant differential expression among the tumors with the presence of lymph vascular (P = 0.255) and perineural invasion (P = 0.160) [Table 2].
Figure 2.
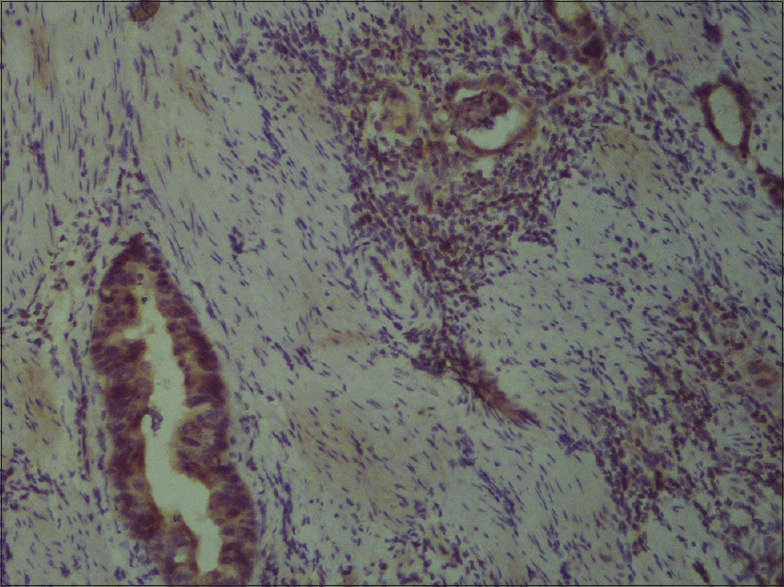
AHRR protein expression in gallbladder cancer surgical specimen by immunohistochemistry
Table 2.
Association of AHRR IHC expression with different clinical parameters
| Clinical Features | AHRR IHC Expression |
P | mRNA AHRR expression |
P | ||
|---|---|---|---|---|---|---|
| Low (n=37) | High (n=11) | No. of Cases | mRNA Expression | |||
| T Status | ||||||
| T1/T2 | 1 (2.7%) | 11 (100%) | 0.001 | 12 | 0.933±0.239 | 0.001 |
| T3/T4 | 36 (97.3%) | 0 | 36 | 0.415±0.181 | ||
| N Status | ||||||
| N0 | 22 (59.5%) | 11 (100%) | 0.001 | 15 | 0.672±0.263 | 0.001 |
| N1 | 15 (40.5%) | 0 | 33 | 0.265±0.139 | ||
| M Status | ||||||
| M0 | 35 (94.6%) | 11 (100%) | 0.431 | 46 | 0.1994±0.00747 | 0.095 |
| M1 | 2 (5.4) | 0 | 2 | 0.5603±0.29597 | ||
| Histology | ||||||
| Well Differentiated | 3 (8.1%) | 11 (100%) | 0.001 | 14 | 0.889±0.247 | 0.001 |
| Moderately Differentiated | 25 (67.6%) | 0 | 25 | 0.478±0.144 | ||
| Poorly Differentiated | 9 (24.3%) | 0 | 9 | 0.195±0.061 | ||
| Lymph Vascular Invasion | ||||||
| Present | 4 (10.8%) | 0 | 0.255 | 4 | 0.2981±0.13508 | 0.084 |
| Absent | 33 (89.2%) | 11 (100%) | 44 | 0.5677±0.30001 | ||
| Perineural Invasion | ||||||
| Present | 9 (24.3%) | 0 | 0.160 | 9 | 0.2675±0.10478 | 0.001 |
| Absent | 28 (75.7%) | 11 (100%) | 39 | 0.6093±0.29241 | ||
Statistical significance at P<0.05
On correlating the AHRR expression at mRNA level with clinical features, it was observed to be positively associated with T, N status, and histology of GBC cases. AHRR mRNA expression was significantly lower in the advanced stage (T3/T4) than in the early stage (T1/T2) (P < 0.001) of GBC. Similarly, down-regulation of AHRR is associated with nodal metastasis status (N1) (P < 0.001). However, no statistical significance was detected with the M stage (P = 0.095). AHRR mRNA expression is significantly associated with histological differentiation (P = 0.001) and perineural invasion (P = 0.001). We did not find a significant association between AHRR mRNA expression and lymphovascular invasion (P = 0.084) [Table 2].
DISCUSSION
The AHRR is a bHLH--PAS protein transcription factor located in chromosome 5 (5p15.3).[6,13] This chromosomal region (containing AHRR) has been reported to be deleted in different cancers, and its promoter region is hypermethylated in various cancers.[13] Recent studies suggest that AHRR may function as a tumor suppressor gene that becomes silenced during tumorigenesis.[17] In the present study, we have hypothesized that inactivation and downregulation of AHRR may have a critical role in gallbladder cancer progression. The expression profile of AHRR could help in early identification and developing new molecular targets for GBC.
In our study, AHRR expression at mRNA and protein levels were evaluated by RT-PCR and immunohistochemical analysis. Concordant to previous reports in other cancers including lung, breast, hepatocellular carcinoma, cervical, colon, and ovarian cancer,[13,18,19,20] we demonstrated that AHRR is down-regulated at both mRNA and protein levels in GBC tissues than in controls (P = 0.002). Zudaire et al., (2008) demonstrated the mRNA expression level in multiple cancers.[13] Our results are consistent with this study showing reduced mRNA expression in all the malignant tissues compared with the normal tissues. A recent study analyzed the levels of AHRR in a cohort of 439 breast cancer patients and compared with the normal breast tissue sample. However, they found a wide range of AHRR mRNA expression (0.0 to 19.8).[21]
We compared the levels of mRNA and protein expression of AHRR with different clinicopathological parameters. In our study, we observed a positive association of AHRR expression with advanced stage (T3/T4) and nodal status of GBC cases. We demonstrated that AHRR expression is significantly associated with tumor differentiation (P = 0.001) and perineural invasion (P = 0.001) while there was no association with lymphovascular invasion (P = 0.084). Similar to our findings, Liang et al. (2011) also found low expression of AHRR, which was significantly correlated with a high grade of hepatocellular cancer.[19] On the contrary, Vacher et al. (2018) did not find any association with any clinical or pathological factor studied, including age, histological, grade, and lymph node status of the breast cancer cases. They suggested that levels of AHRR may represent an independent prognostic factor for breast cancer.[21] Recently, it has been demonstrated that AHRR knock-down with siRNA enhances AHR activity, confirming the assumption that AHRR constitutively represses AHR activity in human mammary tumor cell lines[22] suggesting a critical role in GBC tumorigenesis.
Li et al., (2012) has investigated the AHHR expression and its prognostic significance in primary gastric adenocarcinoma and reported reduced AHRR expression in gastric cancer tissue samples in comparison with adjacent non-tumor tissue samples by RT-qPCR (P = 0.0423) and Western blot analysis (P = 0.004). Furthermore, immunohistochemistry analysis indicated that AHRR expression was significantly decreased in 42.7% of gastric adenocarcinoma cases.[23] Similarly, our results demonstrate decreased AHRR expression in 40% of GBC cases.
Our observations are consistent with the idea that AHRR plays a tumor suppressor role and we further suggest that AHRR might play an important role in the GBC progression, since low AHRR expression is associated with the late T stage (P = 0.001) and nodal N status (P = 0.001).
In conclusion, our study suggests that low AHRR expression may be critical in gallbladder cancer development. In addition, our results suggest that AHRR may be a tumor suppressor gene and its expression status has the potential to be an independent diagnostic marker. However, further research is required to understand the molecular mechanism(s) involved in AHRR regulation in GBC. The study is limited to the analysis of AHRR expression at mRNA and protein levelin a limited sample size. Therefore, a large-scale follow-up study will be required for a better understanding of AHRR in gallbladder cancer.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hickman L, Contreras C. Gallbladder cancer: Diagnosis, surgical management, and adjuvant therapies. Surg Clin North Am. 2019;99:337–55. doi: 10.1016/j.suc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol. 2017;23:3978–98. doi: 10.3748/wjg.v23.i22.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–64. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 4.Krell RW, Wei AC. Gallbladder cancer: Surgical management. Chin Clin Oncol. 2019:836. doi: 10.21037/cco.2019.06.06. [DOI] [PubMed] [Google Scholar]
- 5.Hundal R, Shaffer EA. Gallbladder cancer: Epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;3:20–5. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitra AB, Murty VV, Singh V, Li RG, Pratap M, Sodhani P, et al. Genetic alterations at 5p15: A potential marker for progression of precancerous lesions of the uterine cervix. J Natl Cancer Inst. 1995;87:742–5. doi: 10.1093/jnci/87.10.742. [DOI] [PubMed] [Google Scholar]
- 8.Xu SF, Peng ZH, Li DP, Qiu GQ, Zhang F. Refinement of heterozygosity loss on chromosome 5p15 in sporadic colorectal cancer. World J Gastroenterol. 2003;9:1713–8. doi: 10.3748/wjg.v9.i8.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang VW, Bell DA, Berkowitz RS, Mok SC. Whole genome amplification and high-throughput allelotyping identified five distinct deletion regions on chromosomes 5 and 6 in microdissected early-tage ovarian tumors. Cancer Res. 2001;61:4169–74. [PubMed] [Google Scholar]
- 10.Böhm M, Kleine-Besten R, Wieland I. Loss of heterozygosity analysis on chromosome 5p defines 5p13-12 as the critical region involved in tumor progression of bladder carcinomas. Int J Cancer. 2000;89:194–7. [PubMed] [Google Scholar]
- 11.Peralta RC, Casson AG, Wang RN, Keshavjee S, Redston M, Bapat B. Distinct regions of frequent loss of heterozygosity of chromosome 5p and 5q in human esophageal cancer. Int J Cancer. 1998;78:600–5. doi: 10.1002/(sici)1097-0215(19981123)78:5<600::aid-ijc12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Wieland I, Böhm M, Arden KC, Ammermüller T, Bogatz S, Viars CS, et al. Allelic deletion mapping on chromosome 5 in human carcinomas. Oncogene. 1996;12:97–102. [PubMed] [Google Scholar]
- 13.Zudaire E, Cuesta N, Murty V, Woodson K, Adams L, Gonzalez N, et al. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. Version 2. J Clin Invest. 2008;118:640–50. doi: 10.1172/JCI30024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlezinger JJ, Liu D, Farago M, Seldin DC, Belguise K, Sonenshein GE, et al. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem. 2006;387:1175–87. doi: 10.1515/BC.2006.145. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 16.Pocar P, Fischer B, Klonisch T, Hombach-Klonisch S. Molecular interactions of the aryl hydrocarbon receptor and its biological and toxicological relevance for reproduction. Reproduction. 2005;129:379–89. doi: 10.1530/rep.1.00294. [DOI] [PubMed] [Google Scholar]
- 17.Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, Puga A. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis, Cancer Res. 2010;70:212–20. doi: 10.1158/0008-5472.CAN-09-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanada N, Gotoh Y, Shimazawa R, Klinge CM, Kizu R. Repression of activated aryl hydrocarbon receptor-induced transcriptional activation by 5alpha-dihydrotestosterone in human prostate cancer LNCaP and human breast cancer T47D cells. J Pharmacol Sci. 2009;109:380–7. doi: 10.1254/jphs.08328fp. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Li WW, Yang BW, Tao ZH, Sun HC, Wang L, et al. Aryl hydrocarbon receptor nuclear translocator is associated with tumor growth and progression of hepatocellular carcinoma. Int J Cancer. 2011;130:1745–54. doi: 10.1002/ijc.26166. [DOI] [PubMed] [Google Scholar]
- 20.Monick MM, Beach SR, Plume J, Sears R, Gerrard M, Brody GH, et al. Coordinated changes in AHRR methylation in lymphoblasts and pulmonary macrophages from smokers. Am J Med Genet B Neuropsychiatr Genet. 2012;159:141–51. doi: 10.1002/ajmg.b.32021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vacher S, Castagnet P, Chemlali W, Lallemand F, Meseure D, Pocard M, et al. High AHR expression in breast tumors correlates with expression of genes from several signaling pathways namely inflammation and endogenous tryptophan metabolism. PLoS One. 2018;13:e0190619. doi: 10.1371/journal.pone.0190619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn ME, Allan LL, Sherr DH. Regulation of constitutive and inducible AHR signaling: Complex interactions involving the AHR repressor. Biochem Pharmacol. 2009;77:485–97. doi: 10.1016/j.bcp.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YF, Wang DD, Zhao BW, Wang W, Yuan SQ, Huang CY, et al. Poor prognosis of gastric adenocarcinoma with decreased expression of AHRR. PLoS One. 2012;7:e43555. doi: 10.1371/journal.pone.0043555. [DOI] [PMC free article] [PubMed] [Google Scholar]


