To the Editor:
Over the past year, SARS-CoV-2, the novel coronavirus causing COVID-19, has spread across the globe, infecting an estimated 100 million people and causing >2 million deaths thus far, with hundreds of thousands of additional cases occurring daily (1). Typical symptoms include fever, cough, dyspnea, myalgia, and anosmia, however, there is a growing body of evidence which suggests that a wide range of neurologic symptoms may be more prevalent than previously recognized with corresponding pathologies ranging from nonspecific hypoxic-ischemic injury and microvascular injury with microhemorrhage to large infarcts and findings similar to acute disseminated encephalomyelitis (ADEM) (2–7), although there is considerable debate over which neuropathologic findings can truly be attributed to COVID-19 (8–12). These findings are present despite conflicting evidence for viral particles in the brain, spinal cord, and cerebrospinal fluid of patients with neurologic symptoms (13–15). Here, we present 2 cases of fatal COVID-19 with severe neurologic sequalae and neuropathologic findings of ADEM and acute hemorrhagic leukoencephalopathy (AHLE).
CASE 1
A 51-year-old woman with a past medical history of trisomy 21 and medically refractory seizures, documented history of medical noncompliance, status-post corpus callosotomy in 2004 and vagus nerve stimulator placement in 2015 presented to the emergency department with increased seizure activity (6–10 seizures/day) for the preceding 3 days, fever, diarrhea, incontinence, and aphasia. She was intubated and transferred to the neuro ICU and treated for seizures. She tested positive for COVID-19 and remdesivir was started. A CT scan was performed and demonstrated evidence of her prior corpus callosotomy but no evidence of acute intracranial pathology. On hospital day 4 she became hemodynamically unstable and passed away despite full code measures.
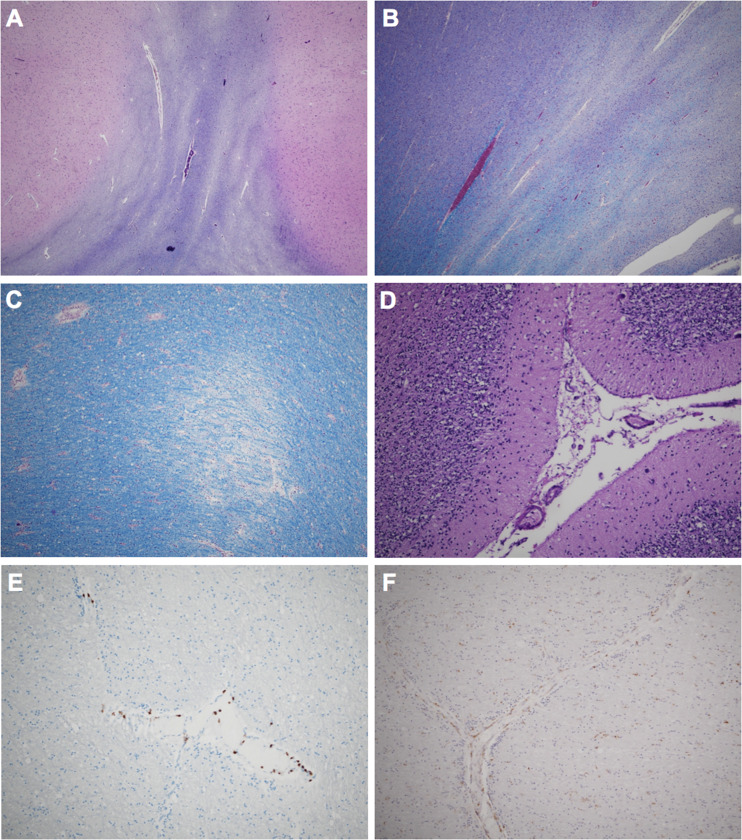
At brain autopsy, there was evidence of a disproportionately small cerebellum, and coronal sections demonstrated evidence of prior corpus callosotomy and multifocal tan-gray discoloration throughout the subcortical white matter. Histologically, there was an irregular pattern of demyelination as seen by Luxol fast blue/periodic acid-Schiff (LFB/PAS) (Fig. 1A, B), which corresponded to the white matter discoloration identified grossly. This demyelination centered primarily around veins and venules (Fig. 1C), and although we observed relatively few perivascular CD3+ T-lymphocytes (Fig. 1E), there were frequent perivascular and parenchymal CD68+ histiocytes and activated microglial cells (Fig. 1F), all features consistent with a final diagnosis of ADEM. There was also evidence of focal cortical dysplasia (FCD) type 1c, significant global hypoxic-ischemic injury, and cerebellar hypoplasia with Bergmann gliosis (Fig. 1D). A full workup for neurodegenerative disease demonstrated high-level Alzheimer disease neuropathologic change ([ADNC]; Braak V, Thal 4, CERAD frequent) and mild cerebral amyloid angiopathy (CAA) (Table). Immunohistochemical staining for SARS-CoV-2 was negative in multiple brain sections (mouse monoclonal SARS-CoV-2 spike antibody [1A9], Cat. No. GTX632604, GeneTex, Irvine, CA) (14).
Figure 1.
Histologic features of Case 1. LFB/PAS stains demonstrating serpiginous myelin pallor in the temporal (A) and peri-hippocampal (B) white matter. Higher-power LFB/PAS demonstrating perivascular myelin loss (C). Loss of Purkinje cells and Bergmann gliosis (D). There are multifocal regions of perivascular CD3+ T-lymphocytes (E) and perivascular and parenchymal CD68+ histiocytes/activated microglial cells (F).
TABLE.
Clinical, Radiologic, and Pathologic Findings
| Case | Patient Demographics | Past Medical History | Radiology Findings | Pathologic Diagnoses |
|---|---|---|---|---|
| 1 | 51-Year-old | Trisomy 21 | No evidence of acute intracranial processes | Histologic features of ADEM |
| Female | Medically refractory epilepsy | High-level ADNC (Braak V, Thal 4, CERAD 3) | ||
| Corpus callosotomy (2004) | Evidence of prior corpus callosotomy | Cerebral amyloid angiopathy, mild | ||
| Vagus nerve stimulator placement (2015) | Focal cortical dysplasia, type 1c | |||
| History of medical noncompliance with seizure medication | Cerebellar hypoplasia with | |||
| Bergmann gliosis | ||||
| Global hypoxic-ischemic injury | ||||
| Evidence of prior corpus callosotomy | ||||
| 2 | 64-Year-old | Hypertension, hypothyroidism | Right occipital lobe IPH | Histologic features of AHLE |
| Male | Heavy alcohol use | Right-sided subdural hematoma | Right occipital lobe IPH | |
| Recent history of MSSA aortic valve endo- carditis with 6-week IV antibiotics, complicated by DVT (July–August 2020) | Right-sided subdural hematoma | |||
| Global hypoxic-ischemic injury | ||||
| Subfalcine and uncal herniation | ||||
| PART (Braak I, Thal 0, CERAD 0) |
ADEM, acute disseminated encephalomyelitis; ADNC, Alzheimer disease neuropathologic change; MSSA, methicillin-susceptible Staphylococcus aureus; DVT, deep vein thrombosis; IPH, intraparenchymal hemorrhage; AHLE, acute hemorrhagic leukoencephalitis; PART, primary age-related tauopathy.
CASE 2
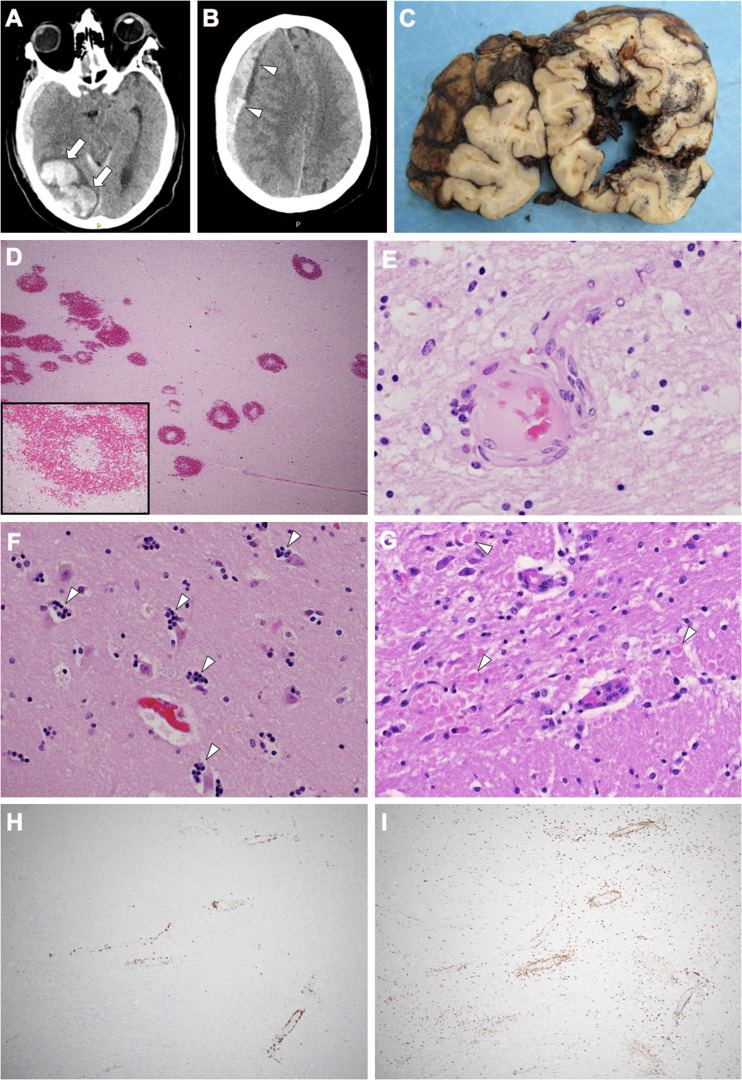
A 64-year-old neurologically intact man with a past medical history of hypertension, hypothyroidism, and heavy alcohol use was admitted to the hospital for persistent fever but tested negative initially for COVID-19. Further workup demonstrated methicillin-susceptible Staphylococcus aureus (MSSA) aortic valve endocarditis and he was placed on a 6-week IV antibiotic regimen (which was complicated by left axillary vein DVT), then transferred to a rehabilitation facility where he tested positive for COVID-19. He was placed on remdesivir, convalescent plasma, and corticosteroids. Initial CT scan showed no acute intracranial pathology. During ambulation, the patient collapsed and was nonresponsive with a fixed and dilated right pupil. Noncontrast CT scan demonstrated a large right occipital lobe intraparenchymal hemorrhage (Fig. 2A) and right-sided subdural hematoma (Fig. 2B) with significant right-to-left midline shift. The patient passed away soon after.
Figure 2.
Radiologic and pathologic features of Case 2. Antemortem transverse CT scans demonstrating right occipital lobe intraparenchymal hemorrhage (arrows) (A) and right cerebral convexity subdural hematoma (arrowheads) (B). Coronal sections of the occipital lobes demonstrating right-sided intraparenchymal hemorrhage (C). Hematoxylin and eosin sections demonstrating ring and ball hemorrhages (D) and vessels showing fibrinoid necrosis (E). Hippocampal sections demonstrate neuronophagia in CA1 neurons (arrowheads) (F) and basis pontis sections demonstrate frequent axonal spheroids (arrowheads) (G). There are multifocal regions of perivascular CD3+ T-lymphocytes (H) and perivascular and parenchymal CD68+ histiocytes/activated microglial cells (I).
Brain autopsy demonstrated the acute subdural hematoma, large acute right occipital lobe intraparenchymal hemorrhage (Fig. 2C), and both subfalcine and uncal herniation, as well as multifocal bilateral cerebral white matter hemorrhages. Histologic examination revealed myelin pallor as well as numerous ring- and ball-hemorrhages throughout the white matter with central blood vessels showing fibrinoid necrosis (Fig. 2D, E), most consistent with a diagnosis of AHLE. In addition, there was global hypoxic-ischemic injury, focal neuronophagia in hippocampal sections (Fig. 2F), multifocal areas of myelin pallor, axonal spheroids (Fig. 2G), numerous perivascular CD3+ T-lymphocytes (Fig. 2H) as well as perivascular and parenchymal CD68+ cells (perivascular macrophages and parenchymal microglia) (Fig. 2I). Neurodegenerative workup demonstrated mild primary age-related tauopathy (PART) (Table). Immunohistochemical staining for SARS-CoV-2 spike protein was also negative in the brain.
These 2 cases represent the most severe end of the COVID-19 neuropathologic spectrum. While previous studies have reported neuropathology findings in patients with neurologic symptoms ranging from a complete lack of histologic findings other than hypoxic-ischemic changes (13) to ADEM (2), it remains unclear whether even the most severe pathology is due to the presence of the virus in the brain itself, direct or indirect vascular damage, or parainfectious mechanisms. The findings in these brains are similar to the handful of previously described cases with severe pathology, namely demyelination and axonal injury centered primarily around vessels with perivascular and parenchymal CD3+ and CD68+ cells, ring- and ball-hemorrhages limited to the white matter, and larger hemorrhages. This suggests that COVID-19 could be added to the list of viral syndromes associated with demyelinating disease in general and specific syndromes, such as ADEM and AHLE. The damage to both myelin and axons, the lack of immunohistochemical staining for SARS-CoV-2 spike protein, as well as the pathology centering around vessels and evidence for endothelial damage in the brain and other organs (7) suggests an indirect etiologic pathway, such as a primary vascular or parainfectious etiology or a mechanistic route secondary to systemic inflammation and coagulopathy, as opposed to direct central nervous system SARS-CoV-2 infection.
FUNDING
The authors received no financial support for the research, authorship, or publication of this article.
COMPETING INTERESTS
The authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. WHO Coronavirus Disease (COVID-19) Dashboard. February 12, 2021. Available at: https://covid19.who.int/. Accessed February 12, 2021.
- 2. Reichard RR, Kashani KB, Boire NA, et al. Neuropathology of COVID‐19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)‐like pathology. Acta Neuropathol 2020;140:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019. JAMA Neurol 2020;77:1018–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020;143:3104–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bodro M, Compta Y, Sánchez-Valle R. Presentations and mechanisms of CNS disorders related to COVID-19. Neurol Neuroimmunol Neuroinflamm 2021;8:e923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020;382:2268–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee M-H, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med 2021;384:481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Weyhern CH, Kaufmann I, Neff F, et al. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet 2020;395:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glatzel M, Hagel C, Matschke J, et al. Neuropathology associated with SARS-CoV-2 infection. Lancet 2021;397:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egervari K, Thomas C, Lobrinus JA, et al. Neuropathology associated with SARS-CoV-2 infection. Lancet 2021;397:276–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nasrallah MP, Mourelatos Z, Lee EB. Neuropathology associated with SARS-CoV-2 infection. Lancet 2021;397:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Weyhern CH, Kaufmann I, Neff F, et al. Neuropathology associated with SARS-CoV-2 infection—Authors' reply. Lancet 2021;397:277–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med 2020;383:989–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol 2020;19:919–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Placantonakis DG, Aguero-Rosenfeld M, Flaifel A, et al. SARS-CoV-2 is not detected in the cerebrospinal fluid of encephalopathic COVID-19 patients. Front Neurol 2020;11:587384. [DOI] [PMC free article] [PubMed] [Google Scholar]