Abstract
Background
The objective of this study was to examine the aggregate rates of antibiotic use at the population level and compare these rates over time against historical averages to identify the effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting control measures on community prescribing.
Methods
We collected antibiotic prescriptions and physician office visits from January 1, 2016, to July 21, 2020. We calculated monthly prescription rates stratified by sex, age group, profession, diagnosis type, and antibiotic class. We looked at monthly prescription rate as a moving average over time. Using the interrupted time series analysis method, we estimated the changes in prescription rates after March 2020.
Results
The moving average of overall monthly prescription rates during January–June 2020 was below the minimum of the historical years’ moving averages (2016–2019). We observed a >30% reduction in overall monthly prescription rates in April, May, and July of 2020 compared with the same months of 2019. We observed that overall monthly prescription rates experienced a significant level change of –12.79 (P < .001) during the coronavirus disease 2019 pandemic after March 2020, with the greatest level change being –18.02 among children 1–4 years of age (P < .001). We estimated an average –5.94 (P < .001) change in respiratory tract infection (RTI)–associated monthly prescription rates after March 2020. Overall prescription rates comparing January–July 2019 and their 2020 counterparts showed a decrease in monthly prescribing ranging from –1 to –5 for amoxicillin, amoxicillin and enzyme inhibitors, azithromycin, clarithromycin, and sulfamethoxazole.
Conclusions
In British Columbia, Canada, overall and RTI-specific monthly antibiotic prescription rates declined significantly during April–July 2020 compared with the same months in prepandemic years.
Keywords: antibiotics, Canada, COVID-19, pandemic, SARS-CoV-2
The impact of the emergence and subsequent pandemic escalation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on health care has been unequivocal. The pandemic has drastically altered modes of health care delivery and access to care. Outpatient care has had to adapt swiftly to diverse limitations and new societal norms imposed by SARS-CoV-2. As public health measures called for social distancing and much of the public sphere abruptly transferred home, telehealth became increasingly utilized in Canada, and globally, to ensure continuity of care for outpatient illness. However, a key limitation underlying this route of care is the physician’s inability to perform a thorough physical examination of the patient.
The potential impact of telehealth on quality of care is especially a concern with respect to the use of antibiotics in the outpatient setting. With the World Health Organization (WHO) officially declaring antimicrobial resistance (AMR) a global health crisis in 2015, outpatient antibiotic prescribing has been increasingly scrutinized for both quantity and quality of use [1–4]. The overuse of antibiotics in the treatment of potential co-infections in coronavirus disease 2019 (COVID-19) inpatients has been well documented in the current literature [5–9]. Despite WHO guidelines supporting the absence of antibiotic use and/or prophylaxis in mild or moderately symptomatic COVID-19 patients [10], recent studies have shown that prescribing has well exceeded historical averages of use [5–9]. These studies, however, have been overwhelmingly targeted to the inpatient setting and COVID-19 patients specifically.
With respect to the current global context of SARS-CoV-2, both the short-term effects on community prescribing and the long-term implications for resistance are still unknown [11]. Prepandemic studies have demonstrated higher rates of suboptimal antibiotic use in direct-to-consumer modes of health care delivery, like telehealth [12–14]. However, given the reduced opportunities for transmission of pathogens as communities continue to isolate, as well as increased measures of infection prevention and control at the individual level (ie, hand washing, usage of nonmedical masks), the direction of effect of SARS-CoV-2 upon the use of antibiotics in the outpatient setting remains a question. The purpose of this study was to examine the rates of prescribing of outpatient antibiotics in British Columbia, Canada, to determine the effects of SARS-CoV-2 on antibiotic prescribing. Using a retrospective cohort design, the goals of this research are 2-fold: first, to examine the aggregate rates of antibiotic use at the population level and, second, to compare these rates over time against historical averages in order to identify the effects of SARS-CoV-2 and the resulting control measures on community prescribing.
METHODS
Antibiotic prescription data from January 1, 2016, to July 30, 2020, were extracted from BC PharmaNet, a centralized database that contains information on all prescriptions dispensed from community pharmacies (with the exception of some medications used for HIV and sexually transmitted infection [STI] treatment) for all residents of British Columbia [15]. We also collected data on physician office visits from the Medical Service Plan (MSP), an outpatient billing system. Indications of interest were identified using diagnostic codes from the ninth revision of the International Classification of Diseases (ICD-9) [16]. We have not used ICD-10, as the outpatient billing system in British Columbia is still using ICD-9 diagnostic codes. Both PharmaNet and MSP data were extracted by a third party within the BC Ministry of Health, and all patient identifiers were replaced with an anonymized study ID. Using the unique ID, a prescription was assigned a diagnostic code using an algorithm that matched the date on which the medication was dispensed to a practitioner service date within the 5 days prior via the office visit billing claim. If a practitioner service date was associated with more than 1 diagnostic code or multiple service dates fell within the 5-day period of a prescription dispensing date, then a hierarchy was applied to link only the most relevant diagnostic code to the prescription. Oral antibiotics were defined by the Anatomical Therapeutic Chemical (ATC) classification system developed by the WHO and included 6 major classes: tetracyclines (J01A), penicillins (J01C), cephalosporins (J01D), sulfonamides and trimethoprim (J01E), macrolides (J01F), and quinolones (J01M) [17]. Population estimates were obtained from the BC Vital Statistics database [18].
For this study, the prepandemic duration included January 2016 to December 2019 (historical 4 years), and the pandemic duration included January 2020 to July 2020. Additionally, we further delineated the antibiotic pandemic year data into 2 distinct segments: January–March 2020 and April–July 2020. In British Columbia, public health measures such as strict physical distancing, the provincial state of emergency, and business shutdowns were all mandated in mid-March 2020 [19]. This segmentation allowed for statistical analysis to observe whether prescription rates were significantly changed by the implementation of provincial public health measures.
Monthly antibiotic prescription rates per 1000 population in British Columbia between January 2016 and July 2020 were calculated. Monthly prescription rates were also stratified by sex, age group, profession, and diagnosis type. Age groups were categorized as: <1, 1–4, 5–19, 20–49, 50–64, and 65+ years. Prescription rates were calculated both for matched and overall prescriptions. Matched prescriptions were those with a successful match to an MSP service date, as defined above. The overall prescription rate included all prescriptions (matched and unmatched). We looked at the monthly prescription rate as a moving average over time, centered and calculated using a 3-month time window. This rate was calculated for January 2020–June 2020 and compared with the maximum, minimum, and the mean of the 4 years (2016–2019) of moving averages in the same months—broken down by sex, age group, profession, and diagnosis type. Prescription rates were calculated for the following diagnosis types: respiratory tract infections (RTIs), skin and soft tissue infections (SSTIs), and urinary tract infections (UTIs). For classes of antibiotics, a heat map was created to visually summarize changes in monthly prescription rates comparing 2020 with 2019 for the months January–July. Color intensity was categorized as 0, <1, 1–5, and 5+ differences in monthly prescription rate per 1000 population.
We also conducted an interrupted time series analysis (ITSA) using the Box and Tiao approach [20] to test for significant changes in prescription rates after March 2020. The ITSA model includes a transfer function (intervention coefficient) capturing the intervention and a seasonal autoregressive integrated moving average (SARIMA) error portion. The transfer function is coded as 0 for periods before March 2020, 0.5 for the month of March 2020 because of COVID-19 restrictions that began mid-month, and 1 for periods after March 2020. The ITSA model was fit for the overall, RTI, age <1 and 1–4 years, physician, and dentist prescription rates.
The structure of the SARIMA model is (p,d,q) × (P,D,Q)S, where p, d, and q are nonseasonal terms for the autoregressive order, number of differences, and the moving average order respectively. P, D, and Q are the seasonal terms for the autoregressive order, number of differences, and seasonal moving average order, respectively. S is the seasonal period, which is 12 in this study due to the annual pattern of prescription rates. We used Box and Jenkin’s SARIMA modeling methodology [21]. First, necessary seasonal and nonseasonal differencing was applied to identify potential (d) and (D) values, and the Augmented Dickey-Fuller (ADF) test was used to check for data stationary [21]. Second, the autocorrelation coefficient (ACF) and partial autocorrelation coefficient (PACF) plots were assessed to identify potential (p,q) and (P,Q) values. Third, optimal coefficients were those that minimized model AIC. This selection of the lowest AIC balances greater model fit and the use of a minimum number of model parameters [21, 22]. Fourth, using the Ljung-Box test, we checked for autocorrelation of residuals. The Ljung-Box tests for each SARIMA model yielded a P > .05, indicating no auto-correlation of residuals.
RESULTS
Overall, the monthly antibiotic prescription rate in 2020 declined starting in the month of March. During prepandemic years, the monthly prescription rate per 1000 population ranged from 39.1 to 57.8, whereas during January–July 2020, the monthly prescription rate ranged from 22.1 to 49.5 per 1000 population. We observed a >30% reduction in overall monthly prescription rates in April, May, and July of 2020 compared with the same months of 2019 (Table 1). January–March 2020 did not see significant reductions in prescription rates when compared with the same months in the previous 4 years (2016–2019); however, a significant reduction was observed in April–July 2020 when compared with the same historical prescribing rates of previous prepandemic years. The decrease in April–July 2020 in comparison with historical prescribing rates remained significant when stratified by sex and across all age groups. The results of ITSA showed that on average overall monthly provincial prescription rates experienced a significant level change of –12.79 (P < .001) after COVID-19 restrictions were put into place in March 2020, with the greatest level change, –18.02, observed among those age 1–4 years (P < .001) (Table 2).
Table 1.
Monthly Overall Antibiotic Prescription Rate From January 2016 to July 2020 and Relative Percent Change in Antibiotic Prescription Rate in British Columbia, Canada
| Year | ||||||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | ||
| Months | Prescription Rate/1000 Population | Relative Percent Change (2019 vs 2020) | ||||
| Jan | 53.0 | 57.9 | 54.3 | 50.5 | 49.6 | –1.8 |
| Feb | 52.3 | 46.9 | 44.4 | 43.2 | 44.6 | 3.5 |
| Mar | 52.0 | 51.2 | 49.0 | 48.7 | 44.5 | –8.6 |
| Apr | 46.7 | 44.4 | 43.6 | 43.3 | 30.2 | –30.1 |
| May | 44.6 | 47.8 | 43.5 | 43.2 | 29.0 | –32.8 |
| Jun | 43.4 | 43.8 | 40.9 | 39.6 | 32.1 | –18.7 |
| Jul | 40.7 | 39.7 | 39.2 | 40.8 | 22.1 | –45.8 |
| Aug | 41.5 | 40.4 | 39.1 | 39.1 | ||
| Sep | 42.8 | 41.3 | 39.9 | 40.4 | ||
| Oct | 45.9 | 45.8 | 45.1 | 46.0 | ||
| Nov | 48.3 | 45.4 | 43.9 | 43.1 | ||
| Dec | 52.5 | 48.3 | 46.7 | 46.1 | ||
Table 2.
Interrupted Time Series Analysis Showing the Monthly Prescription Rate Change After March 2020 in British Columbia, Canada
| Prescription Rate Category | Model Parameters, (p,d,q) s(P,D,Q)12 | Intervention Coefficient (95% CI) | Standard Error | P Value |
|---|---|---|---|---|
| Overall | SARIMA (0,1,1)(0,1,0)12 | –12.79 (–15.848 to –9.743) | 1.557 | <.001 |
| RTI | SARIMA (0,1,2)(0,1,0)12 | –5.94 (–8.241 to –3.648) | 1.172 | <.001 |
| <1 y | SARIMA (0,0,1)(1,1,0)12 | –10.91 (–14.286 to –7.528) | 1.724 | <.001 |
| 1–4 y | SARIMA (0,1,1)(1,1,0)12 | –18.02 (–23.080 to –12.959) | 2.582 | <.001 |
| Physician | SARIMA (0,1,1)(0,1,1)12 | –10.03 (–12.695 to –7.373) | 1.358 | <.001 |
| Dentist | SARIMA (0,1,3)(1,0,0)12 | –1.36 (–1.546 to –1.172) | 0.096 | <.001 |
Prescription rates for categories were selected for time series analysis based on the significant changes observed in the exploratory and descriptive analysis. p, d, and q are nonseasonal terms for the autoregressive order, number of differences, and moving average order, respectively. P, D, and Q are the seasonal terms for the autoregressive order, number of differences, and seasonal moving average order, respectively. S is the seasonal period, which is 12 in this study due to the annual pattern of prescription rates.
Abbreviations: RTI, respiratory tract infection; SARIMA, seasonal autoregressive integrated moving average.
When looking only at prescriptions matched to diagnoses, across all age groups monthly RTI-associated prescription rates decreased significantly during April–July 2020 compared with the same 4 months in the 4 prepandemic years. For January to March 2020, monthly RTI prescription rates decreased significantly for patients aged <1 year, 1–4 years, 50–64 years, and 65+ years. However, for UTI- and SSTI-related prescriptions, the decrease in monthly prescription rates was not observed until July 2020 (Supplementary Figure 1).
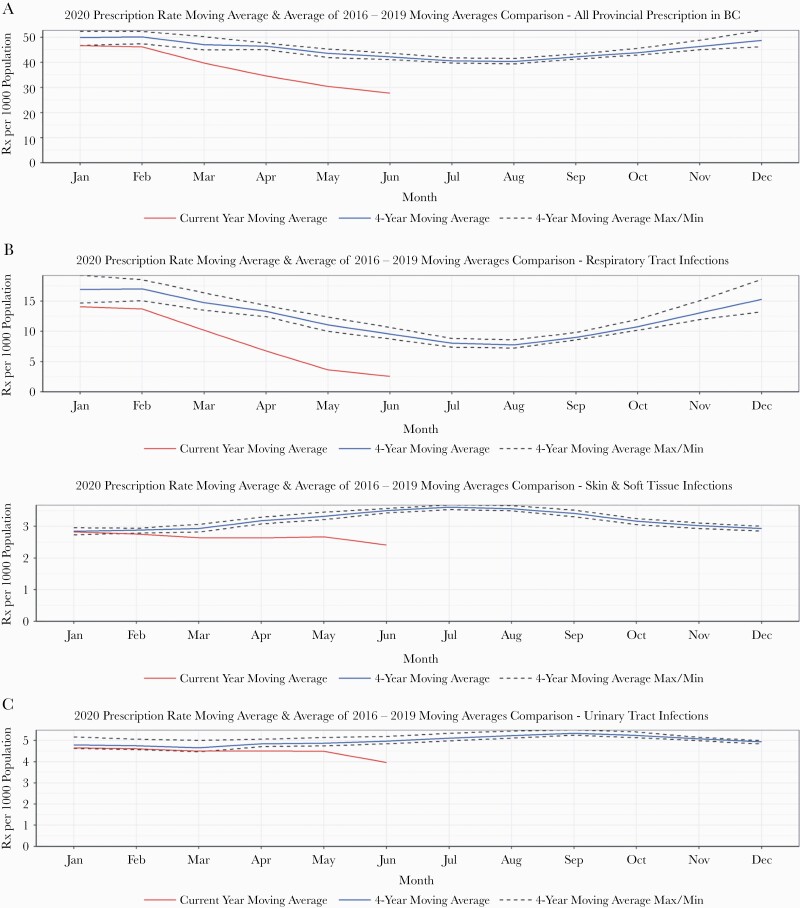
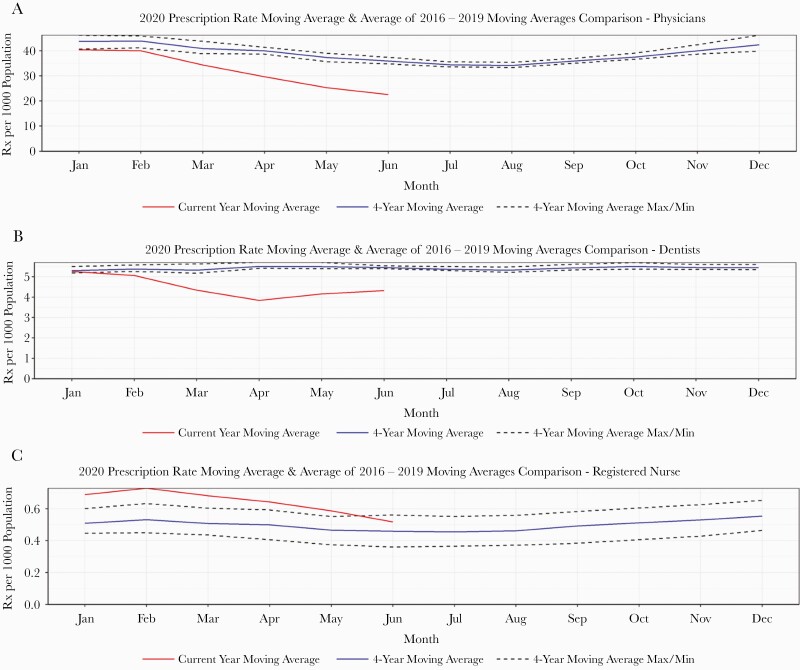
The moving average of overall monthly prescription rates during January–June 2020 was below the minimum of the prepandemic years’ moving averages (Figure 1). However, this trend was not consistent across various diagnoses: For RTI-related prescribing, the trend was very similar to the overall, with a sharp reduction in prescribing rates beginning in April 2020. We estimated an average –5.94 (P < .001) change in RTI-associated monthly prescription rates after March 2020. For UTI- and SSTI-related prescribing, we observed a moving average slightly below the minimum historical moving average, which plateaued throughout January–May 2020 and started to fall in June 2020 (Figure 1). The moving averages of overall monthly prescription rates for 2020 were very similar for males and females and across all age groups—consistently below the minimum historical moving average (Supplementary Figures 1 and 2). When looking at prescription rates by health care profession, we observed a moving average below the historical 4-year moving average for physicians, dentists, and naturopaths during March–June 2020. Although dentists’ average prescription rates were below the historical moving average, we noticed an increasing trend during May–June 2020. For midwives, registered nurses, and pharmacists, the moving average in 2020 was above the historical 4-year moving average (Figure 2). The estimated level changes in monthly prescription rates after March 2020 for physicians and dentists were –10.03 (P < .001) and –1.36 (P < .001), respectively (Table 2).
Figure 1.
Comparison of overall and diagnosis-specific moving average of prescription rates between January and June of 2016–2019 and January and June of 2020 in British Columbia, Canada.
Figure 2.
Profession-specific moving average of prescription rates comparing 2020 with 2016–2019 in British Columbia, Canada.
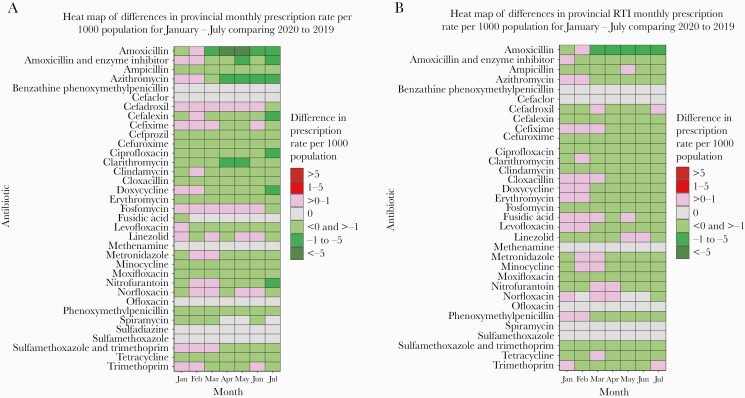
For medications of interest, a heat map of monthly overall prescription rates comparing January–July 2019 and their 2020 counterparts showed a decrease in monthly prescribing with a range of –1 to –5 prescriptions per 1000 population for amoxicillin, amoxicillin and enzyme inhibitors, azithromycin, clarithromycin and sulfamethoxazole, and trimethoprim during April and June (Figure 3). In July, monthly prescription rates decreased for cephalexin, ciprofloxacin, doxycycline, and nitrofurantoin at a range of –1 to –5 prescriptions per 1000 population. When limited to only RTI-related prescribing, amoxicillin had the greatest decrease during March to July of 2020 at a range of –1 to –5 prescriptions per 1000 population. Azithromycin, doxycycline, and levofloxacin showed a more modest decrease at a range of <0 to >–1 prescriptions per 1000 population during the months of April–July 2020.
Figure 3.
Heat map showing the overall and respiratory tract infection–specific monthly prescription rate difference during January to July comparing 2020 with 2019 in British Columbia, Canada.
DISCUSSION
To our knowledge, this is the first study of its kind both in Canada and worldwide that has reviewed the impact of the SARS-CoV-2 pandemic on antibiotic prescribing rates by age, sex, profession, diagnosis, and antibiotic type at the population level, compared with prepandemic years. Most published studies have reported overall antibiotic use during the current pandemic with a specific lens on hospitalized patients, specific health care professions like dentistry, or based on projected data as opposed to actual patient information and were not linked to specific diagnoses [5–7, 22, 23]. This study identified that the overall monthly prescription rates during April–July 2020 were significantly lower when compared with 4 years of historical data, with a level change of 12.79 in monthly prescription rates after March 2020. Furthermore, the overall monthly moving average of prescription rates fell below historical averages in March 2020 and continued to fall through June. In contrast, Buehrle et al. reported that most commonly prescribed antibiotics in the United States saw a reduction in use for the month of April 2020 but rebounded swiftly throughout May to July, ultimately exceeding prepandemic prescribing rates [24]. However, a recent US study reported 39% and 42% reductions in patients dispensed antibiotic prescriptions in April and May of 2020, respectively, compared with 2017–2019 [22].
While in British Columbia, the overall monthly moving averages of prescription rates were significantly lower in 2020, when compared with the minimum historical moving average; this trend was not consistent across the indications of interest included in this study. RTI-associated prescribing saw significant reductions across the monthly moving averages of 2020 when compared with historical trends. However, UTI- and SSTI-associated antibiotic use in 2020 both had monthly moving averages just below historical trends for January to March, with a slight decrease in March and April before plateauing. In 2019, SSTI- and UTI-associated monthly antibiotic use declined after August and October, respectively. This observed decline can be explained by the community antibiotic stewardship program’s targeted diagnosis and age group–specific interventions during that time [25]. We observed a significant reduction in both overall and RTI-specific monthly prescription rates after March 2020. Across all age groups, the overall monthly prescription rates decreased significantly after March 2020, with the highest reduction observed in <1 and 1–4 years of age, both for overall and RTI-related monthly prescription rates.
The abrupt reduction of prescription rates, beginning in April 2020, can be explained by reduced opportunities for infectious disease transmission due to public health measures implemented gradually in British Columbia. Restrictions on mass gatherings, mandatory self-isolation periods for travelers, service restrictions, and the closure of the US–Canada border were all introduced in the latter half of March 2020 [19]. These restrictions further coincided with the shift to a telehealth platform for the delivery of outpatient care. These radical changes in tandem likely led to a multitude of overlapping potential scenarios, all of which resulted in decreased use of antibiotics in British Columbia. Perhaps a number of individuals eventually ended up consulting a physician virtually, while others were less likely to seek care for mild respiratory illnesses that may previously have resulted in antibiotic prescriptions. Clinicians were less pressurized or less likely to prescribe an antibiotic when consulted virtually [26]; we also noted less bacterial infection/coinfection due to public health measures, increased hand washing, and/or hand sanitizer use at the individual level.
This finding in BC is encouraging as most of the previous studies reported that antibiotic use had increased significantly since the beginning of the pandemic even in the absence of bacterial/fungal coinfection among patients with RTI or when public health measures were relaxed and some health care services resumed [5, 6]. On the other hand, this prescription rate reduction in British Columbia could be a reflection of following the BC provincial and WHO guidelines on empirical use of antibiotics among outpatients with mild to moderate respiratory illness [25, 27, 28].
We observed a significant reduction of some of the most commonly prescribed antibiotics, especially for RTI, in British Columbia. Among them, amoxicillin, azithromycin, cefixime, clindamycin, cloxacillin, doxycycline, and levofloxacin had the highest reduction in monthly prescription rate from March/April through July 2020, when compared with the same months of 2019. This is in contrast to the US study that reported no significant changes in prescription fills of azithromycin and levofloxacin from April to July 2020 and further observed a significant increase in the monthly prescription fills of other antibiotics during the same months [6]. This increase in monthly prescription fills reported by the US study, following April 2020, could have resulted from the reinstitution of in-person health care services. However, another US study reported an increase in azithromycin prescriptions from February to March of 2020 with a significant decrease from March to May of 2020 [22]. It should be noted that azithromycin was widely used, along with hydroxychloroquine, which is not currently recommended outside the COVID clinical trials [10]. However, in British Columbia, the relaxation of community control measures occurred in several phases beginning mid-May 2020 and continued over the following months [19].
Overall, monthly prescription rates in British Columbia declined significantly immediately after the implementation of public health measures due to the pandemic and continued to decrease even as control measures were eased. Although causation cannot be assumed, a strong temporal correlation exists. A historically significant transmission vector: The closure of schools and day care centers with gradual re-openings using face masks/shields, policies of physical distancing, and enhanced hand hygiene might also have played a role in children not contracting, and subsequently spreading, SARS-CoV-2 or other transmissible viruses or bacteria and not having bacterial coinfection. As a result, perhaps there were less illness and so fewer antibiotics were needed. Apart from all these pandemic-era mitigation measures, since 2005, a provincial antimicrobial stewardship program has been advocating for a reduction in antibiotic consumption in order to combat antimicrobial resistance [25]. This stewardship program has been successful in reducing overall antibiotic consumption in British Columbia, with a significant reduction among children age 0–5 years during prepandemic years [29].
This study has several limitations. Although PharmaNet captures >90% of all community prescriptions in British Columbia, neither PharmaNet nor MSP had information available on whether the visit was in person or virtual. As such, we were not able to quantify the antibiotic prescription rates for telehealth visits in contrast to in-person evaluation. However, the impact of telemedicine vs in-person visits on the likelihood of prescribing an antibiotic remains unclear in the literature [14, 30]. The PharmaNet data used for this study did not include drugs prescribed and dispensed for HIV and STIs. We did not have information about each of the physician visits, and we could not compare changes between consultation rates and prescription rates per consultation. The proportion of prescription linkage to a single diagnosis in our data set ranged from 77% to 79%. Although we described overall antibiotic prescription rates in British Columbia, we could not include all the prescriptions when the prescription rate was described by diagnosis.
CONCLUSIONS
In British Columbia, Canada, overall and RTI-specific monthly antibiotic prescription rates declined significantly during April to July 2020 compared with the same months in prepandemic years. Prescription rates for the most RTI-associated antibiotics declined significantly in 2020 when compared with 2019, especially amoxicillin, azithromycin, levofloxacin, and doxycycline. Although community prescription rates continued to decline through July 2020, the final month of our study period, it is important that we continue to monitor these trends to better understand the long-term effects of the SARS-CoV-2 pandemic on antibiotic use and their impact on the health care system, especially given the context of increasing use of telemedicine and antimicrobial resistance.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We acknowledge the BC Ministry of Health for funding the Community Antimicrobial Stewardship program, formerly known as the Do Bugs Need Drugs program.
Financial support. The Community Antimicrobial Stewardship program, formerly known as the Do Bugs Need Drugs program, is funded by the BC Ministry of Health.
Potential conflict of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. No written or verbal consent was obtained from patients, as this study used administrative data, which were provided by the BC Ministry of Health. For this study, we received Institutional Research Board approval through the University of British Columbia (certificate H09-00650).
Author contributions. A.M., F.M., and D.P.—conceptualized the study, developed methods of data analysis, critically reviewed the manuscript; A.M.—wrote the manuscript, interpreted the results; A.S., H.L., and E.B.-H.—interpreted results, critically reviewed and helped develop the manuscript; M.X.—analyzed the data, interpreted results, and critically reviewed the manuscript.
References
- 1. World Health Organization. Global Action Plan on AMR. Geneva: World Health Organization; 2020. [Google Scholar]
- 2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 3. Saatchi A, Marra F. Increasing paediatric prescribing rates in British Columbian children: cause for concern? Can J Public Health 2020; 111:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spivak ES, Cosgrove SE, Srinivasan A. Measuring appropriate antimicrobial use: attempts at opening the black box. Clin Infect Dis 2016; 63:1639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abelenda-Alonso G, Padullés A, Rombauts A, et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol 2020; 41:1371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buehrle DJ, Decker BK, Wagener MM, et al. Antibiotic consumption and stewardship at a hospital outside of an early coronavirus disease 2019 epicenter. Antimicrob Agents Chemother 2020; 64:e01011-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nestler MJ, Godbout E, Lee K, et al. Impact of COVID-19 on pneumonia-focused antibiotic use at an academic medical center. Infect Control Hosp Epidemiol 2020: 1–3. doi:10.1017/ice.2020.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaughn VM, Gandhi T, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clin Infect Dis 2021; 72:e533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furukawa D, Garber CJ. Antimicrobial stewardship in a pandemic: picking up the pieces. Clin Infect Dis 2021; 72:e542–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Clinical management of COVID-19 interim guidance—May 2020. 2020. Available at: https://www.who.int/publications-detail/clinical-management-of-covid-19. Accessed 4 June 2020.
- 11. Rawson TM, Ming D, Ahmad R, et al. Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol 2020; 18:409–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Children’s National Hospital. Interventions stem antibiotic prescribing rates in telemedicine. Available at: http://childrensnational.org/news-and-events/childrens-newsroom/2020/decreasing-antibiotic-prescribing-rates-in-telemedicine. Accessed 9 November 2020.
- 13. Martinez KA, Rood M, Jhangiani N, et al. Association between antibiotic prescribing for respiratory tract infections and patient satisfaction in direct-to-consumer telemedicine. JAMA Intern Med 2018; 178:1558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ray KN, Shi Z, Gidengil CA, et al. Antibiotic prescribing during pediatric direct-to-consumer telemedicine visits. Pediatrics 2019; 143:e20182491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. BC Ministry of Health, Data Stewardship Committee. PharmaNet. Data Extract. 2020. Available at: http://www.health.gov.bc.ca/data/. Accessed 6 December 2020. [Google Scholar]
- 16. British Columbia Ministry of Health. Medical Services Plan (MSP) Payment Information File. Data Extract. 2020. Available at: http://www.health.gov.bc.ca/data/. Accessed 6 December 2020. [Google Scholar]
- 17. World Health Organization. Guidelines for ATC Classification and DDD Assignment. Geneva: World Health Organization; 1996. [Google Scholar]
- 18. Province of British Columbia. BC statistics. Available at: https://www2.gov.bc.ca/gov/content/data/statistics/people-population-community/population. Accessed 4 December 2020.
- 19. British Columbia Cenfre Disease Control (BCCDC), BC COVID -19 situation report week 50: December 6–December 12, 2020. Available at: http://www.bccdc.ca/Health-Info-Site/Documents/COVID_sitrep/Week_50_BC_COVID-19_Situation_Report.pdf. Accessed 6 January 2020.
- 20. Box GE, Tiao GC. Intervention analysis with applications to economic and environmental problems. J Am Stat Assoc 1975; 70:70–79. [Google Scholar]
- 21. Box GE, Jenkin GM, Reinsel GC, et al. Time Series Analysis: Forecasting and Control. Hoboken, NJ: John Wiley & Sons; 2015. [Google Scholar]
- 22. Akaike H, et al. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19:716–23. [Google Scholar]
- 23. King LM, Lovegrove MC, Shehab N, et al. Trends in U.S. outpatient antibiotic prescriptions during the COVID-19 pandemic. Clin Infect Dis. 2020; doi: 10.1093/cid/ciaa1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah S, Wordley V, Thompson W. How did COVID-19 impact on dental antibiotic prescribing across England? Br Dental J 2020; 229:601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buehrle DJ, Hong Nguyen M, Wagener MM, et al. Impact of the coronavirus disease 2019 pandemic on outpatient antibiotic prescriptions in the United States. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. British Columbia Cenfre Disease Control (BCCDC). Community antimicrobial stewardship. 2020. Available at: http://www.bccdc.ca/our-services/programs/community-antimicrobial-stewardship. Accessed 6 December 2020.
- 27. Johnson KM, Dumkow LE, Burns KW, et al. Comparison of diagnosis and prescribing practices between virtual visits and office visits for adults diagnosed with sinusitis within a primary care network. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antibioticwise.ca. Available at: https://antibioticwise.ca/. Accessed 6 January 2020.
- 29. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected interim guidance. Available at: http://apps.who.int/iris/bitstream/10665/178529/1/ WHO_MERS_Clinical_15.1_eng.pdf. Accessed 4 June 2020.
- 30.British Columbia Centre for Disease Control (BCCDC). Antibiotic utilization Dashboard, 2021. Available at: http://www.bccdc.ca/health-professionals/data-reports/antimicrobial-resistance-utilization/antimicrobial-utilization-dashboard. Accessed 22 May 2021.
- 31. Yao P, Clark S, Gogia K, et al. Antibiotic prescribing practices: is there a difference between patients seen by telemedicine versus those seen in-person? Telemed J E Health 2020; 26:107–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.