Abstract
Background:
Upper gastrointestinal bleeding (UGIB) remains a healthcare burden and is associated with considerable morbidity and mortality. We aim to describe the presentation, clinical, and laboratory characteristics of patients presenting with UGIB as well as important patient outcomes.
Methods:
This is a retrospective study performed at a tertiary care university hospital in Riyadh. Electronic endoscopic reports of patients undergoing gastroscopies for the indication of UGIB from January 2006 to January 2015 were included. Demographic data, past medical conditions, medications used, symptoms on presentation, as well as the patients' hemodynamic status, laboratory investigations on presentations, the need for blood products, the need for admission to an intensive care unit, rebleeding, and in-hospital mortality rates were retrieved from medical records.
Results:
Two hundred fifty-nine patients were included with a mean age of 57.1 years and males constituted 66.8% of the study cohort. At least one comorbidity was present in 88.2%, while 20.7% had a history of prior UGIB, 12.6% had a history of peptic ulcer disease, and 9.2% had known esophageal varices. A nonvariceal source represented 80.1% of the causes (95% CI: 75.4 to 85.3%), 15.5% required admission to the intensive care unit (ICU), the rebleeding rate was 8.9% (95% CI; 5.7% to 12.2%) while the in-hospital mortality was 4.4% (95% CI; 2.4% to 6.9%). The mean pre-endoscopic Rockall score was 2.6 (range: 0 to 5), while the total Rockall score was 4.4 (range: 1 to 9). There was no association between the pre-endoscopic Rockall score and rebleeding (3.0 vs. 2.5, P = 0.27) or need for ICU admission (3.2 vs. 2.4, P = 0.08), the total Rockall score and rebleeding (5.0 vs. 4.4, P = 0.58) or need for ICU admission (5.0 vs. 4.3, P = 0.36).
Conclusion:
Causes of UGIB in this patient population were predominantly nonvariceal and the rebleeding and mortality rates resembled those of other studies.
Keywords: Nonvariceal bleeding, patient-reported outcomes, peptic ulcer disease, Saudi Arabia, upper gastrointestinal bleeding, variceal bleeding
INTRODUCTION
Despite the recent advances in medical care, upper gastrointestinal bleeding (UGIB) remains a considerable healthcare burden and is associated with a mortality which could be as high as 11% at 30 days in cases of peptic ulcer disease[1] that is either directly related to the bleeding episode or to the consequences of complications during hospitalization. These outcomes are even worse for those who develop UGIB when hospitalized for other reasons[2] and those with severe comorbidities.[3,4] Peptic ulcer disease remains the most common cause of nonvariceal upper gastrointestinal bleeding (NVUGIB) but has been decreasing as a cause of hospitalizations over time.[5,6,7]
Prior studies from Saudi Arabia tend to report a variceal cause of UGIB to be in the range of 38% to 45%,[8,9,10,11] while there is a wide variation of other causes reported.[8,9,10,11,12,13,14,15,16] These studies have spanned a few decades, with the earliest report that we could find dating back to 1988[8] and the most recent in 2019.[11] During this time, the prevalence of H. pylori has changed, as well the age composition of the population, the type and burden of disease in the region, as well as the type of therapies and the management of gastrointestinal diseases.[17] This has also been recognized with the change in the endoscopic lesions that are identified on esophagogastroduodenoscopy (EGDs) for patients with dyspepsia over time.[18,19] The variability in the proportion of causes of UGIB across geographical regions has been described[7] and might be influenced by the prevalence of H. pylori,[20,21] viral hepatitis, as well as the demographic features of populations which reflects the burden of noncommunicable disease and their associated morbidities.[22,23]
In this study, we aim to describe the presentation as well as clinical and laboratory characteristics of patients presenting with UGIB as well as the hospital course, rebleeding, and mortality rates associated with UGIB. We also assessed the performance of the Rockall score in predicting adverse events in our patient population.
MATERIALS AND METHODS
This was a retrospective study that was performed at a tertiary care university hospital in Riyadh. From an electronic endoscopic reporting database, the data of patients undergoing EGDs for the indication of UGIB, hematemesis, coffee-ground emesis, melena, or hematochezia from January 2006 to January 2015 (9 years) were included in the study.
Demographic data were retrieved from medical records and included age, sex, height, weight, body mass index (BMI), comorbidities, history of chronic liver disease, esophageal varices, history of prior UGIB or peptic ulcer disease, smoking status, alcohol consumption, and nationality. The medications used by patients including aspirin, other antiplatelets, anticoagulants, heparin, nonsteroidal antiinflammatory drugs (NSAIDS), and cyclooxygenase-2 (COX-2) inhibitors were also obtained.
Symptoms on presentation and the patients' hemodynamic status (pulse, blood pressure, and respiratory rate) were recorded along with a rectal exam or a nasogastric lavage if performed. Laboratory investigations on presentation including hemoglobin level, platelet count, the international normalized ratio (INR), total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and serum creatinine level were documented.
We also recorded the need for blood products as well as the amount transfused and the need for admission to an intensive care unit and length of stay in the ICU, the rate of rebleeding, and in-hospital mortality.
Endoscopy reports were reviewed and the lesion identified was retrieved along with its location and description if these were actively bleeding or not and the Forrest classification if it were peptic ulcer disease related. The pre-endoscopic as well as the complete Rockall score was calculated based on the data obtained.
No personal identification information or other personal identifiers were recorded. The internal review board of the institution approved the study.
Statistical analysis
Descriptive statistics were computed for continuous variables, including minimum and maximum values, means, standard deviations (SDs), as well as frequencies for categorical variables when appropriate. If hypothesis testing was used, Pearson's Chi-square t-test and, where appropriate, Fisher's exact tests were used. A one-way analysis of variance to test for differences among groups when comparing more than one group was performed when appropriate.
R Studio[24] was used for analysis using the R statistical language. Numerous statistical packages were used for statistical calculations. A statistical significance threshold of P = 0.05 was adopted. No attempt at imputation was made for missing data.
RESULTS
Demographics
A total of 259 patients who underwent an EGD for the evaluation of UGIB were included in the study with a mean age of 57.1 years (SD 18.01, range: 14 to 97), males constituted 66.8%, the mean BMI was 27.3 (SD 8.03), and Saudi nationals comprised 90% of the study population. Of the complete cohort, 10.3% were current smokers, 3.2% were prior smokers, and 2.5% had a history of consumption of alcohol. Of the study population, 20.7% had a history of prior UGIB, 12.6% had a history of peptic ulcer disease, and 9.2% had known esophageal varices.
Of the study cohort, 88.2% had known comorbidities which included: Hypertension (48.9%), diabetes mellitus (43.7%), ischemic heart disease (24.1%), known chronic liver disease (18.8%), cerebrovascular accidents (7.6%), malignancy (6.0%), chronic obstructive pulmonary disease (3.8%), as well as other comorbidities at less frequencies [Table 1].
Table 1.
Characteristics of the study population
| Variable | Proportion OR mean (SD) n=259 | Range or (95% CI) |
|---|---|---|
| Age (years) | 57.10 (18.01) | 14-97 |
| Male | 66.79% | 61.39-72.95% |
| Weight (kg) | 73 (17.92) | 27.00-132.00 |
| Height (cm) | 161.3 (10.29) | 120-184 |
| Body mass index (kg/m2) | 27.32 (8.03) | 14.18 -58.67 |
| Comorbidities | 83.17% | 78.37-88.10% |
| Hypertension | 48.94% | 42.11-56.64% |
| Diabetes | 43.68% | 36.84-51.23% |
| Ischemic heart disease | 24.08% | 18.32-30.23% |
| Chronic liver disease | 18.78% | 13.71-24.23% |
| HCV positive | 8.47% | 5.29-12.55% |
| Cerebrovascular accident | 7.61% | 4.35-11.32% |
| Malignancy | 5.98% | 3.26-9.40% |
| COPD | 3.80% | 1.63-6.44% |
| Smokers | 10.34% | 6.90-14.67% |
| Exsmokers | 3.16% | 1.05-5.28% |
| Alcohol consumption | 2.46% | 0.99-4.60% |
| Medications used | ||
| Aspirin | 39.90% | 33.16-47.20% |
| NSAIDs | 13.66% | 9.29-18.67% |
| Clopidogrel | 12.02% | 7.65-16.43% |
| Warfarin | 8.70% | 5.43-12.88% |
| Steroids | 7.65% | 4.37-11.38% |
| Heparin | 3.26% | 1.09-5.45% |
| COX-2 inhibitors | 1.64% | 0.55-3.52% |
| Prior history of | ||
| Gastrointestinal bleeding | 20.67% | 15.08-26.57% |
| Peptic ulcer disease | 12.57% | 8.20-17.19% |
| Esophageal varices | 9.24% | 9.43-13.10% |
| Nationality | ||
| Saudi | 89.96% | 86.87-93.70% |
| Non-Saudi | 10.03% | 6.95-13.78% |
| Presenting complaints | ||
| Melena | 66.19% | 60.00-72.90% |
| Hematemesis | 56.04% | 49.28-63.09% |
| Abdominal pain | 44.56% | 37.82-52.13% |
| Vomiting | 36.36% | 29.95-43.84% |
| Coffee-ground emesis | 31.12% | 25.00-37.97% |
| Dizziness | 19.67% | 14.21-25.32% |
| Hematochezia | 6.01% | 3.28-9.45% |
| Vital signs on presentation | ||
| Pulse (beats per min) | 90 (22.40) | 12-190 |
| Systolic blood pressure (mmHg) | 124 (21.31) | 76-203 |
| Diastolic blood pressure (mmHg) | 70 (13.82) | 29-107 |
| Respiratory rate (breaths/min) | 21 (2.82) | 12-33 |
| Laboratory findings on presentation | ||
| Hemoglobin on presentation (g/dL) | 10.15 (2.84) | 3.10-17.20 |
| Platelets (×109/L) | 249 (135.43) | 99-881 |
| International normalized ratio (INR) | 1.46 (1.03) | 0.83-8.00 |
| Total bilirubin (umol/L) | 20.68 (39.3) | 1-283 |
| Alanine aminotransferase (ALT) (IU/L) | 61 (119) | 15-1200 |
| Aspartate aminotransferase (AST) (IU/L) | 2 (453) | 5-4880 |
| Alkaline phosphatase (ALP) (IU/L) | 138 (161) | 36-1456 |
| Creatinine (mmol/L) | 130 (160) | 24-1135 |
COPD: Chronic obstructive pulmonary disease, COX-2; cyclooxygenase-2, HCV; hepatitis C virus, NSAIDs; nonsteroidal antiinflammatory drugs
Medications used by patients
From the study population, 39.9% gave a history of using aspirin, 13.7% NSAIDS, 12.0% clopidogrel, 8.7% warfarin, 7.7% steroids, 3.3% heparin, and 1.6% COX-2 inhibitors [Table 1]. There was concomitant use of aspirin and clopidogrel in 6.5%, aspirin and NSAIDs in 4.6%, aspirin and steroids in 2.7%, and NSAIDs and clopidogrel in 1.2% of the study population.
Symptoms, hemodynamics, initial assessment, and laboratory investigations on presentation
Symptom on presentation included melena (66.2%), hematemesis (56.0%), abdominal pain (44.6%), vomiting (36.4%), coffee-ground vomiting (31.1%), dizziness (19.7%), and hematochezia (6.0%) [Table 1].
On presentation, the mean pulse rate was 90 beats/min (SD 22.4), systolic blood pressure 124 mmHg (SD 21.3), diastolic blood pressure 70 mmHg (SD 13.8), and respiratory rate 21 breaths/min (SD 2.8). Of the study population, 8.5% had a systolic blood pressure lower than 90 mmHg.
A rectal examination was only documented in 53 patients and revealed melena in 45, occult blood in 6, and bright blood in two patients. A nasogastric lavage was only performed in 22 patients and revealed coffee-ground material in 13, bright red blood in 8, and was normal in one patient.
The various laboratory values that were obtained on presentation are shown in Table 1.
Pre-endoscopic and total Rockall scores
The mean pre-endoscopic Rockall score was 2.55 (SD 1.47, range: 0 to 5), while the total Rockall score was 4.43 (SD 1.95, range: 1 to 9).
There was no association between the pre-endoscopic Rockall score and rebleeding (3.0 vs. 2.5, P = 0.27) or need for ICU admission (3.2 vs. 2.4, P value 0.08). There were no associations between the total Rockall score and rebleeding (5.0 vs. 4.4, P value 0.58) or need for ICU admission (5.0 vs. 4.3, P value 0.36).
Blood product requirements
Only 13.9% of the cohort received packed red blood cells (PRBCs). The mean blood transfusion rate for those who received PRBCs was 3.53 units (SD 0.97, range: 1 to 6), and when fresh frozen plasma was transfused (almost exclusively for patients with a variceal bleed), the range of the number of units was 2 to 6, mean 3.25 units (SD 1.49). The mean hemoglobin level at discharge was 11.0 g/dL (SD 2.02).
Intensive care unit admission, rebleeding, and in-hospital mortality
Out of the patient cohort, 15.5% required admission to the intensive care unit with a mean duration of stay of 25 days (SD 34.16, range from 2 to 96 days), the length of the ICU stay was skewed, and the median length of ICU stay was 6 days. The rebleeding rate was 8.9% (95%CI; 5.7% to 12.2%), while the in-hospital mortality was 4.4% (95%CI; 2.4% to 6.9%).
Endoscopic diagnoses
Nonvariceal sources of bleeding
NVUGIB represented 80.1% of the causes of UGIB (95%CI: 75.4 to 85.3%). In our cohort, the most common findings in patients presenting with NVUGIB were esophagitis/gastroesophageal reflux disease (GERD) (38.1%), gastric erosions (37.6%), duodenal ulcers (23.6%), and gastric ulcers (12.4%). Less common causes included tumors (3.5%). No endoscopic findings were found in 8.9% of the cohort [Table 2].
Table 2.
Endoscopic findings in those undergoing upper gastrointestinal endoscopy for suspected upper gastrointestinal bleeding
| Variable | Percentage | (95% CI) |
|---|---|---|
| Nonvariceal bleeding | 80.08% | 75.42-85.33% |
| Variceal bleeding | 19.91% | 15.25-25.16% |
| Active bleeding | 6.58% | 3.88-9.39% |
| Endoscopic finding | ||
| Esophagitis/GERD | 38.13% | 32.30-44.40% |
| Gastric erosions | 37.60% | 31.78-43.82% |
| Duodenal ulcer | 23.64% | 18.60-28.82% |
| Esophageal varices | 17.83% | 13.57-22.60% |
| Gastric ulcer | 12.40% | 8.91-16.52% |
| Normal | 8.91% | 5.81-12.26% |
| Portal hypertensive gastropathy | 6.59% | 3.88-9.39% |
| Fundal varices | 4.26% | 2.33-6.72% |
| Mass | 3.49% | 1.55-5.47% |
GERD: Gastroesophageal reflux disease
The correlation between the presentation of patients whether it be hematemesis, coffee-ground emesis, melena, or hematochezia and the lesion found on endoscopy is shown in Figure 1: Active bleeding from these lesions that were identified was more commonly to be encountered in those with duodenal ulcers or masses, while was less likely to be seen in cases with esophagitis/GERD or gastric erosions [Table 2 and Figure 2].
Figure 1.
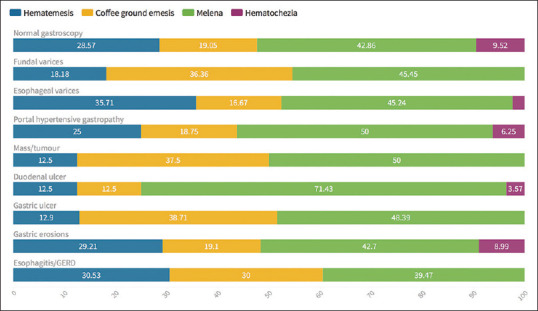
The correlation between the presentation of patients whether it be hematemesis, coffee-ground emesis, melena, or hematochezia and the lesion found on endoscopy
Figure 2.
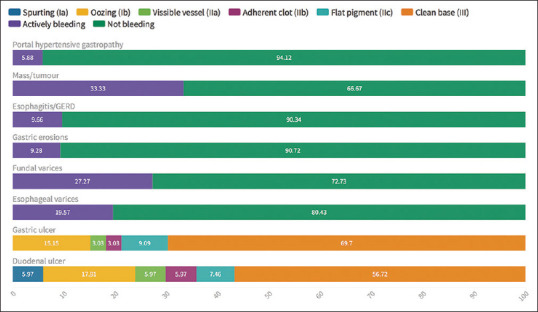
Stigmata of bleeding for the various lesions detected on endoscopy
Variceal bleeding and portal hypertensive gastropathy
Of the complete cohort, 17.8% had esophageal varices, while 4.2% had fundal varices, and three patients (1.16%) had concomitant esophageal and fundal varices. Also, five patients who were found to have esophageal varices and three patients with fundal varices were not known to have liver disease before the episode of UGIB.
Active bleeding was found in 19.6% of those with esophageal varices and 27.3% of those with fundal varices. Also, concomitant duodenal ulcers were found in five patients of those with esophageal varices and three patients with fundal varices.
Portal hypertensive gastropathy was found in 32.4% of patients who were known to have chronic liver disease (CLD) and when present there was known CLD in 80% and almost always with esophageal varices (94.1%). Active bleeding was only present in 5.9% from portal hypertensive gastropathy [Figure 2].
Characteristics of ulcers
Duodenal ulcers were more common than gastric ulcers and most had low-risk stigmata: clean-based ulcers (56.7%) or pigmented spots (7.5%). Active bleeding in the form of a spurting vessel or oozing was present in 6.0% and 17.9%, respectively. Both, a visible vessel and an adherent clot, were present in 5.97% [Table 2 and Figure 2].
Also, the majority of gastric ulcers had low-risk stigmata where clean-based ulcers were found in 69.7% and pigmented spots in 9.1%. Active bleeding gastric ulcers were seen in 15.2% in the form of oozing, while nonbleeding visible vessels were seen in 3.0%. Adherent clots were seen in 3.0% of cases [Table 2 and Figure 2].
DISCUSSION
UGIB remains a significant source of morbidity and mortality on a global level[6,25] as well as within Saudi Arabia.[8,9,11,13,14,15,16] There has been a major shift in the demographic landscape in Saudi Arabia as well as a shift from communicable to noncommunicable diseases[17,26,27] which might explain the variability in the proportion of variceal sources of UGIB that have been reported in prior studies. Also, there is a decreasing prevalence of hepatitis B virus (HBV) and schistosomiasis and an increase in cardiovascular and cerebrovascular diseases and the associated increase in the use of antiplatelets and anticoagulants as well as nonalcoholic steatohepatitis which might contribute to the development of CLD.
International guidelines emphasize the need for proper resuscitation of patients and risk stratification prior to endoscopy.[6,25] The usual practice in our center is that an EGD is performed within 24 h of a patient's presentation with UGIB, or within 12 h of a suspected variceal source, unless EGD is otherwise contraindicated for other reasons. During this time, a patient would be resuscitated and receive an intravenous infusion of a proton pump inhibitor or octreotide based on the clinical suspicion as well as being cross matched and transfused with blood products based on the initial laboratory investigations. A patient would be admitted to an appropriate setting until an EGD is performed after which the patient would be either managed with an oral or intravenous infusion of a proton pump inhibitor or octreotide based on the endoscopic findings, and allocated to the appropriate setting, either an intensive care unit, a general ward, or discharged home.
Compared to a large United Kingdom (UK) audit of UGIB in 2007 that included 6750 patients across 208 hospitals,[2] our patient population had a relatively younger mean age (57.1 vs. 64.4 years) but a higher percent with comorbidities (83.2% vs. 50%), aspirin use (39.9% vs. 28.0%), clopidogrel (12.0% vs. 5.3%), but almost a similar level of hemoglobin on presentation (10.2 vs. 11).[2]
The lesions identified on endoscopy in our cohort had a higher percentage of peptic ulcer disease (36% vs. 27%), esophagitis/GERD (38.1% vs. 25%), gastric erosions (37.6% vs. 22%), but a similar percentage of varices (22% vs. 20%) compared to those described in the UK audit.[2] Despite these differences, the in-hospital mortality rate for the UK audit was 10% compared to 4.4% in our study. Also, the rebleeding rate in our patient population was lower than that in the UK audit (8.9% vs. 13%). Compared to other studies from Saudi Arabia, the mortality rate in our study is in keeping with that reported by Ahmed et al.[12](5.83%), while Alam[9] reported a mortality rate of 15.8% which might represent the high percentage of cases found to have varices. On the other hand, Masoodi et al.[11] reported no mortalities despite having a similar proportion of patients with a variceal source of bleeding [Table 3 and Figure 3]. Alam reported recurrent bleeding in 2.48%[9] all of which resulted in death but it was not clear whether those were the only cases with recurrent bleeding. Masoodi et al.[11] reported recurrent bleeding in 1.2%; both of these results are lower than our experience. It is known that the mortality rate associated with variceal bleeding is high; a study by Fallatah et al. from the western city of Jeddah reported a mortality rate during the first episode of a variceal bleed to be 15.2%.[28]
Table 3.
Studies that have been published in the kingdom on upper gastrointestinal bleeding
| Author | Year | City | Study period | Study size | Males | Saudi nationals | Mean age (years) | Timing of endoscopy |
|---|---|---|---|---|---|---|---|---|
| Laajam et al.[8] | 1988 | Riyadh | 4 years August 1982 to July 1986 | 424 | NA | NA | NA | NA |
| Al-Mofarreh et al.[14] | 1991 | Riyadh | 2 years January 1984 to December 1986 | 747 | 68.9% | 100% | NA | Most of the endoscopies were done within 12 to 36 h of admission |
| Al-Quorain et al.[15] | 1991 | Al-Khobar | 4 years November 1982 to October 1986 | 200 | NA | NA | NA | |
| Barlas[16] | 1992 | Almadinah | NA | 462 | NA | 44.8% | NA | NA |
| Al Karawi et al.[13] | 1995 | Riyadh | 14 years January 1980 to July 1994 | 1246 | 72.71% | Not specified | Not specified | Within 24 h of presentation |
| Ahmed et al.[12] | 1997 | Abha | 2 years May 1991 to 1993 | 240 | 68.8% | Not specified | 44.3±18.1 | Within 24 h of admission. |
| Alam[9] | 2000 | Riyadh | 2 years May 1996 to April 1998 | 564 | 82% | 54% | 52.46±17.8 | Within 24 h in most and within 48 h in all |
| Sibiany[10] | 2013 | Jeddah | 7 years (not further specified) | 1149 | 76.5% | 38.6% | 49.74±1 | NA |
| Masoodi et al.[11] | 2019 | Taif | 3 years January 2015 to December 2017 | 120 | 63.3% | 74% | 58.4±18.7 | - Immediately after admission (25%) - Within 6 h (11.7%) - Within 24 h (63.4%) |
| Alruzug et al. | 2020 | Riyadh | 13 years January 2004 to December 2016 | 2075 | 67.9% | 100% | 56.8 | NA |
| Almadi et al. | 2020 | Riyadh | 9 years January 2006 to January 2015 | 259 | 66.8% | 90% | 57.1±18.0 | Within 24 h of presentation |
Figure 3.
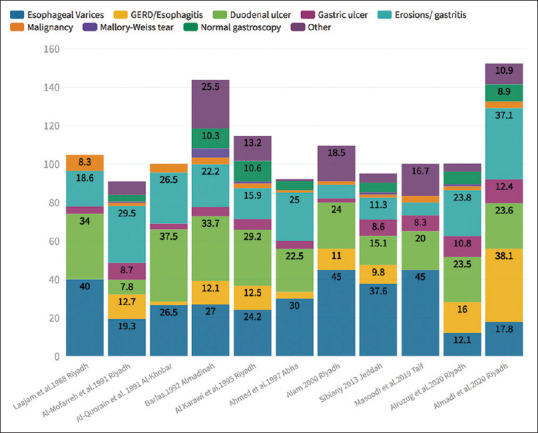
Findings that were reported in cases that presented with upper gastrointestinal bleeding in studies published in Saudi Arabia (Numbers represent percentages and a patient could have more than one finding and thus the total exceeds 100%)
In the UK audit, 43% of patients who were admitted with UGIB received PRBC transfusion and the need for transfusion was directly proportional to the Rockall score, while in our cohort only 13.9% were transfused with PRBCs.[2] This is despite the fact that 57.47% of our study population had a pre-endoscopy Rockall score of 3 or higher, which is more than that reported in the study from the UK (36%).[2] Also, the mean hemoglobin at discharge in our study was somewhat higher than the target recommended level in the most recent guideline[6] but a high proportion of patients had comorbidities including ischemic heart disease and prior cerebrovascular accident, which might explain in part the hemoglobin level at discharge to be 11 g/dL. Additionally, the discharge hemoglobin level might not reflect the target transfusion level during the management of UGIB.
It might seem plausible that the lower rebleeding and mortality rates between our study and the UK audit might be due to the lower proportion of patients receiving blood transfusions, as this has been proven to influence both these outcomes[29] but would be difficult to ascertain owing to the absence of immediate post transfusion hemoglobin level in our study. The only study that reports blood transfusion requirements' in Saudi Arabia was by Masoodi et al.[11] and they reported a mean of 2 ± 1 units of PRBCs being transfused.
A high proportion of the endoscopic findings in our study were due to erosions; in 1990, gastritis and duodenitis ranked as the 14th cause of age-standardized years lived with disability in both sexes in Saudi Arabia but has fallen off the list in 2017 and has been substituted by GERD which ranked 24th.[17] Also, in a study from the same center looking at the prevalence of abnormal findings in patients undergoing an EGD for dyspepsia, 52% had gastritis and 10% had duodenitis as endoscopic diagnoses.[30] In addition, we found that the prevalence of esophagitis and GERD in our study to be relatively high. This might reflect the high underlying prevalence of GERD in the population, where it was demonstrated in a survey that when using a cut-off value of 8 on the GERDQ questionnaire as a definition of GERD, the prevalence of GERD in the surveyed population was 45.4%.[31]
None of the patients in our study were found to need surgery, but this may not have been captured properly through our retrieval methodology, as those with severe bleeding might have been referred to interventional radiology and surgery without undergoing an EGD. However, even if this were true, it would most likely be a small number of cases. The need for surgery was not reported in any of the studies from Saudi Arabia apart from the paper by Alam[9] where at least 9 out of 544 patients required surgery, out of which five died. More patients might have required surgery but it was not clear from the reporting if that was the case.
There was no association between the pre-endoscopic or complete Rockall scores and rebleeding or the need for admission to the intensive care unit. However, it is likely that the study was probably underpowered to detect any association. Also, the number of deaths in our study was small which would preclude any meaningful attempt to explore an association. Unfortunately, we did not capture some variables (e.g., urea and albumin, and mental status) that would have enabled the assessment of other prediction scores like the Glasgow Blatchford score or the AIMS65, although the most recent guidelines did not favor using the AIMS65 as a tool for triaging for early discharge, as it was developed to detect those at risk of death.[6] The only other study from Saudi Arabia that reported the use of a prediction scoring system was the one by Masoodi et al.[11] with a median Rockall score of 3.
In our study population, 20.7% had a history of prior UGIB and 12.6% had a history of peptic ulcer disease, both of which are known risk factors for a second episode of UGIB.[6] Unfortunately, we do not know whether those with peptic ulcer disease had H. pylori infection or eradication. Of those who had a history of peptic ulcer disease, 63.6% were found to have esophagitis/GERD, 27.3% had gastric erosions, 18.2% where found to have a duodenal ulcer, 9.1% a gastric ulcer, and 9.1% were found to have esophageal varices. In a study by Masoodi et al.,[11] the H. pylori prevalence in those presenting with UGIB was 60%.
Examining studies from Saudi Arabia, it is clear that the proportion of the different UGIB endoscopic lesions identified are variable and this variability may reflect changes over time or the different populations that these centers serve [Table 3 and Figure 3].
This study has the strength of having detailed clinical and endoscopic data of patients presenting with UGIB enabling the use and assessment of a predictive score compared to other studies that have described UGIB in Saudi Arabia and that the majority of UGIB cases in our study were due to a nonvariceal source. It also reports some important patient outcomes including rebleeding, intensive care unit utilization as well as in-hospital mortality. Nonetheless, there are limitations that are inherent to the retrospective design including the lack of 30-day mortality parameter as well as the inability to differentiate whether these UGIB episodes were the presenting complaint of patients or UGIB developed while hospitalized for another reason as these might impact outcomes. Also, the small sample size is a limitation. In order to explore whether the number of procedures performed in the center could be a factor for the perceived small number of cases, we reviewed the volume of gastroscopy procedures performed annually and they ranged from 1900 to 2400 gastroscopies per year from 2014 to 2018. When we compare these numbers to those reported in a survey in 2007 in the UK,[32] the average number of gastroscopies was 2551 per center in that year and these ranged from 611 per year in the England independent sector to as high as 4358 per year in the England NHS acute care centers. Also, what might explain the low number of UGIBs in our practice is that we mainly care for a large cohort of IBD patients which is reflected by the fact that we perform almost an identical number of colonoscopies to gastroscopies with the number of colonoscopies during the same period ranging from 1400 to 2200 annually.
Although ERCP volumes have been used as a quality indicator, the volume of gastroscopies was never used as a quality indicator.[33,34] Nonetheless, the volumes of procedures are of importance but they should be judged based on national averages as well as upper and lower bounds. This has been recognized by the Joint Advisory Group (JAG) in the UK and they have commenced a National Endoscopy Database (NED) since 2013 that would capture volumes of procedures as well as selected metrics that could be followed. As of October 2018, 56% of the endoscopy services of the UK have enrolled in the project and 400,000 endoscopic procedures have been uploaded.[35]
A national registry would enable a better understanding of causes, risk factors, and other important patient-reported outcomes to deliver better value-based care to our population.
Furthermore, the low number of clinically important outcomes (rebleeding, mortality) precluded any exploratory analyses for the development of a prediction score that might be better suited for our population. Also, we could not ascertain the specific cause of mortality whether it was directly related to the UGIB episode or not.
This study adds to the knowledge of UGIB outcomes and hospital course in Saudi Arabia as it describes in detail the etiologies and endoscopic findings faced in the region. We believe that the value of the study, in comparison to prior studies, which are larger in number, is the unique manner in which we captured important details of patient characteristics in terms of clinical variables and the hospital course, which has not been described in any of the prior publications.
Financial support and sponsorship
The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project number RGP-279.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rosenstock SJ, Møller MH, Larsson H, Johnsen SP, Madsen AH, Bendix J, et al. Improving quality of care in peptic ulcer bleeding: Nationwide cohort study of 13,498 consecutive patients in the Danish clinical register of emergency surgery. Am J Gastroenterol. 2013;108:1449–57. doi: 10.1038/ajg.2013.162. [DOI] [PubMed] [Google Scholar]
- 2.Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: Patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–35. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- 3.He L, Zhang J, Zhang S. Risk factors of in-hospital mortality among patients with upper gastrointestinal bleeding and acute myocardial infarction. Saudi J Gastroenterol. 2018;24:177–82. doi: 10.4103/sjg.SJG_492_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laine L, Laursen SB, Dalton HR, Ngu JH, Schultz M, Stanley AJ. Relationship of time to presentation after onset of upper GI bleeding with patient characteristics and outcomes: A prospective study. Gastrointest Endosc. 2017;86:1028–37. doi: 10.1016/j.gie.2017.03.1549. [DOI] [PubMed] [Google Scholar]
- 5.Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Quintero E, Perez-Aisa MA, et al. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011;33:585–91. doi: 10.1111/j.1365-2036.2010.04563.x. [DOI] [PubMed] [Google Scholar]
- 6.Barkun AN, Almadi M, Kuipers EJ, Laine L, Sung J, Tse F, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the International Consensus Group. Ann Intern Med. 2019;171:805–22. doi: 10.7326/M19-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sostres C, Lanas A. Epidemiology and demographics of upper gastrointestinal bleeding: Prevalence, incidence, and mortality. Gastrointest Endosc Clin N Am. 2011;21:567–81. doi: 10.1016/j.giec.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Laajam MA, Al-Mofleh IA, Al-Faleh FZ, Al-Aska AK, Jessen K, Hussain J, et al. Upper gastrointestinal endoscopy in Saudi Arabia: Analysis of 6386 procedures. Q J Med. 1988;66:21–5. [PubMed] [Google Scholar]
- 9.Alam MK. Factors affecting hospital mortality in acute upper gastrointestinal bleeding. Saudi J Gastroenterol. 2000;6:87–91. [PubMed] [Google Scholar]
- 10.Sibiany AM. Presentation and endoscopic findings of emergency upper gastrointestinal bleeding: A seven-year experience at King Abdulaziz university hospital, Jeddah, Saudi Arabia. J King Abdulaziz Med Sci. 2013;20:57–64. [Google Scholar]
- 11.Masoodi I, AlQurashi H, Al Sofiyani M. Changing trends in acute upper GI bleeding a single centre study in the western region of Saudi Arabia. Br J Med Pract. 2019;12:a019. [Google Scholar]
- 12.Ahmed ME, al-Knaway B, al-Wabel AH, Malik GM, Foli AK. Acute upper gastrointestinal bleeding in Southern Saudi Arabia. J R Coll Physicians Lond. 1997;31:62–4. [PMC free article] [PubMed] [Google Scholar]
- 13.Al Karawi MA, Ghandour Z, Mohamed A el S. Causes of upper gastrointestinal bleeding: Experience at a major hospital in Riyadh. Ann Saudi Med. 1995;15:606–8. doi: 10.5144/0256-4947.1995.606. [DOI] [PubMed] [Google Scholar]
- 14.Al-Mofarreh M, Fakunle YM, Al-Moagel M. Upper gastrointestinal bleeding among Saudis: Etiology and prevalence the Riyadh central hospital experience. Ann Saudi Med. 1991;11:547–50. doi: 10.5144/0256-4947.1991.547. [DOI] [PubMed] [Google Scholar]
- 15.Al-Quorain A, Satti MB, Al-Hamdan A, al-Ghassab G, al-Freihi H, al-Gindan Y. Pattern of upper gastrointestinal disease in the eastern province of Saudi Arabia. Endoscopic evaluation of 2,982 patients. Trop Geogr Med. 1991;43:203–8. [PubMed] [Google Scholar]
- 16.Barlas S. Upper gastrointestinal bleeding among Saudis: Etiology and prevalence, the Riyadh central hospital experience. Ann Saudi Med. 1992;12:413–4. doi: 10.5144/0256-4947.1992.413. [DOI] [PubMed] [Google Scholar]
- 17.GBD 2017 Saudi Arabia Collaborators. The burden of disease in Saudi Arabia 1990-2017: Results from the global burden of disease study 2017. Lancet Planet Health. 2020;4:e195–208. doi: 10.1016/S2542-5196(20)30075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Humayed SM, Mohamed-Elbagir AK, Al-Wabel AA, Argobi YA. The changing pattern of upper gastro-intestinal lesions in Southern Saudi Arabia: An endoscopic study. Saudi J Gastroenterol. 2010;16:35–7. doi: 10.4103/1319-3767.58766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnenberg A, Turner KO, Genta RM. Low prevalence of helicobacter pylori-positive peptic ulcers in private outpatient endoscopy centers in the United States. Am J Gastroenterol. 2020;115:244–50. doi: 10.14309/ajg.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hussaini AA, Al Jurayyan AN, Bashir SM, Alshahrani D. Where are we today with Helicobacter pylori infection among healthy children in Saudi Arabia? Saudi J Gastroenterol. 2019;25:309–18. doi: 10.4103/sjg.SJG_531_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsohaibani F, Alquaiz M, Alkahtani K, Alashgar H, Peedikayil M, AlFadda A, et al. Efficacy of a bismuth-based quadruple therapy regimen for Helicobacter pylori eradication in Saudi Arabia. Saudi J Gastroenterol. 2020;26:84–8. doi: 10.4103/sjg.SJG_626_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laine L. CLINICAL PRACTICE. Upper gastrointestinal bleeding due to a peptic ulcer. N Engl J Med. 2016;374:2367–76. doi: 10.1056/NEJMcp1514257. [DOI] [PubMed] [Google Scholar]
- 23.Xia HH, Phung N, Kalantar JS, Talley NJ. Demographic and endoscopic characteristics of patients with Helicobacter pylori positive and negative peptic ulcer disease. Med J Australia. 2000;173:515–9. doi: 10.5694/j.1326-5377.2000.tb139318.x. [DOI] [PubMed] [Google Scholar]
- 24.Lau JY, Leung WK, Wu JCY, Chan FKL, Wong VWS, Chiu PWY, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007;356:1631–40. doi: 10.1056/NEJMoa065703. [DOI] [PubMed] [Google Scholar]
- 25.Sung JJ, Chiu PW, Chan FKL, Lau JY, Goh K-L, Ho LH, et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: An update 2018. Gut. 2018;67:1757–68. doi: 10.1136/gutjnl-2018-316276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Memish ZA, Jaber S, Mokdad AH, AlMazroa MA, Murray CJL, Al Rabeeah AA, et al. Burden of disease, injuries, and risk factors in the Kingdom of Saudi Arabia, 1990-2010. Prev Chronic Dis. 2014;11:E169. doi: 10.5888/pcd11.140176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahim HFA, Sibai A, Khader Y, Hwalla N, Fadhil I, Alsiyabi H, et al. Non-communicable diseases in the Arab world. Lancet. 2014;383:356–67. doi: 10.1016/S0140-6736(13)62383-1. [DOI] [PubMed] [Google Scholar]
- 28.Fallatah HI, Al Nahdi H, Al Khatabi M, Akbar HO, Qari YA, Sibiani AR, et al. Variceal hemorrhage: Saudi tertiary center experience of clinical presentations, complications and mortality. World J Hepatol. 2012;4:268–73. doi: 10.4254/wjh.v4.i9.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odutayo A, Desborough MJR, Trivella M, Stanley AJ, Dorée C, Collins GS, et al. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: A systematic review and meta-analysis of randomised controlled trials. Lancet Gastroenterol Hepatol. 2017;2:354–60. doi: 10.1016/S2468-1253(17)30054-7. [DOI] [PubMed] [Google Scholar]
- 30.Azzam NA, Almadi MA, Alamar HH, Almalki LA, Alrashedi RN, Alghamdi RS, et al. Performance of American society for gastrointestinal endoscopy guidelines for dyspepsia in Saudi population: Prospective observational study. World J Gastroenterol. 2015;21:637–43. doi: 10.3748/wjg.v21.i2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almadi MA, Almousa MA, Althwainy AF, Altamimi AM, Alamoudi HO, Alshamrani HS, et al. Prevalence of symptoms of gastroesopahgeal reflux in a cohort of Saudi Arabians: A study of 1265 subjects. Saudi J Gastroenterol. 2014;20:248–254. doi: 10.4103/1319-3767.136982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shenbagaraj L, Thomas-Gibson S, Stebbing J, Broughton R, Dron M, Johnston D, et al. Endoscopy in 2017: A national survey of practice in the UK. Frontline Gastroenterol. 2019;10:7–15. doi: 10.1136/flgastro-2018-100970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ASGE Endoscopy Unit Quality Indicator Taskforce. Day LW, Cohen J, Greenwald D, Petersen BT, Schlossberg NS, et al. Quality indicators for gastrointestinal endoscopy units. VideoGIE. 2017;2:119–40. doi: 10.1016/j.vgie.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rees CJ, Koo S, Anderson J, Morris AJ, East JE, Webster G, et al. British society of gastroenterology Endoscopy quality improvement programme (EQIP): Overview and progress. Frontline Gastroenterol. 2019;10:148–53. doi: 10.1136/flgastro-2018-101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee TJW, Siau K, Esmaily S, Docherty J, Stebbing J, Brookes MJ, et al. Development of a national automated endoscopy database: The United Kingdom National endoscopy database (NED) United European Gastroenterol J. 2019;7:798–806. doi: 10.1177/2050640619841539. [DOI] [PMC free article] [PubMed] [Google Scholar]


