Abstract
Background:
Factors other than pTNM stage have been associated with gastric cancer (GC) prognosis, and several alternative prognostic scores have been constructed. Our aims are to identify prognostic factors in western GC patients and to build clinicopathological prognostic models for overall survival (OS) and disease-free survival (DFS).
Methods:
A Retrospective study of 204 cases of GC resected during the years 2000 to 2014 was conducted in our hospital. Clinicopathological features were assessed, univariate and multivariate analysis were performed and prognostic scores were constructed.
Results:
Most patients were diagnosed at pTNM stages II and III (36.9% and 48.1%, respectively). According to Laurén classification, tumors were intestinal (55.8%), diffuse (35.2%) and mixed (9%). During follow-up, 43.5% of patients had tumor recurrence, and 28.6% died due to tumor. Univariate analysis showed that patient age, Laurén subtype, signet-ring cell morphology, pTNM stage, tumor grade, perineural invasion, growth pattern, intratumoral inflammation, adjuvant therapy, and desmoplasia were significantly related to tumor progression or death. Multivariate analysis showed that Laurén subtype, pT stage, and lymph node ratio (LNR) were significantly and independently associated with GC recurrence. Laurén subtype and LNR were significantly related to patient survival. Prognostic scores for tumor progression and death were developed and patients were classified into four prognostic groups which showed good prognostic performance.
Conclusion:
A prognostic model comprising histological features such as Laurén subtype can be easily applied in clinical practice, and provides more prognostic information than pTNM stage alone. These models can further stratify resected GC patients and have the potential to aid in the individualization of patient management.
Keywords: Gastric cancer, histopathological score, prognosis, TNM classification
INTRODUCTION
Gastric cancer (GC) is the fourth most frequent cancer and the second most cause of cancer-related deaths worldwide.[1] Its incidence depends on factors like patient gender, race, and geographical location.[2]50% of all cases occur in Eastern Asia.[3] Histologically, more than 90% of GC are adenocarcinomas. For this reason, in the present study we used GC as a synonym for gastric adenocarcinoma, therefore excluding squamous cell carcinoma of the gastroesophageal junction and rare histologic types of GC.
As previously mentioned, GC shows substantial geographic variation. In western countries, most cases are advanced at diagnosis[4] and 5-year survival rates are low (approximately 20%). In Japan and other Asian countries, where screening programs have been implemented, survival rates are higher.[2] Reported median overall survival (OS) of advanced disease is less than 1 year.[5,6] The best available tool for evaluating GC prognosis is TNM stage, which includes tumor depth, lymph node metastasis, and distant metastasis.[7] However, GC is a heterogeneous disease with varied clinicopathological and molecular features,[8,9] and factors other than anatomic spread have been identified as prognosticators.[10,11] Moreover, some authors have observed prognostic differences between patients with identical TNM stage tumors, and advanced GC remains a hard-to-predict disease.[5,12] Early recurrences in patients with early GC have also been reported.[9,12] An optimal stratification of GC is essential for determining patient prognosis and management. In addition, the limited benefit of chemotherapy (CT) in advanced GC and its potential harms, such as decreased quality of life or drug toxicity, make it necessary to individualize patient management.[5,13]
With this aim, several prognostic scores have been developed, including mainly clinical (preoperative and postoperative), immunohistochemical, and molecular features.[8,14,15] Clinicopathological scores based only on clinical and histological features have been reported less frequently. These scores are cheaper and easier to apply in clinical practice, but studies on histological features demand a consensual and standardized pathological evaluation. In our literature review, we have found that most studies on clinicopathological scores for GC have been performed in Korea, China, and Japan (46.7%). As far as we know, only five studies have developed clinicopathological scores for European patients with GC. Only one of them studied both OS and disease-free survival (DFS).
In this study, we have reviewed gastric carcinomas resected in our institution with the aim of (1) Identifying significant and independent prognostic factors for western patients with resected GC; (2) Building a clinicopathological prognostic model for the definition of the risk of recurrence and tumor death, after surgical treatment for advanced GC.
METHODS
Patients
We investigated all cases diagnosed with GC and surgically resected in a large tertiary hospital in Madrid (Spain), between the years 2000 and 2014. Ethical approval was obtained from the Ethics Committee at Hospital Clínico San Carlos on January 15, 2016. Two hundred and six resection specimens of patients with GC with or without adjuvant chemotherapy were included in our study. Patients were treated by total or subtotal gastrectomy with D1 or D2 lymphadenectomy. Specimens were formalin-fixed, paraffin-embedded, and stained with hematoxylin and eosin. All slides from these cases were retrospectively reviewed by two independent pathologists blinded to the outcome. A detailed protocol for histologic evaluation was followed, and discordant cases were conjointly reviewed. Histopathological features assessed are described below. Gross findings were retrieved from the database of Surgical Pathology Department (PatWin). Tumor morphology was classified according to Borrmann's classification into four types: Polypoid, flat or diffuse, ulcerative, and fungating.[16] Medical records (both electronic and paper-based) were reviewed and endoscopic and demographic data were collected for the study, including patient age, gender, familial history, presence of clinical symptoms, tumor location, and morphology. Tumor location was defined as the part of stomach which contained the bulk of the tumor, as described in endoscopy and/or pathology reports. Outcome measures were tumor progression (recurrence) and tumor death, after surgical resection with a curative intent.
Histopathological features
All cases included in our study were gastric adenocarcinomas. Microscopical features such as tumor type, percentage of mucin pools and signet-ring cell morphology, tumor grade, presence of perineural infiltration, vascular invasion, necrosis, budding, peritumoral and intratumoral inflammatory infiltrates, desmoplasia, growth pattern at the tumor leading edge, T stage and N stage were assessed. Tumor type was evaluated according to Laurén classification (intestinal or diffuse). Tumor grade was reported as low (well and moderately differentiated, ≥50% gland formation) and high (poorly differentiated, <50% gland formation). Tumor budding was analyzed in hematoxylin and eosin stained slides (CK AE1-AE3 was not performed). It was considered positive when ≥5 single tumor cells or cell clusters of up to 4 tumor cells were seen at the leading edge in one ×20 visual field, as reported by Ueno et al.[17,18] Peritumoral and intratumoral inflammatory responses were scored as positive or negative following the recommendations published by the Association of Directors of Anatomic and Surgical Pathology.[19] We also assessed the type of inflammatory infiltrates (lymphocytic-predominant, eosinophilic-predominant, or neutrophilic-predominant) and their density (mild, moderate, or intense). Tumor growth was scored as pushing and infiltrating, and pTNM stage was reported according to the 8th edition of the AJCC cancer staging manual.
Exclusion criteria
Patients with R1 or R2 resections and metastatic tumors at diagnosis were excluded from our study.
Statistical analysis
All information was stored in an anonymized Excel file and analyzed with the statistical package SPSS 20.0 for Windows. Quantitative data were summarized as mean and standard deviation (SD) after confirming Gaussian distribution or median and range for non-parametric variables. All qualitative data were represented with percentage and absolute numbers. For the analysis of association between variables, we employed either χ2 (Chi)-squared test (qualitative variables) or Student's t-test (to compare means between dichotomic quantitative variables). Statistical significance was settled at a P value <0.05. Multivariate Cox regression models for OS and DFS were calculated. Backward stepwise method was applied, and models were adjusted for potential confounders. Clinicopathological variables were considered categorical covariates. All variables such as age and sex, macroscopic type, tumor staging (T stage and LNR), lymphovascular invasion, perineural infiltration, Laurén subtype, presence of signet-ring cells and tumor grade, which are “classically” related to cancer progression and death, were included as covariates. All variables significantly associated to tumor death and recurrence were included in univariate analyses.
Two prognostic scores for tumor progression and death were developed based on hazard ratios.[20] Receiver-operating characteristic (ROC) analyses were performed and area under the curve (AUC) values were calculated. Kaplan–Meier curves were plotted.
A literature search was performed and our results were compared to those available in the literature.
RESULTS
Two hundred and four cases were included in our study. Clinicopathological features are summarized in Table 1. Males formed 44.6% of all cases. Mean age at diagnosis was 71.4 years, with no significant difference between genders (P = 0.781; male: 71.13, female 71.64). Smokers and former smokers formed 13.9% and 26.9% of all cases, respectively. A familial history of gastrointestinal cancer was reported in 20% of cases. 91% of tumors were symptomatic. 67.1% and 57.4% of all patients showed localized and systemic symptoms, respectively. Tumors were located in the gastric antrum or pylorus, body, fundus, and gastric cardia in 55.7%, 34.3%, 8.4%, and 1.7% of cases, respectively. As for macroscopic features, most tumors were fungoid (35.6%) or ulcerative (29.9%). Polypoid and flat lesions were described in 21.1% and 13.4% of cases. According to Laurén classification, tumors were intestinal (55.8%), diffuse (35.2%), and mixed (9%). Mucin pools, signet-ring cell morphology, tumor necrosis, budding and desmoplasia were seen in 19.1%, 41.7%, 26%, 25.6%, and 52% of cases, respectively. Marked intratumoral inflammatory infiltration was identified in 71.1% of tumors. Peritumoral Crohn-like lymphoid reaction was seen in 36.4% of cases. Lymphovascular and perineural invasion were identified in 44.2% and 50.2% of cases, respectively. All tumors were surgically resected, and 22% of patients received adjuvant therapy. Neoadjuvant therapy and immunotherapy were not administered. GC treatment, staging, and patient outcomes are presented in Table 2. As for pTNM stage, most tumors were T3 (61.9%) and 68.8% showed lymph node metastasis. 15% of GC were stage I, 36.9% stage II, and 48.1% stage III. During follow-up, 43.5% of patients showed recurrences and 28.6% of patients died due to the GC. Median OS and DFS were 29 and 14.5 months, respectively. Tumor recurrences were locoregional in 35.2% and distant in 64.8% of cases.
Table 1.
Clinicopathological features of our series
| FEATURE | n (valid %) | ||
|---|---|---|---|
| Age [mean (SD)] | 71.4 (12.4) | ||
| Male | 90 (44.6%) | ||
| Smoking habit | Ex-smoker | 54 (26.9%) | |
| Active smoker | 28 (13.9%) | ||
| Drinking habit | Ex-drinker | 9 (4.5%) | |
| Active drinker | 22 (11.1%) | ||
| Symptoms | Localized | 104 (67.1%) | |
| Systemic | 89 (57.4%) | ||
| Total | 142 (91%) | ||
| Location | Cardias | 3 (1.7%) | |
| Fundus | 15 (8.4%) | ||
| Body | 61 (34.3%) | ||
| Antrum | 99 (55.7%) | ||
| Macroscopic type | Polypoid | 41 (21.1%) | |
| Flat | 26 (13.4%) | ||
| Ulcerative | 58 (29.9%) | ||
| Fungoid | 69 (35.6%) | ||
| Laurén type | Intestinal | 111 (55.8%) | |
| Diffuse | 70 (35.2%) | ||
| Mixed | 18 (9%) | ||
| Mucin pools | 38 (19.1%) | ||
| Signet-ring cell morphology | 83 (41.7%) | ||
| High grade | 107 (53.8%) | ||
| Tumor necrosis | 52 (26%) | ||
| Infiltrative pattern | 124 (62.6%) | ||
| Budding | 34 (25.6%) | ||
| Desmoplasia | 102 (52%) | ||
| Lymphovascular invasion | 88 (44.2%) | ||
| Perineural invasion | 100 (50.2%) | ||
| Intrat. IIa | Density | None | 11 (5.6%) |
| Mild/moderate | 46 (23.3%) | ||
| Marked | 140 (71.1%) | ||
| Type | Lymphocytic | 178 (94.6%) | |
| Neutrophilic | 3 (1.6%) | ||
| Eosinophilic | 7 (3.7%) | ||
| Peritumoral inflammatory infiltrate | 54 (36.4%) | ||
aIntra. II: Intratumoral inflammatory infiltrate
Table 2.
Tumor treatment, staging and patient outcomes
| Feature | n (valid %) | |||
|---|---|---|---|---|
| Gastrectomy | Subtotal | 141 (69.5%) | ||
| Total | 62 (30.5%) | |||
| Adjuvant therapy | 36 (22%) | |||
| pT | T1 | 10 (5.1%) | ||
| T2 | 41 (20.8%) | |||
| T3 | 122 (61.9%) | |||
| T4 | 24 (12.2%) | |||
| pN | N0 | 59 (31.2%) | ||
| N1 | 37 (19.6%) | |||
| N2 | 50 (26.5%) | |||
| N3 | 43 (22.8%) | |||
| TNM stage | I | 28 (15%) | ||
| II | 69 (36.9%) | |||
| III | 90 (48.1%) | |||
| LNMa [mm, mean (SD)] | 10.5 (7.33) | |||
| LNRb[mean (SD)] | 0.24 (0.28) | |||
| Extracapsular extension | 69 (52.3%) | |||
| Tumor death | 48 (28.6%) | |||
| Recurrence | Total | 87 (43.5%) | ||
| Type | Locorc | 31 (35.2%) | ||
| Distant | 57 (64.8%) | |||
| OSd [months, median (range)] | 29 (0-205) | |||
| DFSe [months, median (range)] | 14.5 (0-186) | |||
aLNM: Lymph node metastases. bLNR: Lymph node ratio. cLocor: Locoregional. dOS: Overall survival. eDFS: Disease-free survival
Univariate analysis (Chi-squared test) results are summarized in Table 3. Patient age, Laurén subtype, perineural invasion, intratumoral inflammatory infiltration, pT, pN, LNR, pTNM stage and adjuvant therapy were significantly associated to tumor recurrence. Presence of signet-ring cells approached significance (P = 0.056). Younger patients and patients with diffuse GC, perineural invasion, no inflammatory infiltration and higher pT, pN, LNR or pTNM stage showed more recurrences. When considering tumor death, patient age, Laurén subtype, presence of signet-ring cells, tumor grade, tumor desmoplasia, pN, LNR, pTNM stage, adjuvant therapy and tumor recurrence were significant prognostic factors. Growth pattern and lymphovascular invasion were significant. (P = 0.069 and 0.059, respectively). Younger patients and patients with diffuse GC, presence of signet-ring cells, high-grade, non-desmoplastic tumors and higher pN, LNR and pTNM stage showed higher death rates.
Table 3.
Univariate analysis (Chi-squared test/T-student test). Variables associated with recurrence and tumor death
| Event | Feature | p | OR (95% CI) | |||
|---|---|---|---|---|---|---|
| Recurrence | Age [mean dif. (SD)] | 0.017 | 4.47 (1.86) | |||
| Laurén subtype | 0.017 | Intestinal | 1 | |||
| Diffuse | 2.32 (1.25-4.3) | |||||
| Mixed | 0.86 (0.3-2.48) | |||||
| Signet-ring cells | 0.056 | 1.69 (0.95-3.03) | ||||
| Perineural invasion | 0.035 | 1.98 (1.11-3.52) | ||||
| Intrat. IIa | 0.035 | None | 1 | |||
| Mild-mod | 0.09 (0.01-0.76) | |||||
| Severe | 0.08 (0.01-0.65) | |||||
| pT | 0.06 | T1 | 1 | |||
| T2 | 1.71 (0.32-9.29) | |||||
| T3 | 4 (0.82-19.62) | |||||
| T4 | 3.67 (0.64-21.15) | |||||
| pN | 0.038 | N0 | 1 | |||
| N1 | 1.75 (0.74.4,15) | |||||
| N2 | 1.95 (0.88-4.33) | |||||
| N3 | 3.37 (1.46-7.81) | |||||
| pTNM stage | 0.007 | I | 1 | |||
| II | 3.16 (1.07-9.35) | |||||
| III | 4.92 (1.71-14.16) | |||||
| LNR [mean dif. (SD)] | 0.006 | 0.11 (0.41) | ||||
| Adjuvant therapy | <0.001 | 4.52 (2-10.21) | ||||
| Tumor death | Age [mean dif. (SD)] | 0.027 | 4.92 (2.21) | |||
| Laurén subtype | 0.003 | Intestinal | 1 | |||
| Diffuse | 3.59 (1.7-7.58) | |||||
| Mixed | 1.66 (0.47-5.88) | |||||
| Signet-ring cells | 0.006 | 2.68 (1.32-5.43) | ||||
| High grade | 0.008 | 2.67 (1.28-5.58) | ||||
| LVb invasion | 0.059 | 1.94 (0.97-3.86) | ||||
| Infiltrative front | 0.069 | 2 (0.94-4.25) | ||||
| Desmoplasia | 0.035 | 0.48 (0.24-0.95) | ||||
| pN | 0.001 | N0 | 1 | |||
| N1 | 1.4 (0.5-3.94) | |||||
| N2 | 0.53 (0.18-1.59) | |||||
| N3 | 3.93 (1.5-10.28) | |||||
| pTNM | 0.072 | I | 1 | |||
| II | 4 (0.84-10.13) | |||||
| III | 5.21 (1.16-24.1) | |||||
| LNR [mean dif. (SD)] | 0.007 | 0.16 (0.048) | ||||
| Recurrence | <0.001 | 20.9 (7.64-57.13) | ||||
| Adjuvant therapy | 0.005 | 3.12 (1.38-7.07) | ||||
aIntrat. II: Intratumoral inflammatory infiltrate. bLV: Lymphovascular
Patients treated by adjuvant therapy showed significantly more recurrences and deaths. A separate univariate analysis was performed and adjuvant therapy was significantly associated with pTNM stage (P = 0.018). Patients receiving adjuvant therapy were stage I (0%), stage II (33.3%), and stage III (66.7%).
Multivariate analysis results are shown in Table 4. We found that Laurén subtype, pT stage, and lymph node ratio were factors independently associated with tumor recurrence. When analyzing OS as the dependent variable, only Laurén subtype and LNR were significant prognostic predictors.DFS curves at mean of covariates and depending on Laurén subtypes and T stage are presented in Figure 1. OS functions at mean of covariates and depending on Laurén subtypes are included in Figure 2.
Table 4.
Multivariate analysis (Cox regression). Features independently associated with overall survival and disease-free survival
| Recurrence | Feature | p | Exp (B), 95% CIa | |
|---|---|---|---|---|
| LNR | <0.001 | 4.97, 2.17-11.37 | ||
| Laurén | Intestinal | 0.046 | 1 | |
| Diffuse | 0.041 | 1.66, 1.02-2.72 | ||
| Mixed | 0.450 | 0.71, 0.29-1.72 | ||
| T | T1 | 0.133 | 1 | |
| T2 | 0.257 | 3.297, 0.42-25.92 | ||
| T3 | 0.078 | 5.954, 0.82-43.25 | ||
| T4 | 0.09 | 6.01, 0.76-47.80 | ||
| Tumor death | Laurén | Intestinal | 0.014 | 1 |
| Diffuse | 0.004 | 2.74, 1.39-5.41 | ||
| Mixed | 0.506 | 1.46, 0.48-4.46 | ||
| LNR | <0.001 | 8.33, 2.85-24.34 | ||
aCI: Confidence interval
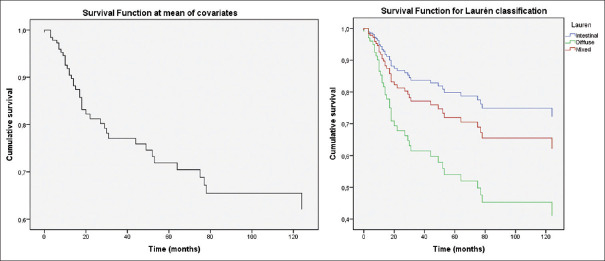
Figure 1.
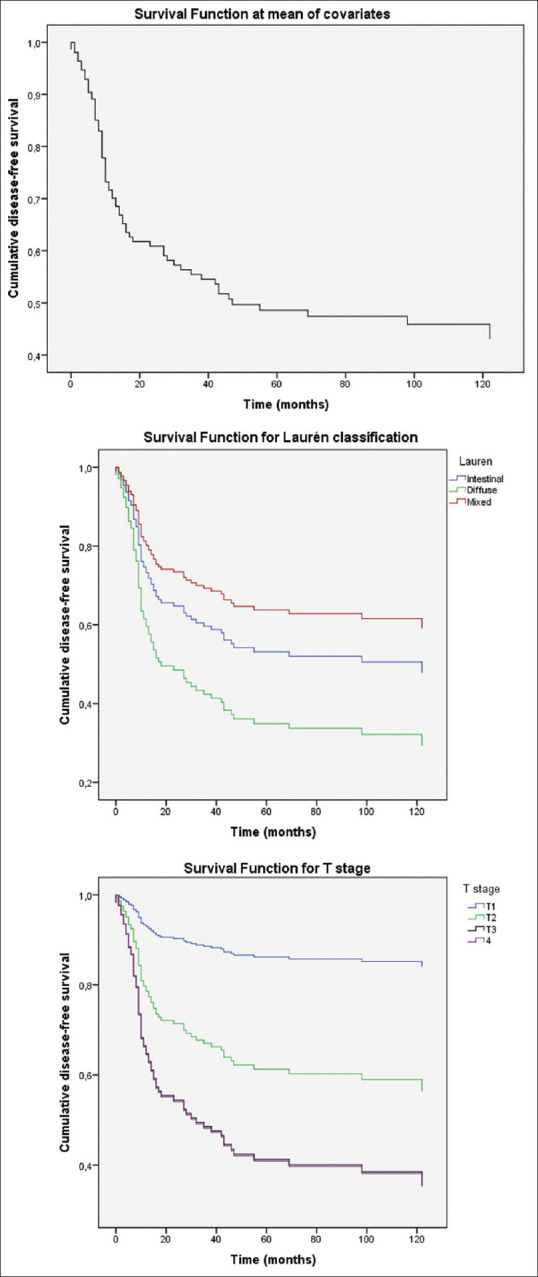
Disease-free survival plots: Disease-free survival function at mean of covariates (top). Survival function for Laurén subtypes (center). Intestinal and diffuse subtypes were independently related to DFS. Survival function for T stage (bottom). T1, T2 and T3-4 tumors showed decreasing DFS rates
Figure 2.
Overall survival plots: Survival function at mean of covariates (left). Survival function for Laurén subtypes (right). Intestinal and diffuse subtypes were independently related to OS
Two simple prognostic scores based on the hazard ratios from the Cox regression analyses were constructed, as seen in other studies [Table 5].[20,21]
Table 5.
Prognostic scores
| Dependent variable | Prognostic score | Total score | ||
|---|---|---|---|---|
| Tumor progression | Laurén subtype | Intestinal | 0 | Range: 0-13 SC1: ≤3 SC2: >3-6 SC3: >6-10 SC4: >10-13 |
| Diffuse | 2 | |||
| Mixed | 0 | |||
| T stage | T1 | 0 | ||
| T2 | 3 | |||
| T3-T4 | 6 | |||
| LNR | LNR x5 | |||
| Tumor death | Laurén subtype | Intestinal | 0 | Range: 0-11 SC1: <1 SC2: 1-<5 SC3: 5-<8 SC4: 8-11 |
| Diffuse | 3 | |||
| Mixed | 1 | |||
| LNR | LNR x8 | |||
In respect of tumor recurrence, total score ranged from 0 to 13. ROC analyses were performed. Area under the curve (AUC) for TNM stage (I–III) was 0.615 (95% CI: 0.534-0.696, P: 0.007). AUC for recurrence score was 0.659 (0.581–0.738, P < 0.001). Cut-off points were defined and patients were classified into four prognostic categories: SC1 (≤3), SC2 (>3–6), SC3 (>6–10), SC4 (>10–13). 11.3%, 23.1%, 52.7%, and 12.9% of patients were SC1, SC2, SC3, and SC4, respectively. Kaplan–Meier curves [Figure 3] showed a good patient stratification into four groups with evenly spaced curves.
Figure 3.
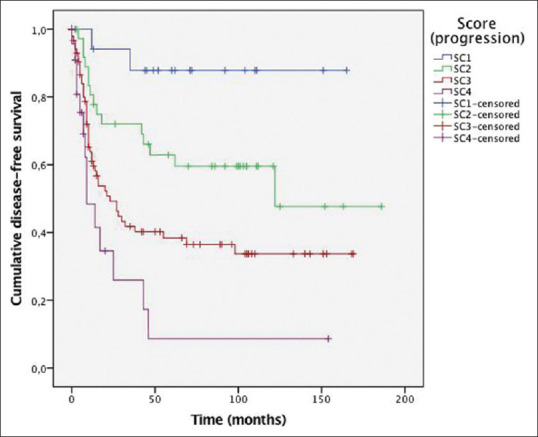
Disease-free survival curves depending on our prognostic score.P value by log-rank test was P < 0.001
Regarding tumor death, total score ranged from 0 to 11. ROC analyses were performed, and AUC for TNM stage (I–III) was 0.594 (95% CI: 0.498–0.690, P = 0.071). AUC for death score was 0.685 (0.593–0.778, P < 0.001). Cut-off points were defined and patients were classified into four prognostic groups: SC1 (>1), SC2 (1-< 5), SC3 (5-< 8), and SC4 (8–11). 28.2%, 48.9%, 13.8%, and 9% of patients were SC1, SC2, SC3, and SC4, respectively. Kaplan–Meier curves [Figure 4] showed good stratification into four categories with evenly spaced curves.
Figure 4.
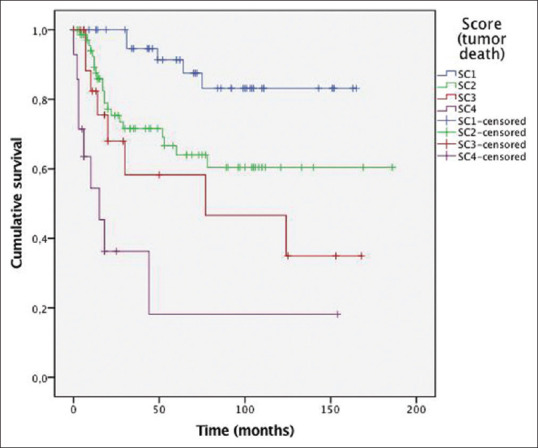
Overall survival curves depending on our prognostic score. Pvalue by log-rank test was P< 0.001
DISCUSSION
Incidence of GC and GC-related mortality have slightly decreased in the last decades, probably due to the emergence of new surgical techniques and management options. However, GC is still diagnosed at advanced stages and shows high mortality rates.[22] In fact, recurrence rates of almost 70% have been reported in patients with advanced disease.[13] Histologically, according to Laurén's criteria, GC can be classified into three types: Intestinal, diffuse, and mixed.[23] The WHO classification of gastrointestinal neoplasms establishes four histologic patterns: Tubular, discohesive, mucinous, and papillary.[24] Mixed GC accounts for approximately 25% of all tumors, a fact which could reflect the heterogeneity of GC. As for its molecular features, GC is a polygenic disease, which results from the combined interaction of multiple genes and tumor microenvironment.[8]
As previously mentioned, prognostic stratification systems of GC need to be refined to provide accurate and individualized information. The pTNM staging system is still the main tool for patient stratification.[7] However, several issues have arisen. First of all, pTNM system only assesses the anatomic extension of GC, taking into account tumor depth and lymph node or distant metastasis.[25] A marked prognostic heterogeneity has been reported among patients with advanced GC, and some authors have observed early recurrences in early GC cases.[5,12] Several prognostic factors other than pTNM stage have been identified, including clinical (age, gender, nutritional status, blood test findings),[6,20,26] pathological (tumor depth, lymphovascular invasion, histologic type, histologic grade),[27,28,29] immunohistochemical and molecular features.[30] These factors have been repeatedly reported in the literature and have demonstrated significant prognostic value in multiple studies. Based on these findings, some authors have built new prognostic models which have shown better prognostic performance than TNM stage.[22] The clinicopathological scoring system proposed by Qian et al. identified high risk patients in stage II or III, and they observed that low-risk stage III patients had higher survival probabilities than stage II patients.[13] Costa et al. suggested that the epidemiological discrepancy between Asian and western countries cannot be explained solely by differences in screening interventions, because this discrepancy persists even when patients are stratified according to their TNM stage.[22] In our opinion, the prognostic role of the TNM staging system should not be underestimated, and new prognostic scores could be used as an adjunct to pTNM stage, in order to individualize management decisions. Specific prognostic scores could also be applied to different populations and patient subgroups.
An optimal prognostic score should be objective, reliable, and practical.[12] Variables included in the score should be standardized and easy to assess.[24] Most published prognostic scores include clinical (preoperative and postoperative) and molecular features.[8,14,15] Immunohistochemical or molecular techniques may be difficult to introduce in certain centers, but models including clinical and histological features are cheap and easy-to-implement in clinical practice.[31,32] Thus, they may be useful to refine the existing TNM classification. Previously published histopathological models have been summarized in Table 6. Almost half of these studies have been performed in Chinese, Korean, or Japanese population. Due to the geographical variations of GC, more studies should be performed in other populations to identify risk factors and to develop and validate specific prognostic equations.
Table 6.
Prognostic scores including histopathological factors reported in the literature
| Author, year pub |
Type of patients included | Predicted end point | Variables included |
|---|---|---|---|
| Becker Germany 2012 Ann Surg |
NAa + Sb | OSc | yT yN Histopathological tumor regression |
| Bria32 Italy 2012 Ann Oncol |
S | CSSd | Sex Stage Margins Tumor location Lymph node involvement APC IHCe expression Fhit IHC expression |
| OS | Age Sex Stage Margins Tumor location Lymph node involvement APC IHC expression HER2 expression |
||
| Kologlu25 Turkey 2000 Am J Surg |
S | OS and DFSf | pT pN (AJCCg 1992, UICCh 1997) pM Metastatic lymph node ratio Resectability Tumor location Lymph node dissection (D1 or D2) Borrman classification Lauren classification |
| Vieira Costa22 Brazil 2006 Ann Surg Oncol |
S | OS | Sex Weight loss Pre-operative lymphocyte count Lymph node ratio Lymph node dissection TNM stage |
| Zhu29 China 2014 BMJ Cancer |
S | OS | Histological grading stage |
| Marrelli27 Italy 2005 Ann Surg |
S | DFS | pN pT Lymph node dissection (D1 vs D2-3) Tumor location Age |
| Sekiguchi28 Japan 2016 J Gastroenterol |
S Early tumors |
Lymph node metastasis | Tumor size Tumor depth Histologic type Ulcerative features Lymphovascular invasion |
| Haraguchi26 Japan 2018 Oncotarget |
S | OS Tumor progression |
Tumor depth and size |
| Qian13 China 2016 Drug Des Devel Ther |
S + Ai | OS Treatment response |
Lymph node rate Lymphovascular invasionpTNM (I-IV) Preoperative CEA level Preoperative hemoglobin |
| Park42 Korea 2015 Gastric cancer |
S in early GC j (stage I) | DFS | Age Sex pTNM (stage) Lymphovascular invasion Perineural invasion CEA level |
| Marubini43 Italy 1993 Eur J Cancer |
S±A | OS | Age Tumor depth Tumor location Lymph node involvement |
| Ichikura41 Japan 1993 Surg Today |
S±A with serosal invasion | DFS | Lymph node involvement Macroscopic typeSerosal invasion (macroscopic) Interstitial conective tissue |
| S±A without serosal invasion | DFS | Lymph node involvement Serosal invasion (macroscopic) Venous invasion |
|
| Kattan44 US 2003 J Clin Oncol |
S | Nomogram for CSS | Age Sex Tumor location Laurén classification Number of positive lymph nodes Number of negative lymph nodes Tumor depth |
| Dikken34 US / Holland 2013 Ann Surg Oncol |
S±A | Nomogram for conditional probability of survival | Sex Age Tumor location Laurén classification Tumor diameter Positive lymph nodes Negative lymph nodes Tumor depth |
| Han35 Korea 2012 J Clin Oncol |
S±A | Nomogram for OS | Sex Age Tumor location Tumor depth Number of metastatic lymph nodes Number of negative lymph nodes |
aNA: Neoadjuvanttherapy. bS: Surgery. cOS: Overall survival. dCSS: Cancer-specific survival. eIHC: Immunohistochemistry. fDFS: Disease-free survival. gAJCC: American Joint Committee on Cancer. hUICC: Union for International Cancer Control. iA: Adjuvant therapy. wjGC: Gastric cancer
Previous clinical prognostic scores included mainly nutritional and inflammatory variables, such as neutrophil/lymphocyte ratio, circulating concentrations of C-reactive protein, albumin or bilirubin.[5,6,12,25] Nutritional or performance status variables like EGOC or PG-SGA could be more subjective.[20,26,33] As for clinicopathological scores, most reported features are gender, age, pTNM stage, Laurén classification, tumor depth, lymphovascular invasion, and lymph node involvement [Table 6]. Some clinicopathological nomograms for predicting survival of patients with GC have also been created.[34,35,36] In our univariate analyses, we have found that patient age, Laurén subtype, presence of signet-ring cells, perineural invasion, intratumoral inflammatory infiltration, pT, pN, pTNM stage, LNR and adjuvant therapy were significantly associated with tumor progression. Histological grade and desmoplasia were associated with death due to GC.
With regard to age of the patient, younger patients showed more recurrences and decreased survival rates. However, the effect of age on GC survival is contradictory. Some studies support our results, but others have observed that younger patients show better survival rates.[37] pTNM stage (including tumor depth and lymph node involvement), tumor grade, and perineural invasion are well-known prognostic factors, which can be extrapolated to almost all tumor types. Intratumoral immune response has gained attention in the last decades, and lymphocytic infiltration has been shown to be a factor of better prognosis in several tumor types.[38,39] In our study, only 5.6% of GC showed no intratumoral inflammation, and these patients developed significantly more recurrences. In 94.6% of tumors, the inflammation was predominantly However, lymph node ratio retained prognostic significance. This could be explained by the fact that the extent of lymph node dissection and lymph node positivity in our series was highly variable, and N ratio shows significant advantages in this circumstance.
Based on these results, we developed prognostic scores for tumor progression and death, and our patients were classified into four prognostic groups which showed good prognostic performance in Kaplan–Meier curves.
In respect of GC management, surgery is the only curative treatment and resectable tumors are treated by total or subtotal gastrectomy.[22,27] Early disease could be treated by endoscopic submucosal dissection or endoscopic mucosal resection.[40] The role of adjuvant therapy in advanced GC depends on patient or tumor features,[6] and neoadjuvant therapy is being increasingly used.[15] In our series, patients with adjuvant therapy showed more recurrences and tumor death than patients without postoperative chemotherapy. In a separate analysis, and we found that adjuvant therapy was significantly associated only with pTNM stage (P = 0.018). Patients receiving adjuvant therapy were stage I (0%), stage II (33.3%), and stage III (66.7%). So, association between adjuvant therapy and tumor death or recurrence seems to be a reflection of pTNM stage, because it was administered in patients with more advanced tumors, and those patients showed higher progression and death rates despite this therapy. Finally, metastatic tumors are treated by palliative chemotherapy regimens.[36]
CONCLUSIONS
In western countries, GC is commonly diagnosed in advanced stages and shows low survival rates. pTNM staging is currently the best prognostic tool available, but factors other than pTNM stage have been consistently reported to be associated with GC prognosis, and patients with the same pTNM stage may show different outcomes. Several clinicopathological models including molecular or immunohistochemical features have been proposed, but models based on clinical and histological features only, are scarce, and most of them were developed in China, Japan, or Korea. We have analyzed patients with resected GC. Univariate analyses showed that patient age, Laurén subtype, presence of signet-ring cells, perineural invasion, intratumoral inflammatory infiltration, pT, pN, pTNM stage, LNR and adjuvant therapy were significantly associated with tumor progression. Histological grade and desmoplasia were associated with death due to tumor. In our multivariate analysis, factors independently related to OS and DFS were lymph node ratio, Laurén subtype, and T-stage. Prognostic scores for tumor progression and death were developed and patients were classified into four prognostic groups for each outcome. Kaplan–Meier curves showed good patient stratification with evenly spaced curves. The development of prognostic scores including other histopathological features, such as Laurén subtype, can improve the prognostic value of pTNM stage and has the potential to aid in the individualization of patient management.44
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–48. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–13. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol. 2014;20:4483–90. doi: 10.3748/wjg.v20.i16.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017;39:1010428317714626. doi: 10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Huang D. The value of the systematic inflammation-based glasgow prognostic score in patients with gastric cancer: A literature review. J Cancer Res Ther. 2014;10:799. doi: 10.4103/0973-1482.146054. [DOI] [PubMed] [Google Scholar]
- 6.Koo DH, Ryoo B-Y, Kim HJ, Ryu M-H, Lee S-S, Moon J-H, et al. A prognostic model in patients who receive chemotherapy for metastatic or recurrent gastric cancer: Validation and comparison with previous models. Cancer Chemother Pharmacol. 2011;68:913–21. doi: 10.1007/s00280-011-1561-8. [DOI] [PubMed] [Google Scholar]
- 7.Wittekind C. The development of the TNM classification of gastric cancer. Pathol Int. 2015;65:399–403. doi: 10.1111/pin.12306. [DOI] [PubMed] [Google Scholar]
- 8.Wang P, Wang Y, Hang B, Zou X, Mao J-H. A novel gene expression-based prognostic scoring system to predict survival in gastric cancer. Oncotarget. 2016;7:55343–51. doi: 10.18632/oncotarget.10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunakawa Y, Lenz HJ. Molecular classification of gastric adenocarcinoma: Translating new insights from the cancer genome atlas research network. Curr Treat Options Oncol. 2015;16:17. doi: 10.1007/s11864-015-0331-y. [DOI] [PubMed] [Google Scholar]
- 10.Marrelli D, Pedrazzani C, Morgagni P, de Manzoni G, Pacelli F, Coniglio A, et al. Changing clinical and pathological features of gastric cancer over time. Br J Surg. 2011;98:1273–83. doi: 10.1002/bjs.7528. [DOI] [PubMed] [Google Scholar]
- 11.Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: Ten-year results of the German gastric cancer study. Ann Surg. 1998;228:449–61. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu J-L, Qu X-J, Li Z, Zhang J-D, Liu J, Teng Y-E, et al. Prognostic model based on systemic inflammatory response and clinicopathological factors to predict outcome of patients with node-negative gastric cancer. PLoS One. 2015;10:e0128540. doi: 10.1371/journal.pone.0128540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian J, Qian Y, Wang J, Gu B, Pei D, He S, et al. A clinical prognostic scoring system for resectable gastric cancer to predict survival and benefit from paclitaxel- or oxaliplatin-based adjuvant chemotherapy. Drug Des Devel Ther. 2016;10:241. doi: 10.2147/DDDT.S88743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrelli D, Morgagni P, de Manzoni G, Marchet A, Baiocchi GL, Giacopuzzi S, et al. External validation of a score predictive of recurrence after radical surgery for non-cardia gastric cancer: Results of a follow-up study. J Am Coll Surg. 2015;221:280–90. doi: 10.1016/j.jamcollsurg.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 15.Becker K, Reim D, Novotny A, Büschenfelde CMZ, Engel J, Friess H, et al. Proposal for a multifactorial prognostic score that accurately classifies 3 groups of gastric carcinoma patients with different outcomes after neoadjuvant chemotherapy and surgery. Ann Surg. 2012;256:1002–7. doi: 10.1097/SLA.0b013e318262a591. [DOI] [PubMed] [Google Scholar]
- 16.Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–61. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horcic M, Koelzer VH, Karamitopoulou E, Terracciano L, Puppa G, Zlobec I, et al. Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. Hum Pathol. 2013;44:697–705. doi: 10.1016/j.humpath.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 18.Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–94. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Jass JR, O'Brien J, Riddell RH, Snover DC. Association of directors of anatomic and surgical pathology. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma. Am J Clin Pathol. 2008;129:13–23. doi: 10.1309/6UHNC7MAD8KWNAWC. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh M-C, Wang S-H, Chuah S-K, Lin Y-H, Lan J, Rau K-M. A prognostic model using inflammation- and nutrition-based scores in patients with metastatic gastric adenocarcinoma treated with chemotherapy. Medicine (Baltimore) 2016;95:e3504. doi: 10.1097/MD.0000000000003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall RE, Porter J, Quan H, Reeves MJ. Developing and adapted charlson comorbidity index for ischemic stroke outcome studies. BMC Health Serv Res. 2019;19:930. doi: 10.1186/s12913-019-4720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa MLV, de Cássia Braga Ribeiro K, Machado MAC, Costa ACLV, Montagnini AL. Prognostic score in gastric cancer: The importance of a conjoint analysis of clinical, pathologic, and therapeutic factors. Ann Surg Oncol. 2006;13:843–50. doi: 10.1245/ASO.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 23.Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World J Gastroenterol. 2014;20:5679. doi: 10.3748/wjg.v20.i19.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosman F, Carneiro F, Hruban R, Theise N. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon (France): IARC; 2010. [Google Scholar]
- 25.Kologlu M, Kama NA, Reis E, Doganay M, Atli M, Dolapci M. A prognostic score for gastric cancer. Am J Surg. 2000;179:521–6. doi: 10.1016/s0002-9610(00)00385-8. [DOI] [PubMed] [Google Scholar]
- 26.Haraguchi N, Arigami T, Uenosono Y, Yanagita S, Uchikado Y, Mori S, et al. Clinical significance of primary tumor score determined by tumor depth and size in patients with resectable gastric cancer. Oncotarget. 2018;9:8512–20. doi: 10.18632/oncotarget.23953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: A scoring system obtained from a prospective multicenter study. Ann Surg. 2005;241:247–55. doi: 10.1097/01.sla.0000152019.14741.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekiguchi M, Oda I, Taniguchi H, Suzuki H, Morita S, Fukagawa T, et al. Risk stratification and predictive risk-scoring model for lymph node metastasis in early gastric cancer. J Gastroenterol. 2016;51:961–70. doi: 10.1007/s00535-016-1180-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z, Sun X, Wang J, Sun Z, Wang Z, Zheng X, et al. Histopathology-based prognostic score is independent prognostic factor of gastric carcinoma. BMC Cancer. 2014;14:663. doi: 10.1186/1471-2407-14-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jian-hui C, Shi-rong C, Hui W, Si-le C, Jian-bo X, Er-tao Z, et al. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumor Biol. 2016;37:11105–13. doi: 10.1007/s13277-015-4191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohri Y, Tanaka K, Ohi M, Yokoe T, Miki C, Kusunoki M. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg. 2010;34:285–90. doi: 10.1007/s00268-009-0302-1. [DOI] [PubMed] [Google Scholar]
- 32.Bria E, De Manzoni G, Beghelli S, Tomezzoli A, Barbi S, Di Gregorio C, et al. A clinical–biological risk stratification model for resected gastric cancer: Prognostic impact of Her2, Fhit, and APC expression status. Ann Oncol. 2013;24:693–701. doi: 10.1093/annonc/mds506. [DOI] [PubMed] [Google Scholar]
- 33.Kelly CM, Shahrokni A. Moving beyond karnofsky and ECOG performance status assessments with new technologies. J Oncol. 2016;2016:1–13. doi: 10.1155/2016/6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dikken JL, Baser RE, Gonen M, Kattan MW, Shah MA, Verheij M, et al. Conditional probability of survival nomogram for 1-, 2-, and 3-year survivors after an R0 resection for gastric cancer. Ann Surg Oncol. 2013;20:1623–30. doi: 10.1245/s10434-012-2723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han D-S, Suh Y-S, Kong S-H, Lee H-J, Choi Y, Aikou S, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012;30:3834–40. doi: 10.1200/JCO.2012.41.8343. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Qu J, Li Z, Che X, Zhang J, Liu J, et al. A prognostic model in metastatic or recurrent gastric cancer patients with good performance status who received first-line chemotherapy. Transl Oncol. 2016;9:256–61. doi: 10.1016/j.tranon.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cormedi MCV, Katayama MLH, Guindalini RSC, FAraj SF, Folgueira MAAK. Survival and prognosis of young adults with gastric cancer. Clinics (Sao Paulo) 2018;73(Suppl 1):e651s. doi: 10.6061/clinics/2018/e651s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, Qu C. The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: A systematic review and meta-analysis. Sci Rep. 2020;10:3360. doi: 10.1038/s41598-020-60255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S, et al. Prognostic effect of tumor lymphocytic infiltration in resectable non-small cell lung cancer. J Clin Oncol. 2016;34:1223–30. doi: 10.1200/JCO.2015.63.0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchet A, Mocellin S, Ambrosi A, Morgagni P, Garcea D, Marrelli D, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy. Ann Surg. 2007;245:543–52. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichikura T, Fujino K, Ikawa H, Tomimatsu S, Uefuji K, Tamakuma S. Proposal of a risk score for recurrence in patients with curatively resected gastric cancer. Surg Today. 1993;23:759–64. doi: 10.1007/BF00311616. [DOI] [PubMed] [Google Scholar]
- 42.Park JH, Ryu M-H, Kim HJ, Ryoo B-Y, Yoo C, Park I, et al. Risk factors for selection of patients at high risk of recurrence or death after complete surgical resection in stage I gastric cancer. Gastric Cancer. 2016;19:226–33. doi: 10.1007/s10120-015-0464-5. [DOI] [PubMed] [Google Scholar]
- 43.Marubini E, Bonfanti G, Bozzeti F, Boracchi P, Amadori D, Folli S, et al. A prognostic score for patients resected for gastric cancer. Eur J Cancer. 1993;29:845–50. doi: 10.1016/s0959-8049(05)80421-6. [DOI] [PubMed] [Google Scholar]
- 44.Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–50. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]