Abstract
Background:
The study aimed at comparing restrictive and liberal transfusion strategy in reducing mortality in patients with upper gastrointestinal bleeding (UGIB).
Methods:
This was a single-center, prospective, open-label, non-inferiority, randomized controlled trial conducted over two years. Patients presenting with UGIB were randomized into restrictive (hemoglobin (Hb) <7 g/dl) or liberal (Hb <8 g/dl) transfusion strategy groups. Transfusion was given till patients achieved target Hb of 9 g/dl in restrictive and 10 g/dl in the liberal arms. Patients with exsanguinating bleeding, transfusion within 90 days, recent history of trauma or surgery were excluded. Primary outcome was mortality rate and the secondary outcomes were morbidity, re-bleeding episodes and the need for intervention.
Results:
A total of 224 patients were randomized to 112 patients in each group. Demographic characteristics were comparable. 45-day mortality was similar between the two groups (restrictive vs. liberal; 10/112 vs. 12/112; P = 0.65). The number of in-hospital bleeding episodes (12 vs. 9; P = 0.25), incidence of re-bleeding during the 45-day follow-up (13 vs. 14; P = 0.84), need for endoscopic banding for varices (37/112 vs. 39/112, P = 0.99), mean hospital stay (days) (3.21 ± 2.78 vs. 2.73 ± 1.29; P = 0.10) were similar between the two groups.
Conclusion:
Restrictive transfusion strategy is non-inferior to liberal transfusion strategy in patients with UGIB.
Keywords: Liberal transfusion, restrictive transfusion, upper gastrointestinal bleeding
INTRODUCTION
Upper gastrointestinal bleeding (UGIB) remains one of the most common surgical emergencies with high mortality rates even in developed countries.[1] Bleeding may be due to variceal or non-variceal causes and majority of the patients require transfusion of blood and blood components. There is a wide variation of the causes of bleeding with studies showing both variceal and ulcer-related bleeding being the most common cause depending on the region.[2,3,4]
Some studies have shown that transfusions may be harmful in hypovolemic anemia and there is a high chance of re-bleeding in portal hypertensive patients, as the redistribution of blood after transfusion may cause a rebound increase in the portal pressure.[5,6] There are reports demonstrating the restrictive transfusions strategy to be as effective as the liberal transfusion strategy in critically ill patients.[7,8]
There is a dearth of studies demonstrating the effects of a restrictive transfusion strategy in patients presenting with UGIB. Some studies postulated that there is clot disruption and derangement of clotting parameters associated with blood transfusion.[9,10] In a landmark study from Spain, an absolute reduction of 4% mortality was documented with restrictive transfusion guidelines when compared to liberal transfusion therapy in patients with upper gastrointestinal bleeding irrespective of variceal or non-variceal causes.[11] The incidence of rebleeding was significantly lower, and the need for rescue with Sengstaken-Blakemore tube or with transjugular intrahepatic portosystemic shunt (TIPS) was also reduced in the restrictive arm. A multi-centric feasibility study found no significant difference in either group after 28 days of follow-up.[12] Recent guidelines recommended a transfusion threshold of 8 g/dl in patients with variceal and non-variceal bleeding.[13,14,15] The European Society of Gastrointestinal Endoscopy Guidelines released in 2015 also suggested a target hemoglobin of 7-9 g/dl. Though few studies have shown the advantages of restrictive transfusion strategy, there is a dearth of well-conducted randomized controlled trials to prove its potential advantage. This study was carried out to assess whether restrictive strategy is safe and effective as liberal transfusion strategy in patients with UGIB.
METHODS
Study design
This study was a single center, prospective, open-label, parallel arm, non-inferiority, randomized controlled trial (RCT) carried out in the Department of Surgery of a tertiary care hospital in South India from June 2015 to May 2017. The study was approved by the Institute Ethics Committee (JIP/IEC/2015/615, dated 25.06.2015). Written informed consent was taken from all the participants and patients were given full freedom to withdraw at any point during the study. All the provisions of Helsinki were followed in this study. The study was registered at www.ctri.gov.in (CTRI no: CTRI/2017/09/009682).
Patient enrolment and randomization
All patients above 18 years, who presented to the emergency surgical unit with a diagnosis of UGIB, were recruited to the study. The diagnosis of UGIB was based on history and clinical examination by the emergency surgical registrar. Patients with massive exsanguinating bleeding, lower GI bleed, acute coronary syndrome, symptomatic peripheral vasculopathy, stroke, transient ischemic attack, transfusion within the past 90 days or a recent history of trauma or surgery were excluded.
Eligible patients were randomly assigned into two groups. Stratified permuted block randomization was done using a computer program with randomly selected unequal block sizes of 4 and 6. Stratification was done based on variceal bleeding vs. non-variceal bleeding. Allocation concealment was performed using a serially numbered opaque sealed envelope (SNOSE) technique. The envelopes were opened by the residents on-duty and allocation was carried out at the time of admission of the patient.
Study procedure
In the restrictive transfusion group, patients were transfused when the hemoglobin (Hb) was <7 g/dl, with a target for post-transfusion hemoglobin level of 9 gm/dl. In the liberal transfusion group, the hemoglobin threshold for transfusion was <8 gm/dl with a target range for the post transfusion hemoglobin level of 10 gm/dl.
The in-hospital practice was to transfuse patients with <8 g/dl and was chosen as the threshold for liberal transfusion. Villanueva et al. demonstrated a safer, lowered transfusion threshold of 7 g/dl.[11] This would save a considerable number of blood products and was chosen as the restrictive transfusion threshold.
In both the groups, hemoglobin level was assessed initially at admission and packed cell units were transfused if the hemoglobin level was below the threshold value. Transfusion protocol was applied till the patient was discharged from the hospital. Hemoglobin levels were measured at admission, every eight hours during the first 48 hours and then every day thereafter till the target hemoglobin level was achieved. Hemoglobin level was estimated using Sodium Lauryl Sulphate method.[16]
The patients were monitored for any further bleeding. SB tube was secured only in patients with the clinical diagnosis of variceal bleeding who had in-hospital bleeding before endoscopy unless the patient was a known case of ulcer disease. The duration of tube placement was recorded. Hemodynamically stable patients were subjected to endoscopy within the next 24 hours. Nine patients in the study population underwent endoscopy after 24 hours due to hemodynamic instability, coagulopathy, obscured vision due to ongoing bleeding during the first attempt of endoscopy and hepatic encephalopathy. However, all these patients underwent endoscopy before 48 hours after the correction of parameters. The number of endoscopies required, and the findings were recorded. In case of variceal bleeding, banding was done if they were grade III or IV and the international normalized ratio (INR) was less than 1.5. Endoscopy-guided adrenaline injections were given for actively bleeding ulcers. Oral fluids were started after 4-6 hours of endoscopic intervention. Patients who were known to have varices and those admitted with a clinical suspicion of varices were started on octreotide. The dose and duration of octreotide administered were recorded. The decision to start octreotide was entirely at the treating team's discretion. If there was an in-hospital death, the last Hb value recorded was treated as Hb at death. Transfusion reactions, both major and minor, were recorded.
Patients were discharged after they tolerated normal diet for at least 24 hours and there was no re-bleeding. Patients with variceal bleeding who were banded during the first endoscopy were asked to come for banding after 2 weeks. Telephonic follow-up was done after 45 days of admission, and it was recorded if there was any re-bleeding or death of the patient.
Data was collected using a specified proforma prepared by the investigators. The patient's age, gender, comorbidities, addictions, previous UGIB and endoscopy history, drug history (propranolol or other diuretics for ascites) were recorded. Similarly, laboratory parameters such as the Hb at the time of admission, liver function tests and INR were recorded. The number of packed cells, platelets, and fresh frozen plasma transfused, need for interventions such as banding, sclerotherapy, adrenaline injections, placement of a Sengstaken-Blakemore (SB) tube and its duration, dose and duration of octreotide, were recorded.
Outcome measures
The primary endpoint was the mortality rate in both the groups. The secondary endpoints included number of days from admission to death, rebleeding episodes (in-hospital bleeding and rebleeding during 45-day follow-up), Hb value before death, number of sessions of endoscopic treatment, requirement for banding/sclerosant treatment, requirement of SB tube placement and duration, incidence of transfusion reaction (major/minor), dose, duration of octreotide infusion and the length of hospital stay.
Statistical analysis
Statistical analysis was done by using SPSS® Statistics software version 23.0 for Windows. Sample size was calculated as 112 in each group based on an inter-group non-inferiority margin of 3.5%, which was considered clinically significant with 80% power. Categorical variables such as mortality, rebleeding episodes, the source of bleeding, Child-Turcotte-Pugh score, endoscopic intervention received, and the total number of deaths in either group were expressed as proportions. Continuous variables such as age, Hb at presentation, Hb at death, Rockall Score, the number of blood products transfused, the total length of hospital stay in days, dose and duration of octreotide, and duration of propranolol intake were expressed as mean ± standard deviation (SD). Differences in the proportion between the two groups were tested using Chi-square test or Fisher's exact test. Continuous variables were compared between the two groups using independent t-test or Mann-Whitney U test. Kaplan-Meier survival analysis was carried out among both the groups. A P value of less than 0.05 was considered statistically significant.
RESULTS
A total of 224 patients recruited from June 2015 to May 2017 were randomized into two groups with 112 in each. There was no loss to follow-up during the study period. The CONSORT flow chart is shown in Figure 1. The two groups were comparable in terms of demographic and clinicopathological characteristics [Table 1]. The various causes of bleeding are summarized in Figure 2 and Table 2. The most common cause of bleeding in both the groups was variceal bleeding followed by erosive lesions.
Figure 1.
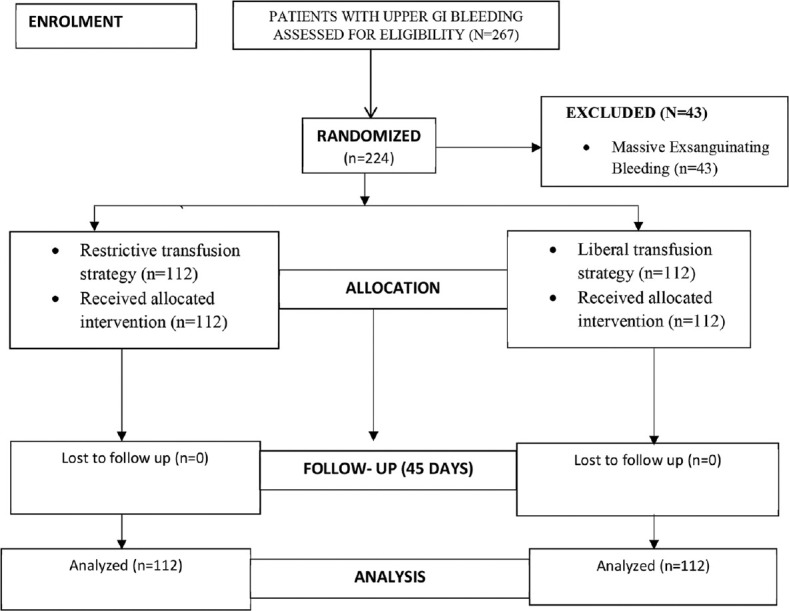
Study flow diagram
Table 1.
Table showing the baseline characteristics of patients in both the groups
| Patient characteristics | Restrictive transfusion group (n=112) | Liberal transfusion group (n=112) | P |
|---|---|---|---|
| Age | 47.86±14.75 | 49.76±14.87 | 0.34* |
| Cirrhosis (%) | 60 (54) | 48 (43) | 0.11† |
| Hypertension (%) | 14 (12) | 20 (18) | 0.26† |
| Diabetes Mellitus (%) | 16 (14) | 12 (11) | 0.26† |
| Alcoholism (%) | 56 (50) | 65 (587) | 0.23† |
| Previous bleeding (%) | 4 (3.6) | 10 (8.9) | 0.16† |
| Hb at admission (g/dL) | 9.31±2.78 | 9.35±2.29 | 0.90* |
| Rockall Score | 5.12±1.01 | 5.15±1.2 | 0.83‡ |
Hb: Hemoglobin. *independent t-test. †Chi-square test. ‡Mann-Whitney U test
Figure 2.
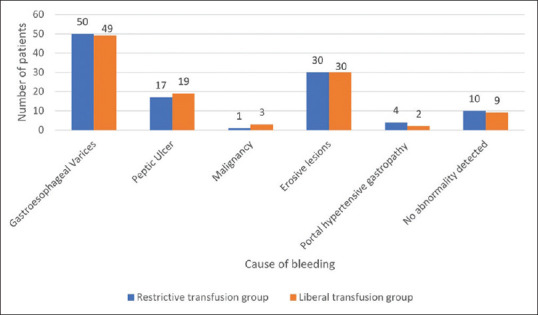
Causes of upper GI bleeding (number) in restrictive and liberal transfusion groups
Table 2.
Table showing distribution of various causes of bleeding among both the groups
| Source of bleeding | Restrictive transfusion group n=112 (%) | Liberal transfusion group n=112 (%) | P |
|---|---|---|---|
| Gastroesophageal Varices | 50 (44.64) | 49 (43.75) | |
| Malignancy | 1 (0.89) | 3 (2.68) | |
| Erosive lesions (Gastritis/esophagitis/duodenitis) | 30 (26.79) | 30 (26.79) | |
| Portal hypertensive gastropathy | 4 (3.57) | 2 (1.79) | |
| Peptic Ulcer† | 17 (15.18) | 19 (16.96) | |
| No abnormality detected on endoscopy | 10 (8.93) | 9 (8.04) | |
| 0.87* | |||
*R by C contingency analysis. †Two peptic ulcer patients had active bleeding upon endoscopy, one in each group, both belonging to Forrest class IIa. The patient in the liberal transfusion group expired
The in-hospital mortality, follow-up mortality and overall mortality rate in both the groups is summarized in Figure 3. There were a total of 22 deaths in the study, 10 in restrictive arm and 12 in liberal arm. In the restrictive transfusion arm, 6 patients died in hospital, and 4 died during follow-up. In the liberal transfusion group, 6 deaths were during the in-hospital stay and 6 during follow-up. All mortality in the study population was due to exsanguinating bleeding leading to hypovolemic shock. There were no signs of circulatory overload in the patients who expired. There was no mortality due to spontaneous bacterial peritonitis (SBP) or due to sepsis. Mortality related to peptic ulcer bleeding was seen in one patient in each group. Exsanguinating variceal bleeding was the cause of mortality in the remaining patients. Upon performing Kaplan-Meier analysis, the difference was however not significant (p = 0.65). The hazard ratio was found to be 0.83 (p = 0.33) [Figure 4].
Figure 3.
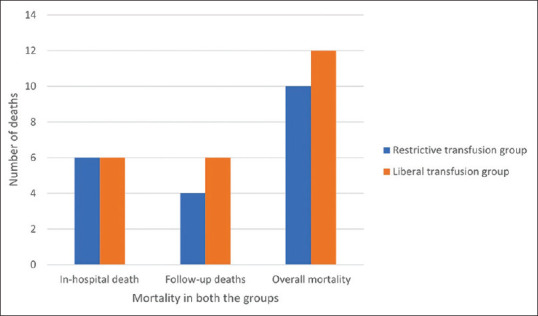
Comparison of in-hospital mortality and death during follow up in both the groups
Figure 4.
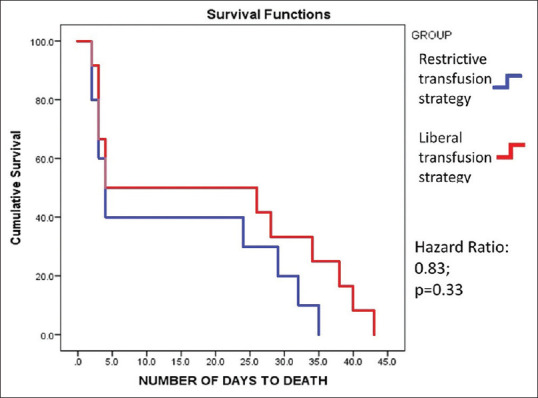
Kaplan-Meier survival analysis among both the groups
The comparison of various secondary outcomes is shown in Table 3. The rates of rebleeding, both in-hospital and bleeding during follow-up, were similar in both the groups. Although the number of packed cells (PC) and fresh frozen plasma (FFP) transfused in the restrictive group was relatively lower, the two groups were comparable. (PC 1.72 ± 1.38 vs. 1.96 ± 1.54, P = 0.22) (FFPs 1.04 ± 2.45 vs. 1.57 ± 2.62, P = 0.12). Patients in the liberal transfusion arm had a shorter duration of hospital stay, although the difference was not significant (3.21 ± 2.78 vs. 2.73 ± 1.29, P = 0.10). The duration of hospital stay was similar in both the arms. Octreotide was received in a significantly higher number of patients in the liberal arm (35 vs. 50, P = 0.04), but there was no difference in the duration for which it was administered. Four patients in restrictive arm and 9 patients in the liberal arm required placement of Sengstaken Blakemore tube. Thirty-seven patients in the restrictive group underwent banding compared to 39 patients in the liberal group. Kaplan Meier survival analysis of the mortality rate among both the groups, demonstrated no significant difference (Hazard ratio: 0.83, 95% confidence interval [CI]: 0.38-1.85; P value: 0.33).
Table 3.
Table comparing the secondary outcomes of both the groups
| Secondary outcomes | Restrictive transfusion group n=112 (%) | Liberal transfusion group n=112 (%) | P |
|---|---|---|---|
| Packed cells transfused | 1.72±1.38 | 1.96±1.54 | 0.22* |
| FFP transfused | 1.04±2.45 | 1.57±2.62 | 0.12* |
| In-hospital bleeding (%) | 12 (10.71) | 9 (8) | 0.25† |
| Bleeding during follow up (%) | 13 (12.26) | 14 (13.21) | 0.84† |
| Banding (%) | 37 (62) | 39 (81) | 0.99† |
| Sclerosant (%) | 4 (7) | 4 (8) | 1.00† |
| Sengstaken Blakemore tube placement (%) | 4 (3.6) | 9 (8) | 0.29† |
| Propranolol (%) | 20 (33.3) | 19 (39.6) | 0.25† |
| Octreotide (%) | 35 (31.25) | 50 (44.64) | 0.04† |
| Duration of octreotide (days) | 2.49±0.82 | 2.16±0.77 | 0.33* |
| Mean length of admission (days) | 3.21±2.78 | 2.73±1.29 | 0.10* |
| Mean Hb at the time of death (g/dl) | 7.17±1.85 | 5.27±2.71 | 0.19* |
FFP: fresh frozen plasma; Hb: Hemoglobin; *independent t-test; †Chi-square test
There were no major transfusion reactions during the study. There were no signs of circulatory overload in either of the groups.
DISCUSSION
In this non-inferiority RCT, the 45-day mortality between the two groups were found to be similar, although a lower trend was noted in restrictive group. Similarly, there was no significant difference in various morbidity parameters such as blood products transfused, bleeding during follow-up, and the need for interventions between the two groups. The incidence of in-hospital bleeding was higher in the restrictive group, although this difference was not significant (12 vs. 9, P = 0.25). Since Hb was estimated every 8 hours in the initial 48 hours, the error in Hb estimation was reduced.
Current protocols dictate a Hb cut-off for transfusion of 7 g/dL for patients with non-exsanguinating massive UGIB. These guidelines are based primarily on a single center Spanish RCT by Villanueva et al.[11] In this study, the 45-day mortality rate was 5% in restrictive transfusion and 9% in the liberal transfusion group. In the TRIGGER trial, the 28-day mortality was 5% in the restrictive policy and 7% in the liberal policy group.[12] In the present study, the mortality rates were comparable between the two groups. The hazard ratio found in the present study was 0.83 (95% CI: 0.38-1.85), compared to that of 0.55 (95% CI: 0.33-0.92) in the previous reports. The centers mentioned above, however, were well-equipped with facilities such as emergency endoscopy and transjugular intrahepatic portosystemic shunts (TIPSS) which was lacking in the present study center.
In the present study, 54% of restrictive transfusion group and 43% of liberal transfusion group were cirrhotics. Anand et al., in a previous report from India in 1983, reported a cirrhotic prevalence of 45%, a prevalence of 30% for ulcer disease and 9% for gastritis.[2] Simon et al. reported the single-center prevalence of 34% for variceal bleeding.[4] Investigators from various parts of the country have reported the prevalence of esophageal varices ranging from 10.8% to 56%.[2,3,4] However, the prevalence of cirrhotics in Western literature was comparatively lesser. Villanueva et al. found a prevalence of 31% in both restrictive and liberal strategies; Jairath et al. found an even lower prevalence of 17% and 11% in restrictive and liberal policies, respectively[11,12] The most common source of bleeding in the present study was due to portal hypertension in 48% of the patients in the restrictive transfusion group and 46% in the liberal transfusion group, whereas peptic ulcer was the most common cause of bleeding in the Western studies. However, in the present study, the incidence of peptic ulcer was 15% and 17% in restrictive transfusion and liberal transfusion groups, respectively. This reflects the varying prevalence of esophageal varices within different geographic regions, and within individual countries as well.
The incidence of in-hospital bleeding in the study by Villanueva et al. was 10% in the restrictive strategy and 16% in the liberal strategy. TRIGGER trial showed an in-hospital bleeding incidence of 5% and 9% for the restrictive and liberal transfusion, respectively.[11,12] However, in the present study, the incidence of in-hospital bleeding was relatively higher in restrictive group, although not statistically significant (10% vs. 8%; P = 0.25). The higher incidence of in-hospital bleeding in the restrictive group in the present study when compared to previous reports may be attributed to the higher number of patients having cirrhosis in the restrictive transfusion strategy when compared to the liberal transfusion strategy groups. The higher prevalence of peptic ulcer bleeds in the previous reports when compared to the present study may also have a contributory role.
The mean number of in-hospital stay in the Spanish trial was 9.6 days in the restrictive arm and 11.5 days in the liberal arm.[11] In the TRIGGER trial, it was 4 days and 5 days respectively for restrictive and liberal transfusion arms. In the present study, the results were comparable to the TRIGGER trial, with 3.21 days and 2.7 days in the restrictive and liberal arms, respectively.[11,12] Unlike the other two studies, the restrictive arm had a longer length of hospital stay in the present study. This may be attributed to one patient in the restrictive arm who was incidentally diagnosed with stomach carcinoma and was operated subsequently during the same admission. However, the median hospital stay in the restrictive group was similar to the liberal group at 3 days. The mean length of hospital stay in the present study was lower in both the groups when compared to the previous reports. This is probably because most of the patients belonged to the variceal group, and hence, were discharged relatively early after the endoscopic therapy.
Despite a higher number of cirrhotic patients diagnosed in the restrictive transfusion group, surprisingly, the number of patients who received octreotide were greater in the liberal transfusion group. This could be attributed to chance as a higher number of patients with cirrhosis were recruited into the restrictive arm (54% vs. 48%), when compared to the liberal transfusion arm. The short duration of therapy with octreotide was in line with recent studies that showed no difference in between 2-day vs. 5-day therapy with octreotide.[17,18]
We found that the mean Hb at the time of death in the restrictive arm was found to be comparable to that of restrictive strategy group of the Spanish trial.[11] The mean Hb of the liberal arm in both the studies were not comparable. It was much lower in the present study at 5.27 g/dL in comparison to an 8 g/dL reported in the Spanish trial. This difference is probably due to us analyzing the last Hb before death in patients of UGIB, while other reports had analyzed the overall mean lowest Hb during hospital stay. Liberal group had lower Hb at death as there was an inherent chance of higher bleeding.
The mean number of packed cells transfused in the present study was comparable to the study by Jairath et al. who had a mean of 1.2 and 1.9 packed cells transfused in restrictive and liberal policies respectively.[12] The Spanish study had a higher number of packed cells transfused in the liberal arm.[11]
We found no difference in the morbidity and mortality among both patient groups. We have also demonstrated the feasibility of the transfusion threshold reported in the Western studies for use in the Indian patients with UGIB. In a setting of limited facilities, especially in developing countries, a shortage of blood and blood products is often encountered. Hence, it becomes important to ensure patient safety as well as reduce the costs.
The present study is not without limitations of its own. The lack of emergency endoscopy services, rescue therapy options such as TIPSS, were limiting factors in the study. Blinding was also not possible due to the inherent nature of the study. Subgroup analysis was not carried out as there was only one death in each group due to ulcer disease and the rest were due to exsanguinating variceal bleeding.
The mortality rate of patients with UGIB in the restrictive transfusion group was similar to the liberal transfusion group. Restrictive transfusion did not increase morbidity, re-bleeding rates and the need for intervention when compared to liberal transfusion in patients with UGIB. In conclusion, restrictive transfusion strategy is as safe and effective as liberal transfusion in patients with UGIB.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928–37. doi: 10.1056/NEJMra0706113. [DOI] [PubMed] [Google Scholar]
- 2.Anand CS, Tandon BN, Nundy S. The causes, management and outcome of upper gastrointestinal haemorrhage in an Indian hospital. Br J Surg. 1983;70:209–11. doi: 10.1002/bjs.1800700407. [DOI] [PubMed] [Google Scholar]
- 3.Singh SP, Panigrahi Mk. Spectrum of upper gastrointestinal hemorrhage in coastal Odisha. Trop Gastroenterol. 2013;34:14–7. doi: 10.7869/tg.2012.85. [DOI] [PubMed] [Google Scholar]
- 4.Simon EG, Chacko A, Dutta AK, Joseph AJ, George B. Acute nonvariceal upper gastrointestinal bleeding—experience of a tertiary care center in southern India. Indian J Gastroenterol. 2013;32:236–41. doi: 10.1007/s12664-013-0305-6. [DOI] [PubMed] [Google Scholar]
- 5.Castañeda B. Effects of blood volume restitution following a portal hypertensive-related bleeding in anesthetized cirrhotic rats. Hepatology. 2001;33:821–5. doi: 10.1053/jhep.2001.23437. [DOI] [PubMed] [Google Scholar]
- 6.McCormick PA, Jenkins SA, McIntyre N, Burroughs AK. Why portal hypertensive varices bleed and bleed: A hypothesis. Gut. 1995;36:100–3. doi: 10.1136/gut.36.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 8.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008;36:2667–74. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 9.Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73:783–5. doi: 10.1002/bjs.1800731007. [DOI] [PubMed] [Google Scholar]
- 10.Duggan JM. Transfusion in gastrointestinal haemorrhage—if, when and how much? Aliment Pharmacol Ther. 2001;15:1109–13. doi: 10.1046/j.1365-2036.2001.01013.x. [DOI] [PubMed] [Google Scholar]
- 11.Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 12.Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): A pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137–44. doi: 10.1016/S0140-6736(14)61999-1. [DOI] [PubMed] [Google Scholar]
- 13.Barkun AN, Almadi M, Kuipers EJ, Laine L, Sung J, Tse F, et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the international consensus group. Ann Intern Med. 2019;171:805–22. doi: 10.7326/M19-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Franchis R. Expanding consensus in portal hypertension. J Hepatol. 2015;63:743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Oakland K, Jairath V, Murphy MF. Advances in transfusion medicine: Gastrointestinal bleeding: Transfusion in gastrointestinal bleeding. Transfus Med. 2018;28:132–9. doi: 10.1111/tme.12446. [DOI] [PubMed] [Google Scholar]
- 16.Karsan A, Maclaren I, Conn D, Wadsworth L. An evaluation of hemoglobin determination using sodium lauryl sulfate. Am J Clin Pathol. 1993;100:123–6. doi: 10.1093/ajcp/100.2.123. [DOI] [PubMed] [Google Scholar]
- 17.Rengasamy S, Ali SM, Sistla SC, Lakshmi CP, Harichandra Kumar KT. Comparison of 2 days versus 5 days of octreotide infusion along with endoscopic therapy in preventing early rebleed from esophageal varices: A randomized clinical study. Eur J Gastroenterol Hepatol. 2015;27:386–92. doi: 10.1097/MEG.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 18.Morales GF, Pereira Lima JC, Hornos AP, Marques DL, Costa CS, Lima Pereira L, et al. Octreotide for esophageal variceal bleeding treated with endoscopic sclerotherapy: A randomized, placebo-controlled trial. Hepatogastroenterology. 2007;54:195–200. [PubMed] [Google Scholar]


