The National Institutes of Health Science of Behavior Change Common Fund Program has accelerated the investigation of mechanisms of behavior change applicable to multiple health behaviors and outcomes and facilitated the use of the experimental medicine approach to behavior change research. This commentary provides a brief background of the program, plans for its next phase, and thoughts about how the experimental medicine approach to behavior change research can inform future directions in two areas of science—reproductive health and COVID-19 vaccine uptake. The incorporation of a mechanisms-based approach into behavior intervention research offers new opportunities for improving health.
Keywords: Science of behavior change, Experimental medicine approach, Mechanisms of behavior change, Reproductive health, COVID-19 vaccine uptake, NIH
Abstract
Background
The National Institutes of Health Science of Behavior Change Common Fund Program has accelerated the investigation of mechanisms of behavior change applicable to multiple health behaviors and outcomes and facilitated the use of the experimental medicine approach to behavior change research.
Purpose
This commentary provides a brief background of the program, plans for its next phase, and thoughts about how the experimental medicine approach to behavior change research can inform future directions in two areas of science—reproductive health and COVID-19 vaccine uptake.
Conclusions
The incorporation of a mechanisms-based approach into behavior intervention research offers new opportunities for improving health.
Implications.
Practice: Systematic mechanisms-focused behavior change research informs and enables the development and implementation of potent real-world behavioral interventions in clinical practice to optimize a variety of health outcomes.
Policy: Conducting systematic research to understand the mechanisms underlying behavior change across multiple health behaviors can inform effective public health policies.
Research: Systematic mechanisms-focused behavior change research should be employed in areas not yet explored by the NIH Science of Behavior Change Program, such as reproductive health and COVID-19 vaccine update.
Introduction
The National Institutes of Health (NIH) Science of Behavior Change (SOBC) Common Fund Program was established to capitalize on emerging basic behavioral science research and existing evidence-based interventions to improve the initiation, personalization, and maintenance of healthful behaviors [1]. The goals for the SOBC Program are to: (a) unify the science of behavior change through a focus on mechanisms of behavior change and by strengthening links between basic and applied behavior change science; (b) strengthen behavioral intervention development by implementing the experimental medicine approach to behavior change research and developing tools and measures to facilitate such an approach; and (c) increase rigor, transparency, and dissemination of common terminology, methods, and measures to advance the field of behavior change research.
The experimental medicine approach to behavior change research
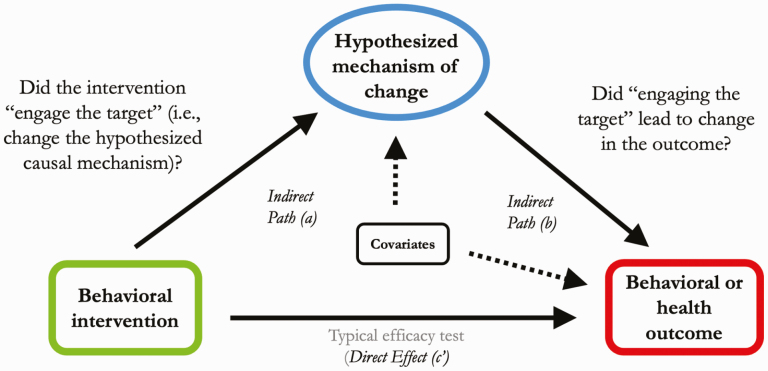
The experimental medicine approach to behavior change research seeks to understand the underlying mechanisms that drive behavior change. The SOBC Program supports and facilitates behavior change research driven by hypotheses of specific malleable target mechanisms or processes, which, if engaged, can lead to changes in the desired health outcome (Fig. 1). A mechanisms-focused approach to behavior change research can help identify the essential components of behavioral interventions, which is crucial for translation, dissemination, and replication of behavior change interventions with fidelity. The steps in the experimental medicine approach include (a) identifying potential malleable targets; (b) leveraging existing or developing new experimental manipulations or interventions to engage the identified targets; (c) identifying or developing measures to permit verification of target engagement; and (d) examining the degree to which target engagement produces a change in the health behavior outcome of interest [2–6].
Fig 1|.
Experimental medicine approach to behavior change research.
Leveraging SOBC insights to inform future research
SOBC-funded projects have used an experimental medicine approach to investigate a variety of mechanistic targets and health behaviors across the domains of self-regulation, interpersonal and social processes, and stress resilience and stress reactivity, with links to health outcomes relevant to multiple NIH Institutes and Centers. These health outcomes include, for example, cardiovascular health [7, 8], sleep health [9], stress and physical activity [10], opioid use disorder [11], obesity [12–14], prediabetes and diabetes management [15, 16], smoking cessation [17], and breast cancer survivorship among African American women [18]. Two areas of research not addressed by current SOBC-funded projects provide promising opportunities to apply a mechanisms-focused approach to behavior change research: reproductive health and COVID-19 vaccine uptake. We first describe how insights learned from SOBC-funded research can inform behavior change research and intervention development in the area of reproductive health. We then elucidate the experimental medicine approach steps as they could be applied to a new behavior change challenge—COVID-19 vaccine uptake.
PROMISING NEW DIRECTIONS
Reproductive health
The NIH mission includes support for research “in the processes of human growth and development” [19]. As for all living organisms, reproduction is a central activity in these domains. More than 25 years ago, delegates to the United Nations’ International Conference on Population and Development in Cairo declared that reproductive health is a fundamental human right [20]. Beyond these deeply significant principles of biology and policy, reproductive health has nearly universal significance, as 99% of American women who have ever had sexual intercourse have used at least one contraceptive method, and more than half of all women of reproductive age are currently using some type of contraception [21]. Poor reproductive health outcomes can be as dire as any disease or condition. Even in the 21st century, infant mortality remains a critical issue in the United States, which has the highest rate among developed nations, and poor infant mortality rates have been linked to a lack of preconception and interpartum care [22].
Reproductive health covers a range of human activity from puberty through menopause and andropause. It includes both preventing and facilitating childbearing as well as the prevention of sexually transmitted infections. A particular challenge in this field is that many of these activities involve healthy people trying to stay healthy. Individuals whose behaviors might but are not yet creating a recognized health problem are likely to be in the precontemplation phase where they are unlikely to participate in preventive behavior change [23]. Additionally, there is often ambivalence about goals such as pregnancy, which leads to inaction or lack of adherence to recommendations for healthful behaviors in anticipation of becoming pregnant [24] or, conversely, medical regimen adherence or device used for avoiding pregnancy [25]. Nearly half of pregnancies to American women in 2011 were unintended [26].
One of the current challenges in contraceptive behavior is one of utilization, not access—effective technologies (e.g., drugs, devices) are available but methods require varying degrees of user adherence to a prescribed regimen. Contraceptive method effectiveness is directly correlated with the degree of user action required [27]. Nevertheless, reversibility is highly valued by users along with effectiveness; therefore, research that informs better adherence of user-dependent methods would advance public health. An extensive body of research on the use of individual theories of behavior change for interventions to improve contraceptive use, particularly studies using a social cognition approach, developed through the 1990s and first decade of the 2000s (e.g., theory of reasoned action). Overall, findings have been inconsistent [28]. Several SOBC-funded projects focused on the imperfections of decision-making processes and may provide insights for new directions to sort through these inconsistencies.
Stress resilience and stress reactivity
Haushofer et al. proposed three targets that may be impacted by stress and consequently change adherence to preventive health practices: self-efficacy, executive control, and temporal discounting [29]. Self-efficacy is the belief that one can perform well enough in a specific situation to produce one’s intended outcome [30]. It has been shown to be strongly related to medical regimen adherence and other health behaviors [31]. For the related area of sexually transmitted infection treatment, Esopo et al. cite a meta-analysis of 207 studies of HIV antiretroviral therapy adherence, which concluded that interventions to boost adherence should target psychological factors such as self-efficacy, as well as beliefs about the efficacy and safety of the medications [32]. Likewise, executive function is positively associated with HIV medication adherence [33].
Temporal discounting leads individuals to assign greater value to a reward nearer in time than one more distant. Thus, a user of a contraceptive method that requires them to pause in the middle of a sexual encounter to insert a device may choose instead to continue with the immediate reward of the encounter. Along similar lines, the behavioral economics principle of loss aversion—preference for avoiding losses over acquiring gains—may lead an individual to avoid interrupting the encounter if they fear that in response their partner may end it.
In an effort to target and measure changes in temporal discounting as a mechanism of behavior change, Haushofer and colleagues used an intervention analogous to a new approach in clinical care: reproductive life planning. The participants visualized the outcomes of different behavioral options and pretended to speak deeply and emotionally with their potential future selves. The team hypothesized that making the future more vivid would reduce temporal discounting and increase self-efficacy [34]. Similarly, in reproductive life planning, the provider encourages the patient—woman or man—to think about their life goals and reproductive goals within that larger context. Together, the provider and patient consider both short- and long-term desires and then discuss contraception options that best fit those goals and timing [35]. The consideration hereof time targets temporal discounting and the focus on planning facilitates self-efficacy.
Interpersonal and social processes
Another complexity in this area is that decisions are often either dyadic or made by one person with impacts on another person who is not yet known to the first person. For example, behavior changes to improve preconception health such as smoking cessation can be taken at the individual level, although reinforcement from a partner is usually helpful. In contrast, preventing sexually transmitted infections is largely a dyadic process because sexual transmission occurs between partners. The partnership may last only as long as the act or may extend for years. This temporal dimension adds to the challenges.
The project led by Slep and Heyman focused on interpersonal processes—specifically, on coercion as a destructive force in family dynamics. Reproductive coercion in couple relationships is associated with poor adherence to healthful behavior patterns resulting in lower levels of condom use for prevention of both sexually transmitted infections and pregnancy and compromised decision-making across many aspects of family planning and reproductive health [36]. The SOBC project developed multimethod measures of coercion that can be obtained from the participant (self-report, biomarker) or a trained observer [37]. The latter type of measure could be an important tool for reproductive health clinicians such as family planning providers.
Self-regulation
Another aspect of reproductive health for which tools and findings from SOBC projects may prove useful is the developmental considerations for self-regulation investigated in a study of diabetes self-management in adolescents [38]. Self-regulation includes the capacity to control one’s thoughts, emotions, and behavior to achieve a goal, which overlaps somewhat with self-efficacy, but the emotion regulation component is unique. Developing and enacting emotion regulation skills are particularly important during adolescence [39] so this population is particularly useful for testing such targets and measures. The role of perceptions of love in sexual experiences is well-documented in the academic and lay literatures, but, overall, the mechanistic processes linking emotion and fertility-related behaviors such as contraceptive use are understudied [40].
These different types of targets—stress resilience and stress reactivity, interpersonal and social processes, and self-regulation—must all be considered within contexts and across moderators. Such a discussion is beyond our scope here. Examples include the context of global health and population characteristics such as gender, race/ethnicity, and sexual orientation. For example, the Haushofer project provides measures that were developed outside the U.S. Information and data about those assays are publicly available through the Open Science Framework (https://osf.io/pf2jh/).
COVID-19 vaccine uptake
As of November 1, 2020, the Centers for Disease Control and Prevention (CDC) reports 9,105,230 confirmed cumulative cases of COVID-19 in the United States (2,751 cases per 100,000 up from 26 per 100,000 on March 26, 2020) and 229,932 COVID-19 deaths (https://covid.cdc.gov/covid-data-tracker/#cases_casesinlast7days), which may undercount the true estimate of excess deaths in the United States due to the novel coronavirus and corresponding gaps in screening and care for other diseases and chronic conditions [41]. COVID-19 morbidity and mortality burden falls disproportionately on Black, Latinx, and Native American persons, widening existing health inequities in the United States [42]. The development and deployment of an effective vaccine is seen as one promising way to end the COVID-19 pandemic [43, 44]. As of September 2020, there are almost 200 vaccines in development, 24 of which are in various stages of clinical trials [45]. Despite the promise of vaccination, significant concern about the successful deployment of vaccination exists. Indeed, nationally representative studies suggest that 30–40% of the U.S. population are unwilling to, or unsure of whether, they will vaccinate [46, 47]. Although COVID-19 vaccination hesitancy may be a rational response, particularly among Black Americans who have reasonable medical mistrust as a result of structural inequities and history of abuses within the medical system and in medical research [48], reducing COVID-19 vaccine hesitancy and increasing vaccine uptake is critical for halting the pandemic and reducing inequities in COVID-19 complications and mortality [49, 50]. As such, reducing vaccine hesitancy is another example of how the approaches promoted in the SOBC Program (i.e., experimental medicine approach to behavior change research) can be leveraged to improve public health.
Many existing approaches to increase vaccination uptake involve developing interventions that target many constructs from health behavior theory (i.e., the “kitchen sink” approach) [51], which often result in diffuse and less efficient and effective interventions [52–54]. There is no strong evidence to support the effectiveness of any one approach for facilitating vaccine adherence, particularly among those who are vaccine hesitant [51, 55]. Indeed, existing interventions designed to reduce vaccine hesitancy by correcting misinformation, facilitating positive attitudes towards vaccination, appealing to emotions, or mandating vaccinations are often ineffective—and sometimes even backfire [56–60]. The potential for such interventions to backfire may be magnified by the increased polarization on beliefs about COVID-19 and the strength of anti-vaccination movements [61–63]. Moreover, even interventions that have been shown to be efficacious in increasing vaccination adherence in other domains are often costly, complex, and time-consuming [61, 64].
An experimental medicine approach can be leveraged to develop more potent, cost-effective, and easy-to-disseminate interventions to increase vaccine uptake. This approach would (a) identify potential intervention targets (i.e., mechanisms of action) based on research on vaccine hesitancy and acceptance in other domains and existing research and theory on COVID-19 vaccine hesitancy and acceptance; (b) conduct formative research to engage the target through experimental manipulation or intervention to examine whether targeting these mechanisms in relevant samples produces reasonable changes in measures; (c) develop measures that would capture the change in the target; (d) examine the degree to which target engagement using methods developed in Step c in relevant samples produces changes in the health behavior outcome - vaccine acceptance and adherence—in randomized controlled trials. Identifying individual and contextual moderators of the effects of targeting key mechanisms on vaccine outcomes is also important. Critically, given the disproportionate COVID-19 burden for Black, Latinx, and Native American persons [42], such studies should ensure there is adequate power to examine whether mechanisms are malleable and lead to behavior change among these groups, and should also involve adequate power to examine heterogeneity and moderators of effects within these groups [65].
Identify potential malleable targets (step a)
Preliminary research and theory on COVID-19 suggest potential mechanisms that may influence vaccine hesitancy and acceptance: misunderstanding of the pandemic [62]; beliefs about post-infection immunity [66]; trust in institutions [67]; motivated reasoning and rationalizing risk behavior [61]; and other biases in risk perception and decision making [62, 68]. Research on vaccine hesitancy and acceptance in other domains (e.g., measles, mumps, rubella [MMR], human papillomavirus, influenza) identifies additional potential mechanisms, including the belief that vaccine can cause disease or other harms [69], ambivalence about risks and benefits [70], and belief in misinformation [71].
Engage the targets (step b) and develop measures (step c)
Once mechanisms are identified, the next step in the experimental medicine approach is to identify experimental manipulations or interventions that would produce a change in the mechanisms, as well as measures to capture such change, and to use these to examine whether mechanisms are malleable. For example, self-persuasion interventions can be used to target vaccine ambivalence [72]. Consideration of future consequences has been linked to H1N1 vaccine uptake [73], and may play an important role in COVID-19 vaccination behavior. Risk perception biases can be targeted with interventions that facilitate a gist, or intuitive, sense of risk [74–76], or with interventions that translate numeric risk into images [76, 77]. Several approaches can be leveraged to reduce biases in decision making [68]. Inoculation strategies can counteract misinformation [64].
Determine whether target engagement leads to health behavior change (step d)
The final step is to examine whether producing changes in the malleable mechanisms identified as potentially underlying vaccination behavior actually lead to vaccination behavior change using randomized controlled trials. This step is comparable to traditional health behavior change research but has the advantage over such approaches because only malleable processes will be targeted, resulting in the potential for more potent interventions than the more traditional “kitchen sink” approach [52–54].
Within this last step, it is important not only to measure changes in the target mechanism, but also to identify potential moderators—for example, characteristics of individuals that would intensify or weaken the effect of a mechanism on the vaccination behavior outcome. Political ideology may be an important moderator, such that although bias can occur regardless of political ideology, the type of bias may cause any given intervention to be more or less effective [78]. People’s prior beliefs about the vaccine may be another moderator to consider, such that herd immunity may be more achievable with interventions targeting those who already have positive attitudes towards the vaccine, and interventions essentially close the intention-behavior gap among those individuals [51, 61]. Numeracy may also moderate the effects of mechanisms like disease risk, severity, and side effects perceptions on vaccination outcomes [79, 80]. Conspiracy beliefs may also modulate intervention effectiveness [81, 82]; for example, one recent study demonstrates individuals high in conspiracy beliefs may be more likely to change their health beliefs in response to misinformation inoculation, whereas individuals low in conspiracy beliefs may not [83]. Moderators related to the information landscape may also be relevant for consideration; for example, research suggests that anti-conspiracy intervention may be effective only prior to misinformation [84]. Identifying moderators is a critical step in developing targeted, “precision medicine” interventions [85].
Many of the hurdles to vaccine uptake, particularly in underserved communities, could require organizational behavior change at the institutional or community level [86, 87]. Systematic exploration of the most effective organizational interventions, such as by incentivizing collaboration and public-private partnerships to improve vaccine access and uptake, is equally important.
Conclusion
The mechanisms-based experimental medicine approach to the development of therapeutic and preventive interventions has a long history in academia and the commercial sector. Drug development relies on determining innovative ways to interrupt or accentuate pathways that control or prevent disease development, progression, and disability. Regulatory agencies review pre-clinical studies of the effect of proposed agents on disease pathways. Purely empiric drug studies are not common; outcomes of clinical studies frequently include determination of the effect of agents on biomarkers or intermediates in relevant disease pathways. In contrast, behavior change research intervention studies have not regularly focused on measuring the effect on known mechanisms underlying the targeted behavior along with the effect on the behavior itself.
The SOBC research community has developed an impressive collection of measures of underlying behavior mechanisms (https://scienceofbehaviorchange.org/measures/). Although additional work is needed to refine and validate these and other measures, the incorporation of a mechanisms-based approach supported by basic behavior research into behavior intervention research offers new opportunities to improve health. The NIH’s continued facilitation of a mechanisms-based approach to behavior change research, in part through its collective support of the renewal of the SOBC Research and Coordinating Center (2U24AG052175-06), and the application of the experimental medicine approach in future research to the development of novel behavioral interventions, such as described here for reproductive health and COVID-19 vaccine hesitancy, will inform strategies to improve the health of individuals and our communities.
Acknowledgements
The authors acknowledge contributions from members of the trans-NIH SOBC Implementation Team, including Will M. Aklin (NIDA), Christine Hunter (OBSSR), Melissa Riddle (NIDCR), and Luke Stoeckel (NIA).
Compliance with Ethical Standards
Funding Sources: Not applicable.
Conflicts of Interest: Chandra Keller, Rebecca Ferrer, Rosalind B. King, and Elaine Collier declare that they have no conflicts of interest.
Human Rights: This article does not contain any studies with human participants performed by any of the authors.
Informed Consent: This study does not involve human participants and informed consent was therefore not required.
Welfare of Animals: This article does not contain any studies with animals performed by any of the authors.
References
- 1. National Institutes of Health. National Institutes of Health Science of Behavior Change Common Fund Program. Available at https://commonfund.nih.gov/behaviorchange (Accessibility verified March 1, 2021).
- 2. Riddle M, Ferrer R. “The Science of Behavior Change,” APS Observer, no. October 30, 2015. Available at https://www.psychologicalscience.org/observer/the-science-of-behavior-change (Accessibility verified March 1, 2021).
- 3. Riddle M; Science of Behavior Change Working Group . News from the NIH: using an experimental medicine approach to facilitate translational research. Transl Behav Med. 2015;5(4):486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aklin WM, Stoeckel LE, Green PA, et al. Commentary: National Institutes of Health (NIH) Science of Behavior Change (SOBC). Health Psychol Rev. 2020;14(1):193–198. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen L, Riddle M, King JW, et al. ; NIH Science of Behavior Change Implementation Team . The NIH Science of behavior change program: transforming the science through a focus on mechanisms of change. Behav Res Ther. 2018;101:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M. Reenvisioning clinical science: unifying the discipline to improve the public health. Clin Psychol Sci. 2014;2(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loucks EB, Nardi WR, Gutman R, et al. Mindfulness-based blood pressure reduction (MB-BP): stage 1 single-arm clinical trial. PLoS One. 2019;14(11):e0223095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birk JL, Cumella R, Lopez-Veneros D, et al. Intervening on fear after acute cardiac events: rationale and design of the INFORM randomized clinical trial. Health Psychology. 2020;39(9):736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brewer JA, Roy A, Deluty A, Liu T, Hoge EA. Can mindfulness mechanistically target worry to improve sleep disturbances? Theory and study protocol for app-based anxiety program. Health Psychol. 2020;39(9):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Almeida DM, Marcusson-Clavertz D, Conroy DE, et al. Everyday stress components and physical activity: examining reactivity, recovery and pileup. J Behav Med. 2020;43(1):108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McHugh RK, Nguyen MD, Fitzmaurice GM, Dillon DG. Behavioral strategies to reduce stress reactivity in opioid use disorder: study design. Health Psychol. 2020;39(9):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lv N, Ajilore OA, Ronneberg CR, et al. The ENGAGE-2 study: engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes in a randomized controlled trial (Phase 2). Contemp Clin Trials. 2020;95:106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosas LG, Azar KMJ, Lv N, et al. Effect of an Intervention for obesity and depression on patient-centered outcomes: an RCT. Am J Prev Med. 2020;58(4):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leahey TM, Gorin AA, Wyckoff E, et al. Episodic future thinking, delay discounting, and exercise during weight loss maintenance: the PACE trial. Health Psychol. 2020;39(9):796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller AL, Lo SL, Albright D, et al. Adolescent interventions to manage self-regulation in type 1 diabetes (AIMS-T1D): randomized control trial study protocol. BMC Pediatrics. 2020;20(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bickel WK, Stein JS, Paluch RA, et al. Does episodic future thinking repair immediacy bias at home and in the laboratory in patients with prediabetes? Psychosom Med. 2020;82(7):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otto MW, Zvolensky MJ, Roenfield D, et al. A randomized controlled trial protocol for engaging distress tolerance and working memory to aid smoking cessation in low socioeconomic status (SES) adults. Health Psychol. 2020;39(9):815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halbert CH, Jefferson MS, Danielson C, Froeliger B, Giordano A, Thaxton JE. An observational study and randomized trial of stress reactivity in cancer disparities. Health Psychol. 2020;39(9):745–757. [DOI] [PubMed] [Google Scholar]
- 19. NIH Mission and Goals. National Institutes of Health. Available at https://www.nih.gov/about-nih/what-we-do/mission-goals. (Accessibility verified March 1, 2021).
- 20.International Conference on Population and Development Programme of Action, in “Twentieth Anniversary Edition,” United Nations, Cairo, Report 978-0-89714-022-5, 2014. Available at https://www.unfpa.org/sites/default/files/pub-pdf/programme_of_action_Web%20ENGLISH.pdf.
- 21. Guttmacher Institute. Contraceptive use in the United States Fact Sheet. Available at https://www.guttmacher.org/fact-sheet/contraceptive-use-united-states. (Accessibility verified March 1, 2021).
- 22. Zeeck E. Infant mortality linked to lack of preconception and interconception care, in ASTHO Experts Blog, Association of State and Territorial Health Officials, 2016. [Google Scholar]
- 23. Prochaska JO, DiClemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychother: Theory Res Pract. 1982;19(3):276–288. [Google Scholar]
- 24. Stephenson J, Heslehurst N, Hall J, et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet. 2018;391(10132):1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaCross A, Smaldone A, Angelson J. Ambivalence toward pregnancy as an indicator for contraceptive nonuse: a systematic review and meta-analysis. J Midwifery Womens Health. 2019;64(4):385–394. [DOI] [PubMed] [Google Scholar]
- 26. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sundaram A, Vaughan B, Kost K, et al. Contraceptive failure in the United States: estimates from the 2006–2010 National Survey of Family Growth. Perspect Sex Reprod Health. 2017;49(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopez LM, Tolley EE, Grimes DA, Chen-Mok M. Theory-based strategies for improving contraceptive use: a systematic review. Contraception. 2009;79(6):411–417. [DOI] [PubMed] [Google Scholar]
- 29. Esopo K, Mellow D, Thomas C, et al. Measuring self-efficacy, executive function, and temporal discounting in Kenya. Behav Res Ther. 2018;101:30–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. [DOI] [PubMed] [Google Scholar]
- 31. Náfrádi L, Nakamoto K, Schulz PJ. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One. 2017;12(10):e0186458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med. 2014;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Solomon TM, Halkitis PN. Cognitive executive functioning in relation to HIV medication adherence among gay, bisexual, and other men who have sex with men. AIDS Behav. 2008;12(1):68–77. [DOI] [PubMed] [Google Scholar]
- 34. Haushofer J, John A, Orkin K. Can simple psychological interventions increase preventive health investment?. Natl Bureau of Econ Res. 2019. Available at https://www.nber.org/papers/w25731 (Accessibility verified March 22, 2021). [Google Scholar]
- 35. Morse JE, Moos MK. Reproductive life planning: raising the questions. Matern Child Health J. 2018;22(4):439–444. [DOI] [PubMed] [Google Scholar]
- 36. Miller E, Jordan B, Levenson R, Silverman JG. Reproductive coercion: connecting the dots between partner violence and unintended pregnancy. Contraception. 2010;81(6):457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith Slep AM, Heyman RE, Mitnick DM, Lorber MF, Beauchaine TP. Targeting couple and parent-child coercion to improve health behaviors. Behav Res Ther. 2018;101:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller AL, Lo SL, Albright D, et al. Adolescent Interventions to Manage Self-Regulation in Type 1 Diabetes (AIMS-T1D): randomized control trial study protocol. BMC Pediatr. 2020;20(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Dev. 2003;74(6):1869–1880. [DOI] [PubMed] [Google Scholar]
- 40. Axinn WG, Ghimire DJ, Smith-Greenaway E. Emotional variation and fertility behavior. Demography. 2017;54(2):437–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess deaths from COVID-19 and other causes, March-July 2020. JAMA. 2020;324(15):1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laurencin CT, McClinton A. The COVID-19 Pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7(3):398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Denworth L. How the COVID-19 pandemic could end. Scientific American. Available at https://www.scientificamerican.com/article/how-the-covid-19-pandemic-could-end1/.( Accessibility verified June 1, 2020). [Google Scholar]
- 44. Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368(6494):948–950. [DOI] [PubMed] [Google Scholar]
- 45. World Health Organization. Draft landscape of COVID-19 candidate vaccines. Available at https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. (Accessibility verified October 19, 2020).
- 46. Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 vaccine: a Survey of U.S. Adults. Ann Intern Med. 2020;173(12):964–973. doi: 10.7326/M20-3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O’Keefe SM. One in three Americans would not get COVID-19 vaccine. Available at https://news.gallup.com/poll/317018/one-three-americans-not-covid-vaccine.aspx. (Accessibility verified August 7, 2020).
- 48. Bogart LM, Ojikutu BO, Tyagi K, et al. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among Black Americans Living with HIV. J Acquir Immune Defic Syndr. 2021;86(2):200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor S, Landry CA, Paluszek MM, Groenewoud R, Rachor GS, Asmundson GJG. A proactive approach for managing COVID-19: the importance of understanding the motivational roots of vaccination hesitancy for SARS-CoV2. Front Psychol. 2020;11(2890):575950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. French J, Deshpande S, Evans W, Obregon R. Key guidelines in developing a Pre-Emptive COVID-19 vaccination uptake promotion strategy. Int J Environ Res Public Health. 2020;17(16):5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149–207. [DOI] [PubMed] [Google Scholar]
- 52. Naar S, Czajkowski SM, Spring B. Innovative study designs and methods for optimizing and implementing behavioral interventions to improve health. Health Psychol. 2018;37(12):1081–1091. [DOI] [PubMed] [Google Scholar]
- 53. Noar SM. A 10-year retrospective of research in health mass media campaigns: where do we go from here?. J Health Commun. 2006;11(1):21–42. [DOI] [PubMed] [Google Scholar]
- 54. Suls JM, Luger T, Martin R. The biopsychosocial model and the use of theory in health psychology. In: Suls JM, Davidson KW, Kaplan RM eds. Handbook of Health Psychology and Behavioral Medicine. The Guilford Press; 2010:15–27. [Google Scholar]
- 55. Dubé E, Gagnon D, MacDonald NE. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33(34):4191–4203. [DOI] [PubMed] [Google Scholar]
- 56. Betsch C, Sachse K. Debunking vaccination myths: strong risk negations can increase perceived vaccination risks. Health Psychol. 2013;32(2):146–155. [DOI] [PubMed] [Google Scholar]
- 57. Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4):e835–e842. [DOI] [PubMed] [Google Scholar]
- 58. Omer SB, Betsch C, Leask J. Mandate vaccination with care. Nature. 2019;571(7766):469–472. [DOI] [PubMed] [Google Scholar]
- 59. Pluviano S, Watt C, Della Sala S. Misinformation lingers in memory: failure of three pro-vaccination strategies. PLoS One. 2017;12(7):e0181640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pluviano S, Watt C, Ragazzini G, Della Sala S. Parents’ beliefs in misinformation about vaccines are strengthened by pro-vaccine campaigns. Cogn Process. 2019;20(3):325–331. [DOI] [PubMed] [Google Scholar]
- 61. Buttenheim AM. SARS-CoV-2 vaccine acceptance: we may need to choose our battles. Ann Intern Med. 2020:M20-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mello MM, Greene JA, Sharfstein JM. Attacks on public health officials during COVID-19. JAMA. 2020;324(8):741–742. [DOI] [PubMed] [Google Scholar]
- 63. Schmidt AL, Zollo F, Scala A, Betsch C, Quattrociocchi W. Polarization of the vaccination debate on Facebook. Vaccine. 2018;36(25):3606–3612. [DOI] [PubMed] [Google Scholar]
- 64. Basol M, Roozenbeek J, van der Linden S. Good news about bad news: Gamified inoculation boosts confidence and cognitive immunity against fake news. J Cogn. 2020;3(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whitfield KE, Allaire JC, Belue R, Edwards CL. Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? J Gerontol B Psychol Sci Soc Sci. 2008;63(5):P301–P308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hallsworth M, Buttenheim AM. Challenges facing a COVID-19 vaccine: a behavioral science perspective. Behav Scientist. 2020. Available at https://behavioralscientist.org/challenges-facing-a-covid-19-vaccine-a-behavioral-science-perspective/ (Accessibility verified March 1, 2021). [Google Scholar]
- 67. Bavel JJV, Baicker K, Boggio PS, et al. Using social and behavioural science to support COVID-19 pandemic response. Nat Hum Behav. 2020;4(5):460–471. [DOI] [PubMed] [Google Scholar]
- 68. Halpern SD, Truog RD, Miller FG. Cognitive bias and Public Health Policy during the COVID-19 pandemic. JAMA. 2020;324(4):337–338. [DOI] [PubMed] [Google Scholar]
- 69. Reyna VF. Risk perception and communication in vaccination decisions: a fuzzy-trace theory approach. Vaccine. 2012;30(25):3790–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thompson EL, Rosen BL, Vamos CA, Kadono M, Daley EM. Human Papillomavirus vaccination: what are the reasons for nonvaccination among U.S. Adolescents? J Adolesc Health. 2017;61(3):288–293. [DOI] [PubMed] [Google Scholar]
- 71. Broadbent JJ. Vaccine hesitancy: misinformation on social media. BMJ. 2019;366:l4457. [DOI] [PubMed] [Google Scholar]
- 72. Baldwin AS, Denman DC, Sala M, et al. Translating self-persuasion into an adolescent HPV vaccine promotion intervention for parents attending safety-net clinics. Patient Educ Couns. 2017;100(4):736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nan X, Kim J. Predicting H1N1 vaccine uptake and H1N1-related health beliefs: the role of individual difference in consideration of future consequences. J Health Commun. 2014;19(3):376–88. [DOI] [PubMed] [Google Scholar]
- 74. Klein WMP, Ferrer RA, Kaufman AR. How (or do) people “think” about cancer risk, and why that matters. JAMA Oncol. 2020;6(7):983–984. [DOI] [PubMed] [Google Scholar]
- 75. Reyna VF. Of viruses, vaccines, and variability: qualitative meaning matters. Trends Cogn Sci. 2020;24(9):672–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Reyna VF. A scientific theory of gist communication and misinformation resistance, with implications for health, education, and policy. Proceedings of the National Academy of Sciences; April 2020. doi: 10.1073/pnas.1912441117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics?. Med Decis Making. 2005;25(4):398–405. [DOI] [PubMed] [Google Scholar]
- 78. Ditto PH, Liu BS, Clark CJ, et al. At least bias is Bipartisan: a meta-analytic comparison of partisan bias in liberals and conservatives. Perspect Psychol Sci. 2019;14(2):273–291. [DOI] [PubMed] [Google Scholar]
- 79. Peters E. Innumeracy in The Wild: Misunderstanding and Misusing Numbers. 1st ed. New York, NY: Oxford University Press; 2020. [Google Scholar]
- 80. Reyna VF, Brust-Renck PG. How representations of number and numeracy predict decision paradoxes: a fuzzy-trace theory approach. J Behav Decis Making. 2020;33:606–628. [Google Scholar]
- 81. Jolley D, Douglas KM. The effects of anti-vaccine conspiracy theories on vaccination intentions. PLoS One. 2014;9(2):e89177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oliver JE, Wood T. Medical conspiracy theories and health behaviors in the United States. JAMA Intern Med. 2014;174(5):817–818. [DOI] [PubMed] [Google Scholar]
- 83. Iles IA, Gillman AS, Klein WMP, Ferrer RA. Investigating the role of psychological inoculation in neutralizing health misinformation effects among individuals with strong conspiratorial beliefs. In preparation.
- 84. Jolley D, Douglas KM. Prevention is better than cure: addressing anti-vaccine conspiracy theories. J Appl Soc Psychol. 2017;47(8):459–469. [Google Scholar]
- 85. Hekler E, Tiro JA, Hunter CM, Nebeker C. Precision health: the role of the social and behavioral sciences in advancing the vision. Ann Behav Med. 2020;54(11):805–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hinnant L, Mednick S. Vaccine storage demands could leave 3B people in virus cold. The Washington Post. 2020. Available at https://apnews.com/article/virus-outbreak-burkina-faso-immunizations-united-nations-7f5526f46ce5e15ba8bb51fe62569ee6 (Accessibility verified March 1, 2021). [Google Scholar]
- 87. Lowe D. Cold chain (and colder chain) distribution. Sci Transl Med. 2020. Available at https://blogs.sciencemag.org/pipeline/archives/2020/08/31/cold-chain-and-colder-chain-distribution (Accessibility verified March 1, 2021). [Google Scholar]