Abstract
Background
Limited data exist on high-sensitivity cardiac troponin (hs-cTn) for risk-stratification in COVID-19.
Methods
We conducted a multicenter, retrospective, observational, US-based study of COVID-19 patients undergoing hs-cTnT. Outcomes included short-term mortality (in-hospital and 30-days post-discharge) and a composite of major adverse events, including respiratory failure requiring mechanical ventilation, cardiac arrest, and shock within the index presentation and/or mortality during the index hospitalization or within 30-days post-discharge.
Results
Among 367 COVID-19 patients undergoing hs-cTnT, myocardial injury was identified in 46%. They had a higher risk for mortality (20% vs 12%, P < 0.0001; unadjusted HR 4.44, 95% CI 2.13–9.25, P < 0.001) and major adverse events (35% vs. 11%, P < 0.0001; unadjusted OR 4.29, 95% CI 2.50–7.40, P < 0.0001). Myocardial injury was associated with major adverse events (adjusted OR 3.84, 95% CI 2.00–7.36, P < 0.0001) but not mortality. Baseline (adjusted OR 1.003, 95% CI 1.00–1.007, P = 0.047) and maximum (adjusted OR 1.005, 95% CI 1.001–1.009, P = 0.0012) hs-cTnT were independent predictors of major adverse events. Most (95%) increases were due to myocardial injury, with 5% (n = 8) classified as type 1 or 2 myocardial infarction. A single hs-cTnT <6 ng/L identified 26% of patients without mortality, with a 94.9% (95% CI 87.5–98.6) negative predictive value and 93.1% sensitivity (95% CI 83.3–98.1) for major adverse events in those presenting to the ED.
Conclusions
Myocardial injury is frequent and prognostic in COVID-19. While most hs-cTnT increases are modest and due to myocardial injury, they have important prognostic implications. A single hs-cTnT <6 ng/L at presentation may facilitate the identification of patients with a favorable prognosis.
Keywords: COVID-19, high sensitivity cardiac troponin T, myocardial injury, myocardial infarction, risk stratification
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected millions of people worldwide (1). It is associated with substantial morbidity and mortality (2, 3). While pulmonary complications are frequent (3), studies suggest that myocardial injury is common, in particular among those with chronic cardiovascular conditions and more severe COVID-19 presentations, and that its presence and magnitude is associated with worse outcomes (4–9).
Cardiac troponin (cTn) is the preferred biomarker for the detection of myocardial injury, which is defined when there is at least one cTn concentration above the 99th percentile upper-reference limit (URL) of a healthy reference cohort (10). Most studies addressing myocardial injury in COVID-19 have been performed outside the US (5–7), used nonguideline definitions of myocardial injury (2, 11, 12), used contemporary cTn assays with thresholds other than the 99th percentile URL for its detection (13), and failed to report the nature of cTn increases, including the incidence of type 1 and 2 myocardial infarction (MI) (9). Further, despite cTn being recognized as a potent risk-stratification tool (14, 15), limited assay-specific actionable data exist on how to use high-sensitivity (hs) cTn assays for risk-stratification in COVID-19.
The goals of our study are 3-fold: first, to determine the incidence of myocardial injury and acute MI, including the frequency of type 1 and 2 MIs, following the recommendations from the Task Force for the Fourth Universal Definition of MI (UDMI) (10) using a hs-cTnT assay with sex-specific 99th percentile URLs in a multicenter COVID-19 US cohort; second, to evaluate the clinical features and outcomes of COVID-19 patients with and without myocardial injury; last, to evaluate the prognostic role of hs-cTnT and its potential use as a risk-stratification tool.
Methods
Study Design
Following Mayo Clinic COVID-19 Research Task Force and Institutional Review Board approval, we retrospectively evaluated consecutive, adult patients with confirmed COVID-19 diagnosis based on detection of SARS-CoV-2 on real-time reverse transcriptase polymerase chain reaction (PCR) swabs that underwent at least one 5th Gen cTnT measurement on clinical indication. The present is a multicenter, observational cohort study involving patients that presented to the emergency department (ED) and/or were admitted to the hospital across 1 of 20 Mayo Clinic sites (Rochester, Florida, and Arizona campuses and 17 Mayo Clinic Health System hospitals across Minnesota and Wisconsin) up to July 13, 2020. Patients without a Minnesota Research Authorization form or permission to use records, <18 years old, with indeterminate PCR results, and those without at least one hs-cTnT during the index hospitalization were excluded. For patients with more than one presentation, we included the first. For patients re-hospitalized within 24 h after index encounter, data were merged and analyzed as single encounter. Data was manually reviewed and extracted by trained physicians using Research Electronic Data Capture (REDCap) (16). The National Early Warning Score (NEWS) was calculated based on vital signs at presentation (17).
Outcomes
The primary outcomes evaluated were 1) short-term mortality, which was defined as either index hospitalization or postdischarge mortality within 30-days, and 2) a composite outcome of major adverse events that included the occurrence of respiratory failure requiring mechanical ventilation, cardiac arrest, circulatory shock requiring pharmacologic and/or mechanical support within the index presentation, and/or mortality during the index hospitalization or within 30-days postdischarge. Other index hospitalization outcomes included: intensive care unit (ICU) admission, acute respiratory distress syndrome (ARDS), pulmonary embolism, super-infection, coagulopathy such as disseminated intravascular coagulation, or severe thrombocytopenia (platelets < 50.000 x 109/L), and clinical heart failure. Outcomes were determined based on clinical notes and discharge summaries. Postdischarge 30-day follow-up was obtained when available and included COVID19-related hospitalizations, acute MI, and death.
Cardiac Troponin Assay
hs-cTnT was measured with the Elecsys Troponin T Gen 5 STAT (Roche Diagnostics) (18) .The lowest reportable clinical value is the <6 ng/L limit of quantification (LoQ). Sex-specific 99th percentiles URLs of 10 ng/L for women and 15 ng/L for men were used (18). Myocardial injury was defined as any hs-cTnT concentration increase above the sex-specific 99th percentile thresholds (10). The Mayo Clinic hs-cTnT protocol for ruling in and out acute myocardial injury and acute MI has been described (18, 19). In brief, patients with suspected MI are evaluated using a 0/2 h hs-cTnT protocol that uses sex-specific 99th percentile URLs to rule-in and rule-out myocardial injury and an absolute delta (serial change) of ≥10 ng/L to identify patients with acute injury. MI is diagnosed when there is objective evidence of myocardial ischemia.
Myocardial Injury and Infarction Event Adjudication
All cases with at least one hs-cTnT above the sex-specific 99th percentile URL underwent clinical adjudication based on all available data following the Fourth UDMI (10). Cases were classified as having either myocardial injury or acute MI based on the presence or absence of clinical features of acute myocardial ischemia (10). Those with clinical evidence of acute myocardial ischemia were classified as having acute MI and further subclassified into one of the 5 MI subtypes, with type 1 MI representative of atherothrombotic MI and type 2 MI due to non-atherothrombotic supply-demand myocardial ischemia (10, 20). For cases adjudicated as either myocardial injury or type 2 MI, etiologies were tabulated. Cases in which event adjudication was considered challenging were further reviewed by the principal investigator (YS), and if needed also reviewed by one of the members from the Task Force for the Fourth UDMI (ASJ).
Statistical Analysis
Categorical variables are presented as number (percentage) and compared using chi-square tests. Continuous variables are presented as median (interquartile range, IQR) and compared using Kruskal-Wallis tests. The risk for short-term mortality was estimated using the Kaplan-Meier method. Hazard ratios (HR) and 95% confidence intervals (CI) for mortality were calculated using Cox proportional hazards models. Outcomes are presented as percentages, and models for these endpoints were estimated using logistic regression models. Adjusted models were performed for mortality adjusting for age, sex, and coronary artery disease (CAD), and for major adverse events adjusting for age, sex, hypertension, CAD, heart failure, chronic kidney disease, and diabetes mellitus, with factors entered per model based on at least 10 events per variable to avoid overfitting. Sensitivity, specificity, negative (NPV) and positive predictive (PPV) values were calculated with corresponding 95% CI. A P value <0.05 was considered statistically significant. Analyses were performed using SAS version 9.4 (SAS Institute) as well as R version 4.0.2.
Results
Clinical Characteristics
Following exclusions (Supplemental Fig. 1), the final study cohort included 367 COVID-19 patients in whom at least one hs-cTnT was obtained, amongst which 46% were identified to have myocardial injury based on hs-cTnT concentrations above the sex-specific 99th percentiles. Baseline characteristics are shown in Table 1 and Supplemental Tables 1 and 2. The mean (SD) age of the cohort was 61 (17) years and most patients presented through the ED (83%). As compared to patients without myocardial injury, those with myocardial injury were more likely to be older and have comorbidities. There were no differences across NEWS score categories between patients with and without myocardial injury, with 38% of the cohort quantified to have medium-to-high illness severity at presentation.
Table 1.
Baseline characteristics and main comorbidities in the overall cohort and by presence or absence of myocardial injury.
|
Overall
N = 367 |
Without myocardial injury
n = 198 |
Myocardial injury
n = 169 |
P value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age—mean (SD) | 61 (17) | 54 (15) | 69 (15) | <0.0001 | |
| Women—n (%) | 147 (40) | 84 (42) | 63 (37) | 0.32 | |
| White—n (%) | 239 (66) | 115 (59) | 124 (74) | 0.003 | |
| Presented to Mayo Clinic Emergency Department—n (%) | 303 (83) | 165 (83) | 138 (82) | 0.67 | |
| Transferred from outside non-Mayo facility—n (%) | 65 (18) | 32 (16) | 33 (20) | 0.40 | |
| Inter-Mayo transfer—n (%) | 13 (3.5) | 6 (3.0) | 7 (4.1) | 0.57 | |
| Repeat Mayo Clinic encounter within 24-hours—n (%) | 9 (2.5) | 5 (2.5) | 4 (2.4) | 0.92 | |
| Medical history | |||||
| Hypertension—n (%) | 214 (58) | 83 (42) | 131 (78) | <0.0001 | |
| Obesity—n (%) | 150 (41) | 90 (46) | 60 (36) | 0.05 | |
| Current or prior tobacco use or vaping—n (%) | 131 (36) | 60 (30) | 71 (42) | 0.02 | |
| Coronary artery diseasea—n (%) | 49 (13) | 10 (5.1) | 39 (23) | <0.0001 | |
| Cerebrovascular disease—n (%) | 35 (9.5) | 7 (3.5) | 28 (17) | <0.0001 | |
| Atrial dysrhythmias—n (%) | 40 (11) | 12 (6.1) | 28 (17) | 0.001 | |
| Heart failure—n (%) | 32 (8.7) | 4 (2.0) | 28 (17) | <0.0001 | |
| Dyslipidemia—n (%) | 170 (46) | 64 (32) | 106 (63) | <0.0001 | |
| Diabetes mellitus—n (%) | 117 (32) | 42 (21) | 75 (44) | <0.0001 | |
| Chronic kidney disease—n (%) | 78 (21) | 10 (5.1) | 68 (40) | <0.0001 | |
| Obstructive sleep apnea—n (%) | 60 (16) | 27 (14) | 33 (20) | 0.13 | |
| COPD/asthma—n (%) | 58 (16) | 32 (16) | 26 (15) | 0.84 | |
| History of solid organ transplant—n (%) | 33 (9.0) | 6 (3.0) | 27 (16) | <0.0001 | |
| HIV—n (%) | 2 (0.5) | 0 (0.0) | 2 (1.2) | 0.13 | |
| Prior ACEI/ARB/ARNI use—n (%) | 105 (29) | 41 (21) | 64 (38) | 0.0003 | |
a: coronary artery disease was defined as reported history of coronary artery disease, prior myocardial infarction, or prior revascularization including coronary artery bypass graft surgery or percutaneous coronary intervention.
Abbreviations: SD, standard deviation; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor.
Patients with myocardial injury had significantly higher creatinine concentrations and lower estimated glomerular filtration rate, as well as lower hemoglobin and platelet counts. Among those undergoing adjunctive laboratory testing, patients with myocardial injury had significantly higher D-dimer, lactate, procalcitonin, interleukin- 6, and NT-proBNP, and lower lymphocyte counts (Supplemental Table 3). The majority of patients (n = 350) underwent chest x-ray imaging at presentation and most (73%) were identified to have infiltrates or findings indicative of pneumonia, with no difference observed between patients with and without myocardial injury. However, among those undergoing chest computed tomography (CT) (n = 166), patients with myocardial injury were more likely to demonstrate bilateral ground-glass opacities (95% vs 87%, P = 0.05) and parenchymal consolidation (54% vs 38%, P = 0.04).
Cardiac testing including 12-lead electrocardiogram (ECG) and echocardiographic findings are shown in Supplemental Table 4. Patients with myocardial injury were less likely to have normal ECGs (43% vs 60%, P = 0.005). Among the 36% of patients undergoing echocardiography, those with myocardial injury were more often detected to have left ventricular dysfunction (left ventricular ejection fraction <50%) (24% vs 7%, P = 0.02), diastolic dysfunction (37% vs 14%, P = 0.009), moderate-to-severe right ventricular dysfunction (13% vs 0%, P = 0.02), and valvular heart disease (14% vs 0%, P = 0.01). Only 2 patients underwent coronary CT angiography; none had obstructive CAD. One had invasive angiography that demonstrated severe CAD requiring percutaneous revascularization.
High-Sensitivity Cardiac Troponin T Concentrations and Myocardial Injury Etiologies
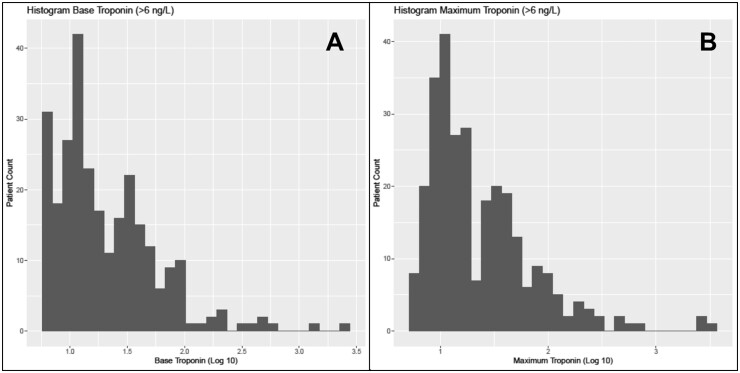
The distribution for baseline and maximum hs-cTnT concentrations is shown in Fig. 1. Median baseline hs-cTnT concentrations were 11 (IQR 5, 29) ng/L, with men having significantly higher baseline concentrations than women (14 vs 7.5 ng/L, P < 0.0001). Baseline hs-cTnT measurements were available in 98% of patients within 24-h of presentation. At baseline, hs-cTnT was below the LoQ in 26% of patients (men 15% vs women 42%, P < 0.0001), quantifiable (LoQ to 99th percentile) in 31% (men 39% vs women 19%, P < 0.0001), and increased above sex-specific 99th percentiles in 43% of patients (men 46% vs women 40%, P = 0.3) (Supplemental Fig. 2).
Fig. 1.
Histogram for the distribution of baseline (panel A) and maximum (panel B) hs-cTnT concentrations.
Most patients (n = 234, 64%) underwent serial hs-cTnT testing (2 or more measurements). Among the subset (n = 129) of patients undergoing 0/2 h hs-cTnT measurements within 24 h of presentation, only 5.4% of patients had serial changes (delta) ≥10 ng/L. Overall median maximum concentrations for the entire cohort across all hs-cTnT measurements were 12 (IQR 6, 32) ng/L, with men also having significantly higher maximum concentrations than women (15 vs. 9 ng/L, P < 0.0001). The distribution of all hs-cTnT concentrations over time for men and women in relationship to sex-specific 99th percentiles is shown in Supplemental Fig. 3. Multivariate analyses identified age (odds ratio, OR, 1.06, 95% CI 1.04–1.08, P < 0.0001), chronic kidney disease (OR 7.61, 95% CI 3.44–16.8, p < 0.0001), and diabetes mellitus (OR 2.17, 95% CI 1.21–3.90, P = 0.009) as independent predictors of hs-cTnT increases.
Event adjudication showed that 95% (n = 161) of elevations were adjudicated as isolated hs-cTnT increases without myocardial ischemia consistent with myocardial injury. The most common adjudicated etiology was chronic myocardial injury (e.g., due to chronic heart failure, cardiomyopathy, or chronic kidney disease) (n = 88) followed by acute etiologies such as critical illness (n = 65) and sepsis (n = 72). Myocarditis was rare, with clinical suspicion in only 3 patients in whom there was no definite confirmatory testing with cardiac magnetic resonance imaging or biopsy. The remaining 5% (n = 8) were adjudicated as acute MI, with 3 classified as type 1 MI and 5 as type 2 MI. Among the latter, cases were classified as being triggered by hypoxia, hypotension, and/or tachyarrhythmias.
Clinical Outcomes and Risk-Stratification
In-hospital and postdischarge outcomes are presented in Table 2. Short-term mortality occurred in 12% of the cohort. A total of 8.7% patients died during the index hospitalization. At postdischarge follow-up (available in 93% of those who survived the index hospitalization; median 49 days), 3.5% of patients died. The major adverse event composite outcome occurred in 22% (n = 81) of patients, which in addition to short-term mortality, included 59 patients requiring mechanical ventilation, 12 suffering cardiac arrest (2 due to ventricular tachycardia/fibrillation, and 10 due to pulseless electrical activity/asystole), and 46 shock requiring pharmacologic and/or mechanical support.
Table 2.
Outcomes and short-term follow-up for the overall cohort and by myocardial injury.
| Overall (n = 367) | Without myocardial injury (n = 198) | Myocardial injury(n = 169) | P value | |
|---|---|---|---|---|
| In-hospital outcomes | ||||
| Length of stay (days) – median (IQR) | 7 | 5 | 9 | <0.0001 |
| ARDS—n (%) | 66 (18) | 22 (11) | 44 (26) | 0.0002 |
| Cardiac arrest—n (%) | 12 (3.3) | 5 (2.5) | 7 (4.1) | 0.39 |
| Coagulopathy—n (%) | 8 (2.2) | 3 (1.5) | 5 (3) | 0.35 |
| Shock—n (%) | 46 (13) | 10 (5.1) | 36 (21) | <0.0001 |
| Respiratory failure requiring mechanical ventilation—n (%) | 59 (16) | 17 (8.6) | 42 (25) | <0.0001 |
| Pulmonary embolism—n (%) | 14 (3.8) | 4 (2) | 10 (5.9) | 0.0521 |
| ICU admission—n (%) | 102 (28) | 36 (18) | 66 (39) | <0.0001 |
| Clinical heart failure—n(%) | 24 (6.5) | 3 (1.5) | 21 (12) | <0.0001 |
| Co-infection—n (%) | 79 (22) | 27 (14) | 52 (31) | <0.0001 |
| In-hospital death—n (%) | 32 (8.7) | 7 (3.5) | 25 (15) | <0.0001 |
| Postdischarge short-term outcomes among survivals | ||||
| COVID-19 related hospitalization—n (%) | 30(9.6) | 14 (8) | 16(12) | 0.28 |
| Acute MI—n (%) | 0 (0) | 0 (0) | 0 (0) | NA |
| Death within 30-days postdischarge—n (%) | 11(3.5) | 2 (1.1) | 9(6.6) | 0.01 |
ARDS, acute respiratory distress symptoms. ICU: intensive care unit. COVID-19: coronavirus disease 2019. MI: myocardial infarction.
Besides a higher incidence of in-hospital (15% vs 3.5%, P < 0.0001) and 30-days postdischarge death (6.6% vs 1.1%, P = 0.01), patients with myocardial injury had more prolonged lengths of stay (LOS) and were more likely to have ARDS, shock, respiratory failure requiring mechanical ventilation, clinical heart failure, and co-infection than those without myocardial injury.
Patients with myocardial injury had a higher risk for short-term mortality (Fig. 2) (20% vs 12%, P < 0.0001; unadjusted HR 4.44, 95% CI 2.13–9.25, P < 0.001) and major adverse events (35% vs 11%, P < 0.0001; unadjusted OR 4.29, 95% 2.50–7.40, P < 0.0001). Following multivariate modeling adjustment, myocardial injury was associated with major adverse events (adjusted OR 3.84, 95% CI 2.00–7.36, P < 0.0001) but not short-term mortality (adjusted HR 1.56, 95% CI 0.68–3.57, P = 0.3) (Table 3).
Fig. 2.
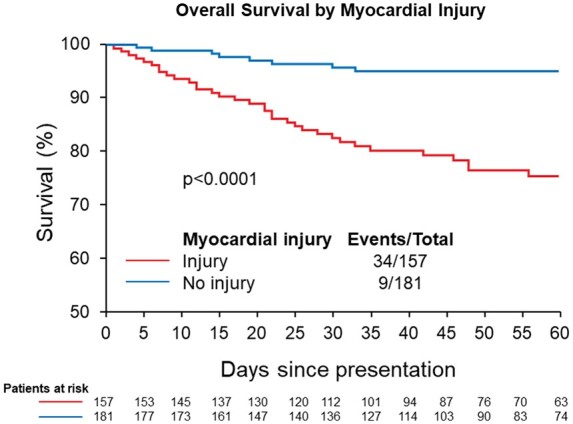
Survival among COVID-19 patients with and without myocardial injury.
Table 3.
Hazard ratio (HR) for short-term mortality and odds ratio (OR) for major adverse events for myocardial injury and for hs-cTnT as a continuous variable (both at baseline and maximum value).
| Short-term mortality (index and 30-days postdischarge) |
Major adverse events |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Myocardial injury |
4.44 (2.13–9.25) |
<0.0001 |
1.56 (0.68–3.57) |
0.3 |
4.29 (2.50–7.40) |
<0.0001 |
3.84 (2.00–7.36) |
<0.0001 |
|
Baseline s-cTnT |
1.001 (1.00–1.002) |
0.005 |
1.001 (1.00–1.002 |
0.1 |
1.004 (1.001–1.008) |
0.022 |
1.003 (1.000–1.007) |
0.047 |
|
Maximum hs-cTnT |
1.001 (1.000–1.001) |
0.002 |
1.001 (1.00–1.002) |
0.002 |
1.006 (1.002–1.01) |
0.002 |
1.005 (1.001–1.009) |
0.012 |
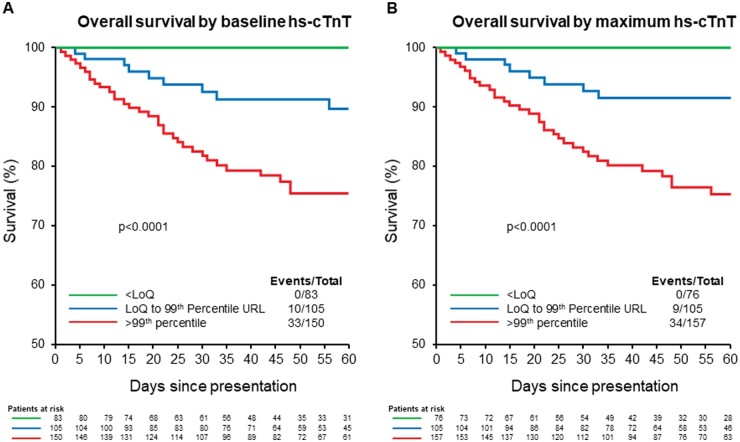
Both baseline and maximum hs-cTnT concentrations were associated with a higher risk for short-term mortality and major adverse events (Table 3). Following adjustment, maximum hs-cTnT was identified to be an independent predictor of both short-term mortality and major adverse events, whereas baseline hs-cTnT was an independent predictor of major adverse events but not short-term mortality. While both the NEWS score and baseline hs-cTnT were predictors of short-term mortality, their evaluation in a joint model demonstrated that baseline hs-cTnT provided additional independent prognostic information beyond the NEWS score.
Outcomes stratified according to analytical hs-cTnT concentration thresholds are shown in Supplemental Table 5 and Fig. 3. As compared to patients with both quantifiable (above the LoQ) and increased concentrations above sex-specific 99th percentiles, those with unquantifiable baseline hs-cTnT (<LoQ) (26%) had shorter LOS and were less likely to have adverse events. As compared to the significantly higher incidence of in-hospital and postdischarge mortality observed in those with quantifiable (7.1% and 2.1%) and increased (15% and 6.9%) baseline hs-cTnT concentrations, there were zero in-hospital and postdischarge (median follow up 46 days) deaths in patients with unquantifiable baseline hs-cTnT. Among those presenting to the ED (n = 303), a baseline hs-cTnT <6 ng/L had a negative predictive value 94.9% (95% CI 87.5–98.6) and sensitivity of 93.1% (95% CI 83.3–98.1) for major adverse events (Supplemental Table 6). While lower baseline hs-cTnT concentrations were associated with higher NPVs and sensitivities, higher concentrations were not associated with higher PPVs due to small number of patients (i.e., only 12 patients presenting to the ED had baseline concentrations >100 ng/L).
Fig. 3.
Survival among COVID-19 patients according to baseline (A) and maximum (B) hs-cTnT concentrations based on analytical ranges: below the LoQ, LoQ to 99th percentile, and above sex-specific 99th percentiles.
Discussion
The present US-based multicenter cohort study of COVID-19 patients presenting to the ED and/or admitted to the hospital undergoing hs-cTnT measurements across 20 centers offers several important findings. First, using a uniform hs-cTnT assay with sex-specific 99th percentile URLs, we determined that myocardial injury is common in COVID-19 patients undergoing hs-cTnT, particularly in older patients with comorbidities, with 46% identified to have myocardial injury based on all available hs-cTnT measurements obtained during the index hospitalization. Second, we demonstrate that myocardial injury has important prognostic implications with 20% of COVID-19 patients with myocardial injury having short-term-mortality and 35% major adverse events, and an independent association observed between myocardial injury and the occurrence of major adverse events. Third, our study is among the first to provide systematic adjudication of all cases with at least one hs-cTnT increase above the sex-specific 99th percentile following the Fourth UDMI (10) and demonstrates that most (95%) events are due to isolated myocardial injury (i.e., hs-cTnT increases without clinical evidence of myocardial ischemia). Fourth, while hs-cTnT increases were modest, maximum hs-cTnT concentrations were found to be an independent predictor of both mortality and major adverse events, with baseline hs-cTnT also associated with major adverse events. Last, our study is the first, to the best of our knowledge, to demonstrate that a single hs-cTnT measurement <6 ng/L at presentation can facilitate the identification of 26% of COVID-19 patients with a more favorable prognosis and potentially help with patient triage.
Our study has several important and unique strengths. First, it evaluates myocardial injury in COVID-19 in a large US healthcare system. Second, it evaluates a uniform hs-cTn assay with sex-specific 99th percentile thresholds across 20 sites. Third, it involved systematic adjudication of all hs-cTnT increases following the Fourth UDMI. Last, the use of a uniform assay facilitated the identification of a risk-stratification threshold that may help triage COVID-19 patients.
While several previous studies have suggested that myocardial injury is frequent and associated with adverse outcomes, prior investigations have often used nonguideline definitions that allowed for ECG or imaging abnormalities (9) to determine the diagnosis, as well as used thresholds other than the 99th percentile URL for its definition (21). Further, several studies have used ‘contemporary’ (not high-sensitivity) assays, and despite recognition that assays vary widely in analytical and diagnostic performance, some have merged various assays in their analyses (7, 21, 22). Studies specifically evaluating hs-cTnT have either not clearly reported the incidence (23, 24) or reported the proportion of increases only using baseline measurements (25). In a smaller study of 101 COVID-19 patients undergoing baseline hs-cTnT, myocardial injury occurred in 15.8%, with patients studied being much younger and less comorbid than those in the present analysis (25). Similar to our findings, a New York study demonstrated that baseline hs-cTnT was ≥20 ng/L (not the 99th percentile) in 43% (361/830) of COVID-19 patients (24).
Complementing previous studies showing that COVID-19 patients with myocardial injury are at higher risk for morbidity and mortality (4–8), we demonstrate that myocardial injury defined using hs-cTnT with sex-specific thresholds has important prognostic implications. While adjusted analyses showed that myocardial injury was not independently associated with short-term mortality, they did demonstrate a significant association between myocardial injury and the risk for major adverse events. These patients had more prolonged hospitalizations and were more likely to require ICU admission, as well as had higher incidence of ARDS, clinical heart failure, and co-infection.
Our study is among the first to provide a systematic adjudication following the Fourth UDMI (10). We demonstrate that most (95%) events are due to isolated myocardial injury. Most increases are due to either chronic illness such as heart failure or renal disease, followed by acute etiologies such as critical illness or sepsis. Myocarditis was rare; it was suspected as a potential cause of myocardial injury in only 3 patients and the diagnosis was not proven with cardiac magnetic resonance or biopsy. While acute infection from COVID-19 has been plausibly associated with a potential higher risk for type 1 MI due to the increased inflammatory, prothrombotic, and procoagulant conditions (9), as well as a potential higher risk for type 2 MI due to the many possible alterations in supply-demand mismatch (20), our study demonstrates that acute MI is infrequent (5% of all hs-cTnT increases) among COVID-19 patients with hs-cTnT increases.
Recognizing the established role of hs-cTn assays as a continuous marker of risk with the potential to facilitate triage and risk-stratification (9, 14, 15), we evaluated the prognostic implications of hs-cTnT in COVID-19. Critically, despite almost 40% of the cohort quantified to have medium-to-high illness severity at presentation, maximum hs-cTnT concentrations were modest. While modest, maximum hs-cTnT concentrations were found to be an independent predictor of both mortality and major adverse events, underscoring the value of serial measurements. Baseline hs-cTnT concentrations were also associated with the major adverse event composite endpoint. Further, we demonstrate that the higher the hs-cTnT concentration (either baseline or maximum), including concentrations within the reference range, the higher the risk for mortality. These findings complement previous studies using contemporary assays (4, 5, 22).
While several COVID-19 studies have often emphasized the association between increased cTn concentrations and adverse events (4–8), our study demonstrates that similar to its application in patients with suspected MI (14), a single hs-cTnT measurement at presentation can facilitate the identification of lower risk COVID-19 patients and potentially help with triage, with our study demonstrating that a baseline hs-cTnT <6 ng/L identifies 26% of COVID-19 patients in whom short-term mortality did not occur and the risk for major adverse events was significantly lower. While it identifies low-risk patients in whom morbid outcomes are less likely, it should be noted that it does not imply a completely benign COVID-19 course for all patients, with several patients with unquantifiable hs-cTnT concentrations developing ARDS (9.6%), requiring ICU admission (11%), or mechanical ventilation (5.3%). Given that most hs-cTnT increases are modest and marked increases infrequent, higher baseline concentrations were not associated with higher PPVs.
Limitations exist. First, the present is an observational study with a retrospective design. Second, potential for selection bias exists, as not all COVID-19 patients undergo systematic hs-cTnT measurements. Electronic-health record data suggests that approximately 67% of COVID-19 patients admitted to the hospital undergo at least one hs-cTnT measurement. Despite the latter, our findings are unique in that they suggest that even in those selected to undergo hs-cTnT testing, maximum hs-cTnT concentrations are modest and conditions such as acute MI or myocarditis are infrequent. Third, not all patients had serial measurements available for analyses. Prospective studies with systematic cTn measurements across all COVID-19 patients are needed; the latter could provide insights about the timing of increases and its relationship with outcomes. Fourth, our findings are specific to the hs-cTnT assay evaluated and studies are needed using other assays. Fifth, potential for misclassification between myocardial injury and infarction exists when adjudicating following the UDMI; our results, however, are unlikely to be impacted given that most increases are due to myocardial injury. Sixth, while postdischarge follow-up was available in most patients (93%), there is a small subset of patients for whom follow-up was not available. Last, given study/event size, multivariate modeling and diagnostic performance analyses are limited, with larger studies needed to validate our findings.
Conclusions
Myocardial injury is frequent in COVID-19 patients undergoing hs-cTnT measurement and associated with adverse outcomes. While most hs-cTnT increases are modest and due to isolated myocardial injury, they have significant prognostic implications, with both baseline and maximum hs-cTnT concentrations associated with outcomes. To facilitate triage of COVID-19 patients, a single hs-cTnT <6 ng/L at presentation may help identify patients with a favorable prognosis.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Nonstandard Abbreviations:
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- US
United States
- cTn
cardiac troponin
- URL
upper-reference limit
- MI
myocardial infarction
- hs
high sensitivity
- UDMI
Fourth Universal Definition of Myocardial Infarction
- hs-cTnT
high sensitivity cardiac troponin T
- PCR
polymerase chain reaction
- ED
emergency department; REDCap, Research Electronic Data Capture
- NEWS
National Early Warning Score
- ICU
intensive care unit
- ARDS
acute respiratory distress syndrome
- LoQ
limit of quantification
- IQR
interquartile range
- HR
hazard ratios
- CI
confidence interval
- CAD
coronary artery disease; NPV, negative predictive value; PPV, positive predictive value
- CT
computed tomography
- ECG
electrocardiogram
- OR
odds ratio
- LOS
length of stay
- COPD
chronic obstructive pulmonary disease
- HIV
human immunodeficiency virus
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- ARNI
angiotensin receptor–neprilysin inhibitor
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
R.A. Mehta, statistical analysis; A.S. Jaffe, financial support, administrative support.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
A.S. Jaffe, Abbott, Siemens, Roche, Beckman-Coulter, Radiometer, ET Healthcare, Sphingotec, Medscape, Amgen, Novartis; Y. Sandoval, Advisory Boards for Roche Diagnostics and Abbott Diagnostics without personal compensation and speaker without personal financial compensation for Abbott Diagnostics.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
This publication was made possible in part by the Mayo Clinic CTSA through grant number UL1TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
Expert Testimony
None declared.
Patents
None declared.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University (JHU). https://coronaravirus.jhu.edu (Accessed January 2021).
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Mount Sinai COVID informatics center. prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020;41:2070–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, et al. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol 2020;5:1274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raad M, Dabbagh M, Gorgis S, Yan J, Chehab O, Dagher C, et al. Cardiac injury patterns and inpatient outcomes among patients admitted with COVID-19. Am J Cardiol 2020;133:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandoval Y, Januzzi JL Jr, Jaffe AS.. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC Review Topic of the Week. J Am Coll Cardiol 2020;76:1244–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD.. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 11. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He XW, Lai JS, Cheng J, Wang MW, Liu YJ, Xiao ZC, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:456–60. [DOI] [PubMed] [Google Scholar]
- 14. Sandoval Y, Smith SW, Apple FS.. Present and future of cardiac troponin in clinical practice: a paradigm shift to high-sensitivity assays. Am J Med 2016;129:354–65. [DOI] [PubMed] [Google Scholar]
- 15. Chapman AR, Bularga A, Mills NL.. High-sensitivity cardiac troponin can be an ally in the fight against COVID-19. Circulation 2020;141:1733–5. [DOI] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith GB, Prytherch DR, Meredith P, Schmidt PE, Featherstone PI.. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation 2013;84:465–70. [DOI] [PubMed] [Google Scholar]
- 18. Sandoval Y, Jaffe AS.. Using high-sensitivity cardiac Troponin T for acute cardiac care. Am J Med 2017;130:1358–65.e1. [DOI] [PubMed] [Google Scholar]
- 19. Sandoval Y, Askew JW 3rd, Newman JS, Clements CM, Grube ED, Ola O, et al. Implementing high-sensitivity cardiac troponin T in a US regional healthcare system. Circulation 2020;141:1937–9. [DOI] [PubMed] [Google Scholar]
- 20. Sandoval Y, Jaffe AS.. Type 2 myocardial infarction: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:1846–60. [DOI] [PubMed] [Google Scholar]
- 21. Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol 2020;76:2043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smilowitz NR, Jethani N, Chen J, Aphinyanaphongs Y, Zhang R, Dogra S, et al. Myocardial injury in adults hospitalized with COVID-19. Circulation 2020;142:2393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poterucha TJ, Elias P, Jain SS, Sayer G, Redfors B, Burkhoff D, et al. Admission cardiac diagnostic testing with electrocardiography and troponin measurement prognosticates increased 30-day mortality in COVID-19. J Am Heart Assoc 2021;10:e018476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart 2020;106:1154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.