Abstract
Background
Healthcare personnel (HCP) are at increased risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We posit that current infection control guidelines generally protect HCP from SARS-CoV-2 infection in a healthcare setting.
Methods
In this retrospective case series, we used viral genomics to investigate the likely source of SARS-CoV-2 infection in HCP at a major academic medical institution in the Upper Midwest of the United States between 25 March and 27 December 2020. We obtained limited epidemiological data through informal interviews and review of the electronic health record and combined this information with healthcare-associated viral sequences and viral sequences collected in the broader community to infer the most likely source of infection in HCP.
Results
We investigated SARS-CoV-2 infection clusters involving 95 HCP and 137 possible patient contact sequences. The majority of HCP infections could not be linked to a patient or coworker (55 of 95 [57.9%]) and were genetically similar to viruses circulating concurrently in the community. We found that 10.5% of HCP infections (10 of 95) could be traced to a coworker. Strikingly, only 4.2% (4 of 95) could be traced to a patient source.
Conclusions
Infections among HCP add further strain to the healthcare system and put patients, HCP, and communities at risk. We found no evidence for healthcare-associated transmission in the majority of HCP infections evaluated. Although we cannot rule out the possibility of cryptic healthcare-associated transmission, it appears that HCP most commonly become infected with SARS-CoV-2 via community exposure. This emphasizes the ongoing importance of mask wearing, physical distancing, robust testing programs, and rapid distribution of vaccines.
Keywords: SARS-CoV-2, precision epidemiology, healthcare personnel, viral sequencing, infection control
The majority of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in 95 healthcare personnel (HCP) could not be linked to a patient or coworker. Infection control procedures, consistently followed, offer significant protection to HCP caring for patients with SARS-CoV-2.
Despite the use of personal protective equipment (PPE) and other strategies to mitigate risk, front-line healthcare workers are at increased risk for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) compared with the general population [1–3]. Healthcare-associated SARS-CoV-2 infections negatively affect healthcare personnel (HCP) through direct health impacts, lost wages, and secondary consequences for their close contacts [4]. Additional repercussions include staffing shortages, environmental contamination, low morale, and other mental health impacts on HCP. Each of these can impact overall quality of care [5, 6]. In the current study, we used rapid viral sequencing and forensic genomics to investigate the likely sources of infection in 95 confirmed cases of coronavirus disease 2019 (COVID-19) in HCP. We further describe how the results of these investigations informed infection control recommendations within a large academic medical system in the midwestern United States.
The US Centers for Disease Control and Prevention have released guidelines for preventing infection in HCP interacting directly with patients with SARS-CoV-2 [7]. These guidelines include recommendations for the proper use of PPE, hand hygiene, precautions to be taken during aerosol-generating procedures, environmental infection control practices, and many others. These guidelines, and additional institution-specific infection control measures [8], were in place at the institution evaluated in our study. We posit that these guidelines are generally successful in protecting HCP from SARS-CoV-2 infection in a healthcare setting. We tested this hypothesis using viral sequences collected from infected HCP, as well as concurrent viral sequences collected from the broader community, to investigate possible sources of infection in a series of HCP.
With a few exceptions [9–11], viral sequencing is not currently standard practice for investigating healthcare-associated SARS-CoV-2 infections, although we and others have highlighted the potential utility of this approach [12–15]. It is currently estimated that SARS-CoV-2 acquires approximately 2–2.5 consensus mutations per month [16, 17]. Viral sequences can therefore be used to infer likely epidemiological relationships. Viruses collected from transmission pairs or from individuals with a shared source of infection are expected to share higher levels of genetic diversity than individuals who become infected at similar times, but from distinct sources. This was especially true from March to December 2020 in the United States, when transmission rates were high and multiple viruses of distinct genetic lineages cocirculated in many areas [18]. By increasing the resolution of inference, rapid viral sequencing can facilitate a targeted approach to examine SARS-CoV-2 nosocomial outbreaks at the level of the individual and the institution, which others have referred to collectively as “precision epidemiology” [19].
METHODS
Sample Approval and Sample Selection Criteria
From 12 March 2020 to 10 January 2021, approximately 1172 HCP tested positive for SARS-CoV-2 at a major academic medical institution in the Upper Midwest. Whenever possible, informal interviews and contact tracing information was collected for each HCP infection. HCP viruses and viruses from other individuals involved in each outbreak (patients, coworkers) were sequenced if epidemiological data did not reveal a likely exposure source and if residual swab was available. Individuals who had high-risk exposures to family or community members with confirmed COVID-19 were not sequenced. Individuals who reported high-risk community activities, such as attending a wedding, funeral, indoor bar, or plane travel, were also not sequenced.
Relevant patient contacts of individuals with no likely exposure source were identified in the Epic electronic medical record, using a comprehensive caregiver trace. This function identifies all patient records accessed by an HCP being traced. Diagnostic assays for the samples included in this study were performed in a clinical laboratory using the diagnostic reverse-transcription polymerase chain reaction assay from the Centers for Disease Control and Prevention [20], the Hologic Panther SARS-CoV-2 assay [21], or the Aptima SARS-CoV-2 assay [22].
Accession numbers for all healthcare-associated samples can be found in Supplementary File 1. The University of Wisconsin–Madison Institutional Review Board deemed this study quality improvement, rather than research, and considered it exempt from review. Data and metadata were collected as part of routine infection control policy in nosocomial outbreaks, and all data were deidentified before analysis.
Infection Control Measures Used to Prevent Transmission of SARS-CoV-2
Detailed descriptions of all infection control measures implemented to prevent transmission of SARS-CoV-2 at the medical institution evaluated here can be found in a recent report by Lepak et al [8]. Briefly, these guidelines include a universal testing policy for all patients, negative air pressure in all locations where SARS-CoV-2 patients are treated, a limit of 1 visitor or primary support person per patient per day (required to undergo screening before entry), establishment of an employee testing site with required employee self-monitoring for symptoms, maintenance of a log of persons entering the room of a patient with confirmed or suspected COVID-19 for contact tracing purposes, and detailed PPE guidelines, among others.
Sample Preparation and Sequencing
Methods are described in detail by Moreno et al [23]. Briefly, viral RNA was extracted using the Viral Total Nucleic Acid Purification kit (Promega) on a Maxwell RSC 48 instrument. Complementary DNA was synthesized using SuperScript IV reverse-transcriptase [24, 25]. SARS-CoV-2–specific multiplex polymerase chain reaction was performed using ARTIC v3 primers [24, 25]. DNA was made compatible for sequencing using the one-pot native ligation protocol with an Oxford Nanopore kit SQK-LSK109 and its native barcodes (EXP-NBD104 and EXP-NBD114) [25]. Up to 23 samples, with 1 no-template control (water), were pooled before being run on the appropriate Nanopore flow cell (FLO-MIN106) using the 72-hour run script.
Processing Raw Oxford Nanopore Technologies Data
Sequencing data was processed using the ARTIC bioinformatics pipeline (https://github.com/artic-network/artic-ncov2019), with a few modifications. Briefly, we have modified the ARTIC pipeline so that it demultiplexes raw fastq files, using the qcat tool as each fastq file is generated by the GridION platform (https://github.com/nanoporetech/qcat). Once a barcode reaches 100 000 reads, it maps to the Wuhan-Hu-1 reference (GenBank no. MN908947.3) using minimap2 software. This alignment will then be used to generate consensus sequences and variant calls using medaka software version 1.0.1 (https://github.com/nanoporetech/medaka). The analysis pipeline is available online (https://github.com/gagekmoreno/SARS-CoV-2-in-Southern-Wisconsin).
Consensus Sequence Analysis—Clade and Lineage Generation
Samples were excluded from downstream analysis if gaps in the consensus sequence totaled ≥20% of the genome. Each sample’s consensus sequence was visually inspected using Geneious Prime software (https://www.geneious.com) and/or Nextstrain’s Nextclade online tool (https://clades.nextstrain.org/). We used Pangolin’s command-line tool to assign sequences to Pangolin lineages (https://github.com/cov-lineages/pangolin).
Consensus Sequence Analysis—Southeast Wisconsin Phylogenetic Tree
Wisconsin-centric time-resolved and divergence phylogenetic trees (seen in Supplementary File 1) were built using the standard Nextstrain tools and scripts [26]. Laboratories responsible for obtaining and genetic sequence data included here, if not our own, are documented in Supplementary File 2. An interactive view of this Nextstrain phylogenetic tree can be found at https://nextstrain.org/community/gagekmoreno/Wisconsin-SARS-CoV-2/ncov/wisconsin/2021-1-8.
Genetic Distance Comparisons
Full-length SARS-CoV-2 sequences available on GISAID (Global Initiative on Sharing All Influenza Data) as of 10 March 2020 were obtained and filtered on “Wisconsin” and parsed by date of collection into month bins. We used this data set as a community comparator set. Consensus mutations were called against Wuhan-Hu-1 reference (GenBank no. MN908947.3) using Varscan software (version 2.4.3). HCP and patient samples were similarly binned by month. We performed a permutation test comparing the percentage overlap in mutation identities in 100 000 randomly selected pairs from the community comparator set and plotted these values as a distribution. We plotted the genetic diversity of n-choose-2 random pairs for a healthcare-associated sample, where n is the number of HCP and patient samples available for comparison each month. Code to replicate the genetic distance analyses can be found at GitHub [27].
RESULTS
HCP began testing positive for SARS-CoV-2 at a major academic biomedical institution in the American Upper Midwest in early March 2020. We began sequencing viral genomes from residual nasopharyngeal specimens from the individuals involved in these infection clusters. We focused our analyses on HCP infections and infection clusters that were highest risk for nosocomial transmission, as when healthcare-associated transmission could not be ruled out using epidemiological data alone (see Methods for details). Each investigation included HCP (≥1), all known direct and indirect SARS-CoV-2–positive patient contacts where residual swab samples were available, and occasionally extended to epidemiologically linked household contacts.
We consider 3 potential sources of HCP infection: “patient source” (via HCP-patient interactions), “employee source” (via HCP-HCP interactions), and “no evidence of healthcare-associated transmission.” Some HCP infections did not fit neatly into these categories, so we have included 3 additional categories, which are defined in full in the Supplementary File 1. These additional categories are “combined patient and employee cluster,” “outside community,” and “inconclusive.” In each category, for us to conclude that person A was a likely source of infection for person B, persons A and B must have had known contact with each other, must have been tested within 14 days of each other, and must have been infected with viruses differing by no more than a single mutation [28].
From 12 March 2020 to 10 January 2021, approximately 1172 HCP tested positive for SARS-CoV-2 at the institution we evaluate in this study. In total, we investigated 95 HCP (8.1%) and 137 possible patient contacts collected between 25 March and 27 December 2020 (n = 232). Of these, we were able to generate 87 complete HCP sequences and 87 complete patient contact sequences that were used in downstream analyses (n = 174). Of the 87 patient sequences, 4 were included in ≥2 outbreak investigations.
We did not find a closely related virus among coworker and patient contacts in 55 HCP infections. We identified a specific household or community source of infection in an additional 3 cases (total, 58 of 95 [61.1%]). We found that a smaller percentage could be traced to a coworker (10 of 95 [10.5%]) or were part of a patient-employee cluster (12 of 95 [12.6%]). Strikingly, the smallest proportion of HCP infections could be clearly traced to a patient source (4 of 95 [4.2%]). The remaining HCP infections could not be definitively traced to a single source and were therefore inconclusive (11 of 95 [11.6%]) (Table 1). Below, we describe a representative example of 3 distinct transmission scenarios: no evidence of healthcare-associated transmission, HCP-to-HCP transmission, and patient-to-HCP transmission.
Table 1.
Likely Sources of Severe Acute Respiratory Syndrome Coronavirus Disease 2 Infection in the Healthcare Personnel Evaluated
| Likely Source of Infection in HCPa | SARS-CoV-2 Cases, No. (%) |
|---|---|
| No evidence of healthcare-associated transmission | 55 (57.9) |
| Combined patient and employee cluster | 12 (12.6) |
| Inconclusive | 11 (11.6) |
| Employee source (via employee-employee interactions) | 10 (10.5) |
| Patient source (via employee-patient interactions) | 4 (4.2) |
| Outside community | 3 (3.2) |
| Total | 95 (100) |
Abbreviations: HCP, healthcare personnel; SARS-CoV-2, severe acute respiratory syndrome coronavirus. 2.
aFull definitions for each transmission bin can be found in Supplementary File 1. Briefly, “no evidence of healthcare-associated transmission” includes cases in which available sequences do not support transmission in the healthcare setting, and “outside community” includes cases in which transmission outside the healthcare setting could be reasonably established. “Inconclusive” includes cases in which no consensus sequence was available for the HCP and/or there were no appropriate comparator sequences.
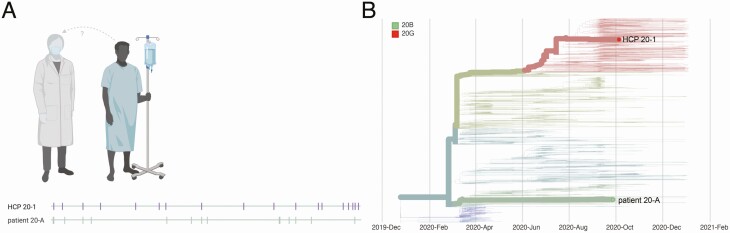
In case 20, we compared the viral sequence of an HCP (HCP 20–1), who tested positive on 5 October, with that of a patient contact who tested positive 8 days earlier. A comprehensive caregiver trace of HCP 20–1 revealed a single patient contact with diagnosed COVID-19 (patient 20-A) within the 14 days before symptom onset in HCP 20–1. HCP 20–1 provided direct care to patient 20-A while wearing appropriate PPE and with no reported lapses in PPE. HCP 20–1 was infected with a virus clustering with the 20G clade, whereas patient 20-A was infected with a 20A-clade virus. The sequences of these viruses differed at >20 sites, so we concluded that these individuals were unlikely to represent a transmission pair (Figure 1).
Figure 1.
Graphic representation of case 20. A, Viral sequences are aligned against severe acute respiratory syndrome coronavirus 2 reference sequence Wuhan-Hu-1 (MN908947.3). Vertical markers denote the location of consensus nucleotide differences between patient viruses and the reference. B, Time-resolved phylogenetic tree built using Nextstrain tools with all Wisconsin sequences available as of 15 January 2021. Viruses involved in this case are denoted with thick branches and labeled tips. Color denotes clade. Abbreviation: HCP, healthcare personnel.
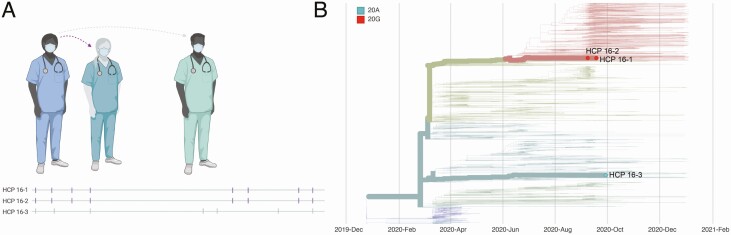
In case 16, we investigated infections in 3 HCP who worked in the same department and tested positive on 8 September (HCP 16–2), 18 September (HCP 16–1), and 29 September (HCP 16–3). Contact tracing revealed that HCP 16–2 worked for 2 days before symptom onset and may have had unmasked contact with HCP 16–1 during overlapping meal breaks. Contact tracing also revealed that HCP 16–3 had an exposure event lasting >15 minutes in the outside community before testing positive. Viral sequencing in this cluster showed that HCP 16–1 and 16–2 were infected with 20G-clade viruses identical at the consensus level, while HCP 16–3 was infected with a genetically dissimilar 20A-clade virus. We therefore concluded that HCP 16–2 was a likely source of infection for HCP 16–1, while HCP 16–3 was likely infected elsewhere (Figure 2).
Figure 2.
Graphic representation of case 16. A, Viral sequences are aligned against severe acute respiratory syndrome coronavirus 2 reference sequence Wuhan-Hu-1 (MN908947.3). Vertical markers denote the location of consensus nucleotide differences between patient viruses and the reference. Purple vertical markers indicate identical viral sequences. B, Time-resolved phylogenetic tree built using Nextstrain tools with all Wisconsin sequences available as of 15 January 2021. Viruses involved in this case are denoted with thick branches and labeled tips. Color denotes clade. Abbreviation: HCP, healthcare personnel.
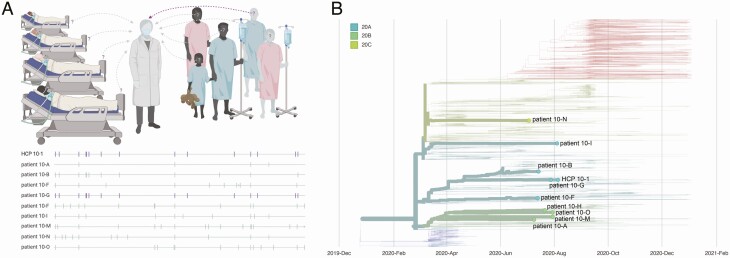
Case 10 involved an HCP (HCP 10–1) who provided care for 15 patients with COVID-19 diagnosed in the 14 days before symptom onset for HCP 10–1. HCP 10–1 provided direct care to each of these patients while wearing appropriate PPE, with no reported lapses in PPE. We generated consensus sequences from HCP 10–1 and 9 patient contacts. There was insufficient viral RNA in the remaining 6 patient contacts to generate high-quality consensus sequences for comparison. The virus isolated from patient 10-G was identical to the virus from HCP 10–1. Given the known epidemiological association between these 2 individuals, the time separating sample collections (28 July and 5 August), and identical viral sequences, we concluded that patient 10-G was a likely source of infection for HCP 10–1 (Figure 3). However, we cannot rule out the possibility that another patient whose sample could not be sequenced also shared an identical virus.
Figure 3.
Graphic representation of case 10. A, Viral sequences are aligned against severe acute respiratory syndrome coronavirus 2 reference sequence Wuhan-Hu-1 (MN908947.3). Vertical markers denote the location of consensus nucleotide differences between patient viruses and the reference. Purple vertical markers indicate identical viral sequences. B, A time-resolved phylogenetic tree built using Nextstrain tools with all Wisconsin sequences available as of 15 January 2021. Viruses involved in this case are denoted with thick branches and labeled tips. Color denotes clade. Abbreviation: HCP, healthcare personnel.
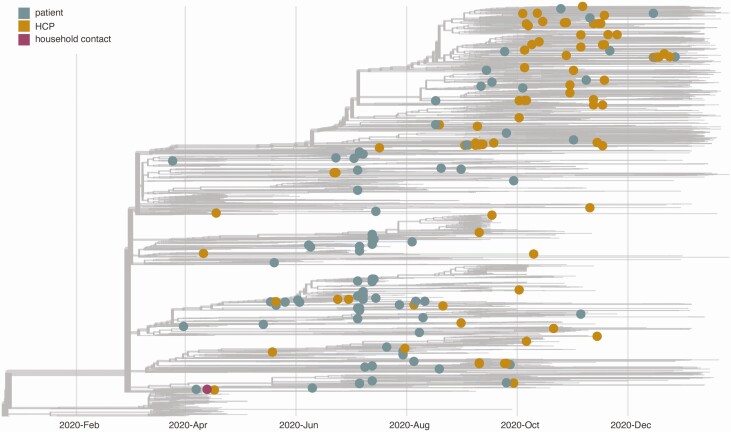
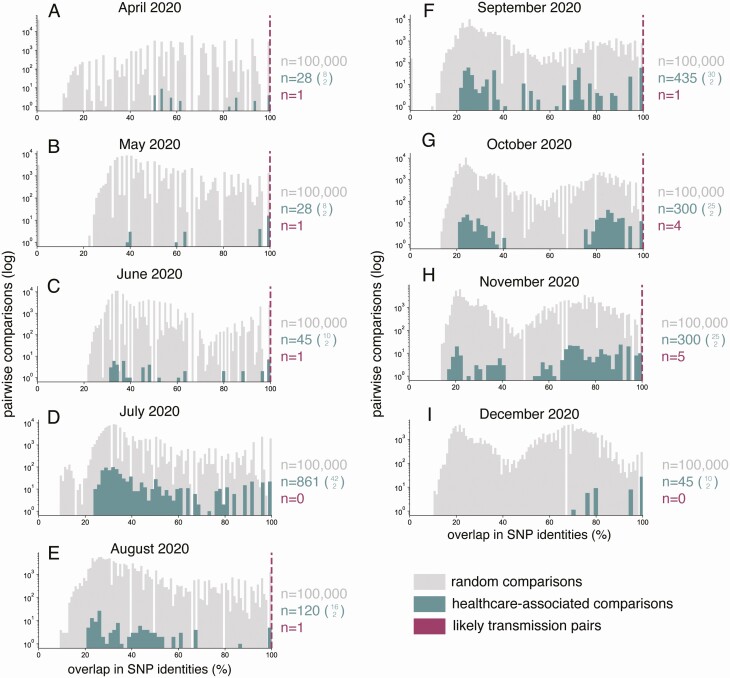
HCP and patient viruses were broadly distributed throughout a phylogenetic tree showing the diversity of circulating viruses collected from the areas surrounding the academic medical center (Figure 4). To investigate the possibility that we missed cryptic healthcare-associated transmission, we compared genetic distances between random pairs of healthcare-associated samples against the genetic distances between randomly paired sequences from the community data set (gray tips in Figure 4) for each month in our study period (Figure 5). Overall, healthcare-associated pairs did not share substantially greater sequence identity than randomly paired sequences from the community. This is consistent with a relatively limited role for nosocomial spread of SARS-CoV-2. We also plotted 14 pairs that were very likely to be true transmission pairs based on epidemiological data (eg, HCP 2–1 and the household contact) and showed these pairs are uniformly genetically identical (dashed magenta lines in Figure 5).
Figure 4.
A time-resolved phylogenetic tree built using Nextstrain tools for all samples collected and shared in Wisconsin from March to December 2020. Healthcare-associated samples are denoted with enlarged tips and colored according to sample type. The gray tips reflect the community surveillance samples. It is likely additional healthcare personnel (HCP) and patient sequences are represented in the community data set, but we do not have access to sufficient metadata to make these designations. Laboratories responsible for obtaining and genetic sequence data included here, if not our own, are documented in Supplementary File 2.
Figure 5.
Genetic diversity among pairwise comparisons of healthcare-associated viruses (healthcare personnel [HCP] and patient samples) is generally similar to that of viruses circulating in the areas surrounding the academic medical center evaluated in this study. Gray distribution reflects 100 000 pairwise random comparisons of the community data set per month. Turquoise distribution shows n-choose-2 comparisons from the healthcare-associated data set per month, where n is the total number of HCP and patient sequences available within each month. The specific value equal to n is shown as the upper value in the turquoise parenthetical expression of n-choose-2. Dashed magenta dashed lines reflect the shared genetic diversity in healthcare-associated pairs where we have high confidence, based on epidemiological data, that these are true transmission pairs. The number of pairs represented in each magenta line is shown in magenta text to the right of each plot. Abbreviation: SNP, single-nucleotide polymorphism.
The center where we conducted this case series implemented a number of changes to their institutional infection control guidelines based on these sequencing results [8]. The recommendations for extended reuse of medical grade face masks were clarified and now instruct HCP to consider barrier mask replacement after 3 days of wear and to inspect the barrier mask before each use and to replace if soiled or damaged. N95 masks or powered air-purifying respirators are now universally required on inpatient units housing patients with confirmed or suspected COVID-19. In addition, medical-grade face masks, instead of cloth masks, are now required for HCP in all clinical areas, not just direct patient care areas. This final recommendation was based on likely HCP-to-HCP transmission involving an HCP who was not directly involved in patient care of patients with COVID-19 (case 14 in Supplementary File 1).
DISCUSSION
HCP across the hospital are involved in caring for people with COVID-19, whether or not they work on an actual COVID-19 ward. With the shifting guidelines and PPE shortages that persist today, it is critical to assess the risk that HCP treating people with known SARS-CoV-2 infection will become infected themselves. We used viral genome sequencing to assess the risk that HCP in a large academic medical system treating patients with COVID-19 would acquire nosocomial infections. Our results suggest that caring for patients with COVID-19 accounted for a minority of HCP infections (n = 4). In contrast, HCP at this institution were much more likely to acquire SARS-CoV-2 from infected coworkers (n = 10) or outside the healthcare system (n = 58).
This result suggests that infection control procedures, consistently followed, offer significant protection to HCP caring for patients with COVID-19 in the United States. A similar conclusion was drawn by studies evaluating healthcare-associated infections in the Netherlands and in the United Kingdom, suggesting that this conclusion may hold across healthcare systems [5, 29]. These results are further supported by those of another study finding that the most important risk factor for HCP SARS-CoV-2 seropositivity was cumulative COVID-19 incidence in surrounding communities, not workplace factors [30].
This study has important limitations. We were able to generate high-quality sequence information for a minority of documented COVID-19 cases in HCP (87 of 1172 [7.4%]) during our study period (25 March to 27 December 2020). Our data set is therefore incomplete and may not be entirely representative of viruses circulating in this healthcare setting, particularly for asymptomatic cases. Similarly, we did not sequence viruses from all SARS-CoV-2–positive patients treated at the medical center where we conducted this study. Given this limitation, we were often able to exclude patient contacts and coworkers as likely sources of infection in HCP, but we were rarely able to pinpoint the exact source of infection.
It is therefore possible that we have underestimated the true rate of SARS-CoV-2 transmission in this healthcare setting. However, the finding that randomly paired HCP and patient sequences do not have greater sequence identity than randomly paired sequences from across the surrounding community suggests s that we have not severely underestimated nosocomial transmission. Our ability to determine the source of infections in these outbreaks was also often limited by incomplete contact tracing data; undocumented exposures between HCP may have occurred inside and outside the workplace.
The current study examined SARS-CoV-2 infections in HCP from a single academic medical center, so our conclusions may not be broadly generalizable. However, another study evaluated healthcare-associated infections in the Netherlands and similarly found no evidence for widespread nosocomial transmission of SARS-CoV-2, suggesting that our conclusions may hold across institutions and healthcare systems [29]. Furthermore, we were not able to differentiate between routes of infection (airborne, droplet, contact) with the limited epidemiological data available to us in our study.
Sampling and contact tracing of nosocomial outbreaks is often coordinated by local hospitals and/or departments of health, while expertise in viral sequencing, bioinformatics, and phylogenetics can more often be found in academic laboratories. Successful application of precision epidemiology requires the integration of these areas. This is possible now at academic medical institutions like ours, but it presents more of a challenge at smaller, rural, and private patient care centers. Federal support should be provided to help establish and maintain these collaborations in the current pandemic and in anticipation of future outbreaks.
In the current study we demonstrated how rapid whole-genome sequencing of current SARS-CoV-2 outbreaks in hospitals can be used retrospectively to reconstruct the likely source of HCP infection and prospectively to adjust and improve infection control practices and guidelines. The approach we describe here need not be limited to investigation of pandemic virus outbreaks. Key concepts from genome sequencing and routine pathogen surveillance can be applied to any nosocomial pathogen and inform changes to infection control practices. Overall, while we do find examples of patient-to-HCP and HCP-to-HCP spread, we found no evidence of healthcare-associated transmission in a majority of HCP infections, emphasizing the importance of ongoing measures to reduce community spread through mask wearing, physical distancing, robust testing programs, and rapid distribution of vaccines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Anna Heffron for assisting with sample transport. They also thank all healthcare workers and infection control teams for their ongoing dedication to patient and community health and wellness. The authors gratefully acknowledge the originating laboratories responsible for obtaining the specimens and the submitting laboratories where genetic sequence data were generated and shared via GISAID (Global Initiative on Sharing All Influenza Data) (Supplementary File 2). Figures 1A, 2A, and 3A were created with BioRender (http://biorender.com).
Financial support. This work was supported by the Wisconsin Partnership Program, University of Wisconsin School of Medicine and Public Health (coronavirus disease 2019 response grants to D. H. O. and T. C. F.) and the National Institute of Allergy and Infectious Diseases (grant 1DP2AI144244-01 to N. S.). G. K. M. is supported by a National Library of Medicine training grant to the Computation and Informatics in Biology and Medicine Training Program (NLM 5T15LM007359).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Nguyen LH, Drew DA, Graham MS, et al. ; COronavirus Pandemic Epidemiology Consortium . Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020; 5:e475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med 2020; 173:120–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open 2020; 3:e203976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng VC, Wong SC, Yuen KY. Estimating coronavirus disease 2019 infection risk in health care workers. JAMA Netw Open 2020; 3:e209687. [DOI] [PubMed] [Google Scholar]
- 5.Meredith LW, Hamilton WL, Warne B, et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis 2020; 20:1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arpacioglu S, Gurler M, Cakiroglu S. Secondary traumatization outcomes and associated factors among the health care workers exposed to the COVID-19. Int J Soc Psychiatry 2020: 0020764020940742. Published 8 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID-19) pandemic. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed 10 May 2021.
- 8.Lepak AJ, Shirley DK, Buys A, Stevens L, Safdar N. Implementation of infection control measures to prevent healthcare-associated transmission of severe acute respiratory coronavirus virus 2 (SARS-CoV-2). Infect Control Hosp Epidemiol 2021; 42:229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houldcroft CJ, Roy S, Morfopoulou S, et al. Use of whole-genome sequencing of adenovirus in immunocompromised pediatric patients to identify nosocomial transmission and mixed-genotype infection. J Infect Dis 2018; 218:1261–71. [DOI] [PubMed] [Google Scholar]
- 10.Greninger AL, Zerr DM, Qin X, et al. Rapid metagenomic next-generation sequencing during an investigation of hospital-acquired human parainfluenza virus 3 infections. J Clin Microbiol 2017; 55:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houlihan CF, Frampton D, Ferns RB, et al. Use of whole-genome sequencing in the investigation of a nosocomial influenza virus outbreak. J Infect Dis 2018; 218:1485–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deurenberg RH, Bathoorn E, Chlebowicz MA, et al. Application of next generation sequencing in clinical microbiology and infection prevention. J Biotechnol 2017; 243:16–24. [DOI] [PubMed] [Google Scholar]
- 13.Tang P, Croxen MA, Hasan MR, Hsiao WW, Hoang LM. Infection control in the new age of genomic epidemiology. Am J Infect Control 2017; 45:170–9. [DOI] [PubMed] [Google Scholar]
- 14.Safdar N, Moreno GK, Braun KM, Friedrich TC, O’Connor DH. Using virus sequencing to determine source of SARS-CoV-2 transmission for healthcare worker. Emerg Infect Dis 2020; 26:2489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikkens JJ, Buis DTP, Peters EJG, et al. Serologic surveillance and phylogenetic analysis of SARS-CoV-2 infection in hospital health care workers. medRxiv [Preprint: not peer reviewed]. 12 January 2021. Available at https://www.medrxiv.org/content/10.1101/2021.01.10.21249440v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biek R, Pybus OG, Lloyd-Smith JO, Didelot X. Measurably evolving pathogens in the genomic era. Trends Ecol Evol 2015; 30:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rai B, Shukla A, Dwivedi LK. Estimates of serial interval for COVID-19: a systematic review and meta-analysis. Clin Epidemiol Glob Health 2021; 9:157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.auspice. Available at: https://nextstrain.org/ncov/north-america. Accessed 15 February 2021.
- 19.Ladner JT, Grubaugh ND, Pybus OG, Andersen KG. Precision epidemiology for infectious disease control. Nat Med 2019; 25:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. CDC diagnostic tests for COVID-19.2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/testing.html. Accessed 19 March 2021.
- 21.Hologic. Panther Fusion® SARS-CoV-2 assay. Available at: https://www.hologic.com/package-inserts/diagnostic-products/panther-fusionr-sars-cov-2-assay. Accessed 19 March 2021.
- 22.Hologic. Aptima® SARS-CoV-2 Assay (Panther® System). Available at: https://www.hologic.com/package-inserts/diagnostic-products/aptimar-sars-cov-2-assay-pantherr-system. Accessed 19 March 2021.
- 23.Moreno GK, Braun KM, Riemersma KK, et al. Revealing fine-scale spatiotemporal differences in SARS-CoV-2 introduction and spread. Nat Commun 2020; 11:5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quick J, Grubaugh ND, Pullan ST, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc 2017; 12:1261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quick J. nCoV-2019 sequencing protocol v1 (protocols.io.bbmuik6w). protocols.io. doi: 10.17504/protocols.io.bbmuik6w. Available at: https://www.protocols.io/view/ncov-2019-sequencing-protocol-bbmuik6w?version_warning=no. Accessed 10 May 2021. [DOI]
- 26.Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 2018; 34:4121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun K. SARSCoV2_sequencing_healthcare-association_infections. GitHub. Available at: https://github.com/katarinabraun/SARSCoV2_sequencing_healthcare-association_infections. Accessed 19 March 2021.
- 28.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 29.Sikkema RS, Pas SD, Nieuwenhuijse DF, et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis 2020; 20:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacob JT, Baker JM, Fridkin SK, et al. Risk factors associated with SARS-CoV-2 seropositivity among US health care personnel. JAMA Netw Open 2021; 4:e211283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.