Abstract
The initial report of the multisystem inflammatory syndrome in children (MIS-C) was from the UK in April 2020; since then, cases have been reported worldwide. Renal involvement has been seen commonly, ranging from 10% to 46%. Kidney involvement following severe acute respiratory syndrome coronavirus 2 infection in children with MIS-C is more common than initially thought and is associated with higher morbidity and mortality. There are several reports of a direct viral tropism of coronavirus disease 2019 and MIS-C-associated renal damage. This study’s objective was to systematically review the current understanding of kidney involvement in children suffering from MIS-C. Based on our systemic literature search, 19 studies have either partially or fully discussed kidney involvement in MIS-C patients. Furthermore, we discuss the multifactorial pathogenesis contributing to acute kidney injury (AKI) development in MIS-C. The current review gives a pediatric nephrologist’s perspective of the renal involvement in MIS-C, the incidence of AKI, the pathophysiology of AKI in MIS-C and the proposed therapeutic regimens available, including the need for kidney replacement therapy for a child with AKI associated with MIS-C. As the disease is rapidly evolving, more detailed clinical prospective studies are required to understand MIS-C and its role in AKI better.
Keywords: acute kidney injury, COVID-19, MIS-C, nephrology
INTRODUCTION
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic [1]. As per the literature [2, 3], with underlying kidney disease, the disease presents with worse outcomes than in those without kidney disease. However, children are mildly affected, and although rare, severe cases have also been documented [4]. There is a lack of strong evidence relating to underlying conditions with severe illness in children [5].
Cases reported from the UK in April 2020 showed a picture similar to incomplete Kawasaki disease (KD) or toxic shock syndrome in children [6]. Since then, reports from different parts of the world describe similarly affected children [7–10]. This clinical disorder has been termed multisystem inflammatory syndrome in children (MIS-C); also known as pediatric inflammatory multisystem syndrome (PIMS) [11, 12], PIMS temporally associated with SARS-CoV-2 (PIMS-TS), pediatric hyperinflammatory syndrome or pediatric hyperinflammatory shock.
Clinicians face a diagnostic dilemma distinguishing KD from MIS-C due to the overlapping clinical features of these two entities and the lack of a definitive diagnostic test for either condition [13]. The signs and symptoms are temporally associated with COVID-19 but presumed to have been developed 2–4 weeks after acute COVID-19, albeit serologic evidence of infection with SARS-CoV-2 [6]. In children, MIS-C occurs as a rare complication of COVID-19 with uncertainty. In a report by Dufort et al., the estimated incidence of laboratory-confirmed SARS-CoV-2 infection in individuals <21 years old was 322/100 000, and the incidence of MIS-C was 2/100 000 [14]. According to the Center of Disease Control and Prevention (CDC), a total of 1659 case of MIS-C have been reported as of 8 January 2021. Acute kidney injury (AKI) is frequently reported in MIS-C as outlined in Table 1, with its incidence ranging from 10% to 60% across studies [15, 16].
Table1.
Kidney and MIS-C
| Study | Study type | Location | Number of MIS-C patients in study | Sex (male) | Age mean (SD) (years) | AKI incidence, n (%) | Use of KRT, n (%) | Additional remarks |
|---|---|---|---|---|---|---|---|---|
| Gonzalez-Dambrauskas et al. [15] | Multicenter | Chile, Colombia, Italy, Spain, and the USA | 17 | 11 | 6.52 ± 2.99 | 3 (18) | Not reported | Different countries patients were recruited; low mortality 3% |
| Stewart et al. [17] | Single center | London, UK | 52 | NA | 4 ± 2.67 | 24 (46) | Not reported | Dedicated study for AKI |
| Derespina et al. [18] | Multicenter | New York, USA | 70 | 43 | 14.5 ± 1.67 | 9 (12.9) | 1 (1.4) | ARDS associated with poor outcome |
| Riphagen et al. [6] | Single center | London, UK | 8 | 8 | 9.29 ± 3.77 | 1 (12.5) | 1(12.5) | All children tested negative for SARS-CoV-2 on bronchoalveolar lavage or nasopharyngeal aspirates |
| Whittaker et al. [9] | Multicenter | UK | 58 | 38 | 9.43 ± 1.38 | 13 (22) | Not reported | Uncontrolled case series |
| Feldstein et al. [10] | Multicenter | USA | 186 | 115 | 8.1 ± 1.53 | 17 (9.1) | Not reported | High use of IVIG—79% cases, low mortality—2% |
| Dufort et al. [14] | Multicenter | New York, USA | 99 | 53 | 9.33 ± 1 | 10 (10) | Not reported | High incidence in Blacks and Hispanics |
| Davies et al. [19] | Multicenter | UK | 78 | 52 | 11 ± 1 | – | 1 (1) | – |
| Pereira et al. [20] | Single center | Sao Paulo, Brazil | 6 | 5 | 8.30 ± 2.94 | 50 | 100% PICU admission, 100% cardiac involvement and 67% death | |
| Lee et al. [21] | Single center | 28 | 16 | 8.77 ± 2.82 | 6 (21) | Cytopenia, level of hyperferritinemia and pattern of cytokine production MIS-C from KD distinguished | ||
| Pouletty et al. [22] | Multicenter | Paris | 16 | 8 | 9.3 ± 1.3 | 9 (56) | Not reported | Older age children were more affected compared with younger children in ‘classic’ KD (10 years versus 2 years, P < 0.0001), respectively |
| Capone et al. [23] | Single center | New York, USA | 33 | 20 | 8.83 ± 1.183 | 23 (70) | Not reported | – |
| DeBiasi et al. [24] | Multicenter | Washington, USA | 9 | NA | 15.08 ± 4.25 | 1 (11) | Not reported | – |
| Dionne et al. [25] | Single center | Boston, USA | 25 | 15 | 9.28 ± 2.05 | 2 (8) | Not reported | – |
| Godfred-Cato et al. [16] | Multicenter | District of Columbia, New York, USA | 570 | 316 | 9.01 ± 3.33 | 105 (18.4) | 2 (0.4) | – |
| Mamishi et al. [26] | Multicenter | Iran | 45 | 24 | 6.98 ± 0.98 | 13 (29) | Not reported | Wide spectrum of sign and symptoms and abnormal inflammatory markers were noted |
| Shahbaznejad et al. [27] | Case series | Iran | 10 | 6 | 5.37 ± 3.9 | 2 (20) | Not reported | |
| Deep et al. [28] | Multicenter observational study | UK | 116 | 76 | 10.5 ± 3.5 | 101 (87) | 3 (2.97) | Short-term outcomes of AKI in PIMS-TS appears good, long-term outcomes are unknown |
ARDS, acute respiratory distress syndrome; NA, not available.
Definition
The case definitions made by the US CDC in association with the American Academy of Pediatrics, the World Health Organization (WHO), American College of Rheumatology (ACR) and Royal College of Pediatrics and Child Health (RCPCH) are summarized in Tables 2–5. The criteria used for case definition vary slightly between these agencies. The presence of fever remains common along all case definitions except for a much broader duration of fever (>3 days) in WHO guidelines, in contrast to CDC, ACR and RCPCH. The CDC case definition adds several points that are unique to their guideline. Notably, severe illness requiring hospitalization for clinical symptoms and evidence of COVID-19 exposure within 4 weeks are important factors not present in other case definitions. While CDC and WHO case definitions require prior SARS-CoV-2 infection or exposure, RCPCH does not. These definitions are constantly evolving and likely to change as more updated information becomes available (Table 6).
Table 2.
Case definition of MIS-C as per the CDC
| CDC case definition | |
|---|---|
| All four criteria must be met: | |
| (1) Age <21 years | |
| (2) Clinical presentation consistent with MIS-C (including all of the following): | (a) Fever: |
| Documented fever >38.0°C (100.4°F) for ≥24 h | |
| or | |
| Report of subjective fever lasting ≥24 h | |
| (b) Laboratory evidence of inflammation including, but not limited to (any of the following): | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| (c) Multisystem organ involvement (two or more of the following): | |
| |
| |
| |
| |
| |
| |
| |
| (d) Severe illness requiring hospitalization | |
| (3) No alternative plausible diagnoses | |
| (4) Recent or current SARS-CoV-2 infection or exposure (any of the following): |
|
| |
| |
| |
Centers for Disease Control and Prevention Health Alert Network (HAN). MIS-C associated with COVID-19. https://emergency.cdc.gov/han/2020/han00432.asp (15 May 2020, date last accessed).
BNP, brain natriuretic peptide; ESR, erythrocyte sedimentation rate; GI, Gastrointestinal; LDH, lactate dehydrogenase; Na, sodium.
Table 6.
Case definition across different associations comparing MIS-C
| Criteria | RCPCH PIMS-TS | ACR | AAP/CDC MIS-C | WHO MIS-C |
|---|---|---|---|---|
| Age | All children (age not defined) | All children (age not defined) | <21 years | 0–19 years |
| Fever | Persistent fever ≥38.5°C | Unremitting fever > 38°C | ≥38.0°C for ≥24 h OR subjective fever ≥24 h | Fever ≥3 days |
| Clinical symptoms | (i) Single or multi-organ dysfunction AND (ii) additional features |
At least two suggestive clinical features: (i) Rash; (ii) GI symptoms; (iii) edema of hands/feet; (iv) oral mucosal changes; (v) conjunctivitis; (vi) lymphadenopathy; (vii) neurologic symptoms |
(i) Severe illness (hospitalized) AND (ii) two or more organ systems involved |
Two of the following: (i) Rash, conjunctivitis, mucocutaneous inflammation; (ii) hypotension or shock; (iii) cardiac involvement; (iv) coagulopathy; (v) acute GI symptoms |
| Inflammation |
(i) Neutrophilia, (ii) increased CRP AND (iii) lymphopenia |
Laboratory evidence: (i) CRP ≥5 mg/dL OR (ii) ESR ≥40 mm/h At least one suggestive laboratory feature: (i) ALC <1000/μL; (ii) platelet count <150 000/μL; (iii) Na <135 mmol/L; (iv) neutrophilia; (v) hypoalbuminemia |
Laboratory evidence of inflammation not limited to one or more of the following: (i) increased CRP; (ii) increase ESR; (iii) increased fibrinogen; (iv) increased procalcitonin; (v) increased D-dimer |
Elevated inflammatory markers such as: (i) increased ESR; (ii) increased CRP; (iii) increased procalcitonin |
| Link to SARS-CoV-2 | PCR+ or − | SARS-CoV-2 PCR and/or serologies |
Current or recent: (i) +PCR; (ii) +serology; (iii) +antigen test OR (iv) COVID-19 exposure within prior 4 weeks |
Current or recent: (i) +PCR; (ii) +serology; (iii) +antigen test OR (iv) likely COVID-19 contact |
| Exclusion | Exclusion of other infections | Exclusion of other infections | No alternative diagnosis | No obvious microbial cause |
AAP, American Academy of Pediatrics; GI, gastrointestinal; ESR, erythrocyte sedimentation rate; ALC, absolute lymphocyte count; Na, sodium.
Table 3.
Case definition of MIS-C as per WHO
| WHO case definition | |
|---|---|
| All six criteria must be met: | |
| (1) Age 0–19 years | |
| (2) Fever for ≥3 days | |
| (3) Clinical signs of multisystem involvement (at least two of the following): |
|
| |
| |
| |
| |
| (4) Elevated markers of inflammation (e.g. ESR, CRP or procalcitonin) | |
| (5) No other obvious microbial cause of inflammation, including bacterial sepsis and staphylococcal/streptococcal TSSs | |
| (6) Evidence of SARS-CoV-2 infection any of the following: |
|
| |
| |
| |
ESR, erythrocyte sedimentation rate; BNP, brain natriuretic peptide. MIS-C and adolescents with COVID-19: Scientific Brief. 2020. https://www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (17 May 2020, date last accessed).
Table 4.
Case definition of MIS-C as per ACR
| ACR | |
|---|---|
| (1) All children (age not defined) | |
| (2) Unremitting fever >38°C | |
| (3) At least two suggestive clinical features: |
|
| |
| |
| |
| |
| |
| |
| (4) Laboratory evidence: | 1) CRP ≥5 mg/dL OR |
| 2) ESR ≥40 mm/h | |
| 3) At least one suggestive laboratory feature: | |
| |
| |
| |
| |
| |
| (5) SARS-CoV-2 PCR and/or serologies | |
| (6) Exclusion of other infections | |
ALC, absolute lymphocyte count; BNP, brain natriuretic peptide; ESR, erythrocyte sedimentation rate; GI, gastrointestinal; LDH, lactate dehydrogenase; Na, sodium.
Table 5.
Case definition of MIS-C as per RCPCH
| RCPCH PIMS-TS | |
|---|---|
| (1) All children (age not defined) | |
| (2) Persistent fever ≥38.5°C | |
| (3) Clinical symptoms | (1) Single or multi-organ dysfunction AND |
| (2) Additional featuresa | |
| (4) Laboratory evidenceb: | (1) Neutrophilia |
| (2) Increase CRP | |
| AND | |
| (3) Lymphopenia | |
| (5) SARS-CoV-2 PCR and/or serologies | |
| (6) Exclusion of other infections | |
Abdominal pain, confusion, conjunctivitis, cough, diarrhea, headache, lymphadenopathy, mucus membrane changes, neck swelling, rash, resp symptoms, sore throat, swollen hands and feet, syncope, vomiting.
High d-dimers, high ferritin, hypoalbuminemia.
MATERIALS AND METHODS
Search
A systemic literature search was performed in PubMed/Medline from January 2020 to February 2021 to include all the studies related to MIS-C and AKI. Medical subject headings (MeSH) terms including ‘Multisystem inflammatory syndrome’, ‘pediatric inflammatory multisystem syndrome’, ‘Kawasaki-like disease’, ‘Acute kidney injury’, ‘Acute renal injury’ and ‘renal dysfunction’ were used in the search strategy. We excluded foreign language and case reports during our analysis.
Data selection and extraction
Two independent reviewers assessed the literature based on the inclusion and exclusion criteria. A third independent reviewer evaluated articles with conflict. The studies were selected based on selecting any incidence of AKI or the use of renal replacement therapy in patients with MIS-C. A standardized data collection form was used to extract the following information from each included article: the last name of the first author, study type, location, number of MIS-C patients in the study, AKI incidence, use of renal replacement therapy.
RESULTS
Search results
The initial search returned 91 studies; 18 studies met the selection criteria; 7 were multicenter studies; 8 were single-center studies and 1 was a case series. Details of the included studies are provided in Table 1 and Supplementary Figure S1.
CLINICAL FEATURES AND PRESENTING SYMPTOMS
MIS-C presents with persistent fever >38.5°C, variable rash, conjunctivitis, peripheral edema, severe abdominal pain and diarrhea. Fever is the most common presenting symptom, lasting for 3–5 days, though fewer days of fever have been reported [10]. Respiratory distress does not seem to be one of the main presenting symptoms of COVID-19, although a significant proportion of patients require respiratory support [8]. More significantly, many of these children present with hypotension and shock, requiring vasopressor support and pediatric intensive care unit (PICU) admission [6]. Echocardiogram findings include ventricular dysfunction and coronary artery abnormalities [29]. Patients with MIS-C also have evidence of severe inflammatory state, including elevation in C-reactive protein (CRP), ferritin, d-dimer, lactate dehydrogenase, pro-brain natriuretic peptide and cardiac enzymes. Additionally, patients showed elevated pro-calcitonin levels, liver transaminases, anemia, along with thrombocytosis or thrombocytopenia (Table 7) [27]. Constitutional gastrointestinal symptoms like abdominal pain, vomiting and diarrhea are particularly prominent, mimicking appendicitis in some children [26, 30], and may be associated with higher mortality [20].
Table 7.
Moderate to severe signs and symptoms of MIS-C
|
BNP, brain natriuretic peptide; CA, coronary artery.
PATHOGENESIS
The pathophysiology of MIS-C is still not well established. It is proposed that MIS-C occurs secondary to an abnormal immune reaction to the virus, much like KD and cytokine release syndrome (CRS), causing this disease [22, 21]. The mechanisms through which SARS-CoV-2 triggers the abnormal immune reaction are unknown. Unabated cytokine storm leading to endothelial dysfunction associated with SARS-CoV-2 infection has also been hypothesized [31, 32]. The mechanisms underlying the exaggerated immune reaction in MIS-C are an area of active investigation (Figure 1).
FIGURE 1:

Pathophysiology of MIS-C. ACE-2, angiotensin converting enzyme-2.
Children tend to be less susceptible to severe respiratory infections than adults. Milder reactions could be attributable to their robust innate immune response, healthier respiratory tracts and few underlying illnesses. In addition to that, children are overprotected by their parents; many are involved in fewer outdoor activities and undertake less international travel. Notably, decreased gene expression of the angiotensin converting enzyme-2 (ACE-2) receptor (the target of SARS-CoV-2) in the pediatric population could result in the age-related difference in the incidence of COVID-19 [33–36].
However, one of the major challenges is to understand the post-viral lag in symptom presentation in MIS-C patients. MIS-C cases predominantly occur 4 weeks post-COVID-19 peak in the population [37]. One-third of reported MIS-C cases test positive for SARS-CoV-2 using reverse transcriptase–polymerase chain reaction (RT-PCR). Most cases test positive for an antibody test immunoglobulin gamma (IgG), indicating immune response development against the virus. This condition’s lag in symptom presentation may be attributed to a higher ratio of antibody positivity with a lower ratio of SARS-CoV-2 positivity by RT-PCR, which suggests a late inflammatory process owing to antibody- and/or immune complex-mediated development to SARS-CoV-2 [38]. Although the pathophysiology of MIS-C remains unclear, the conversion of mild disease to a multisystem inflammatory response in the post-infectious period is thought to emerge from a dysregulated immune response resembling CRS [10, 13].
The timing of the interferon (IFN) response to SARS-CoV-2 infection plays a critical role in the presentation of MIS-C. A hypothesis by Rowley suggests that early IFN response may lead to rapid viral clearance during the early stages of the disease, inducing a mild illness, when the viral burden is low. Conversely, with a high viral burden, the replicating virus may delay the IFN response, resulting in a marked cytokine storm before the body’s adaptive response can clear the virus, driving a severe infection including MIS-C [13].
Furthermore, Martinez et al. theorize that some children with MIS-C could harbor viral RNA in the nasopharynx, which may provide a continuous antigen source for T cells leading to chronic viral exposure in the respiratory system. These theories, however, should be tested in future longitudinal studies [39].
Antibodies against SARS-CoV-2 can play a functional role in MIS-C. Gruber et al. suggest the role of neutralizing antibodies against SARS-CoV-2 in patients with MIS-C and its association with interleukin-18 (IL-18) and IL-16 activation, myeloid chemotaxis and activation of lymphocytes, monocytes and natural killer cells [40]. Suboptimal levels of antibodies to SARS-CoV-2 might exacerbate the disease course through antibody-dependent enhancement of viral entry and amplification of viral replication, as observed in other infections like dengue [41]. The formation of antigen–antibody immune complexes results in the enhanced activation of the immune cascade in the lung tissue leading to the COVID-19 pathology [42].
Evolving pathogenesis of AKI in MIS-C
The pathogenesis of AKI in MIS-C is a complex, multifaceted entity with overlapping mechanisms. The means through which SARS-CoV-2 triggers the abnormal immune reaction are unknown. Investigators have reported several mechanisms for AKI in COVID-19. They are thought to occur secondary to an abnormal immune response to the virus leading to tubular injury and podocytopathy, inflammatory process, hemodynamic instability and vascular endothelial dysfunction [21]. The underlying exaggerated immune reaction in MIS-C is a place of active investigation.
COVID-19 exhibits viral tropism and can directly affect the kidney. The spike (S) glycoprotein of SARS-CoV-2 binds to the ACE-2 receptor on host cells. Afterward, the active S protein is cleaved by transmembrane serine proteases (TMPRSSs), resulting in membrane fusion facilitated by fusion peptides released by the virus. Finally, the virus penetrates the proximal tubule cells and the podocytes leading to renal impairment (podocytopathy and tubular epithelial cell injury) [43, 44]. This is suggested by a co-expression of ACE2 and TMPRSS2 genes in the podocytes and proximal convoluted tubules, like that in the lung, small intestine and colon [44].
Sub-Saharan African descent with high-risk apolipoprotein-1 (APOL1) genotype (presence of two risk alleles) could be at increased risk of kidney disease in the setting of COVID-19 [45]. Case reports of patients presenting with heavy proteinuria associated with collapsing glomerulopathy with a predisposition in the form of APOL-1 polymorphism suggest a crucial role of innate immunity pathways, which are upregulated in viral illnesses [45].
The development of a hyperinflammatory state as a response to the virus in MIS-C suggests a macrophage activating syndrome (MAS) like cytokine storm or secondary hemophagocytic lymphohistiocytosis (HLH). MAS or secondary HLH is a life-threatening condition triggered by an autoimmune condition or viral illness [46]. The findings indicative of MAS include cytopenias, coagulopathy, tissue damage and hyperferritinemia, leading to a fatal multi-organ failure [21, 47, 48]. Similar findings have been noticed in MIS-C patients, specifically hyperferritinemia on admission, associated with severe AKI contributing to the acute inflammatory state in MIS-C [28]. Furthermore, data from an adult study on secondary HLH demonstrate increased AKI incidence adversely affecting patient survival [49].
Another cardinal finding of MAS bearing similarity with MIS-C is the involvement of the plasma mediators driving the hypercytokinemia state. Cytokine profiling studies show that IFN-γ-induced response markers (including IFN-γ, IL-18, IP-10) are the main triggers of inflammation in both MIS-C and MAS [50]. Other important cytokines involved are IL-2, IL-6, IL-7, IL-16, granulocyte-colony stimulating factor, macrophage inflammatory protein-1a, tumor necrosis factor-alpha (TNF-α), MCP-1, IL-1α and IL-1 receptor agonist (IL-1Ra) [51].
The immunological mechanisms of MAS/secondary HLH overlap with the complex hyperinflammatory pathology of MIS-C in AKI. Extensive clinical and translational studies are required to better understand the cytokine mediators role and their efficacious use and safety as a target for future therapeutics.
IL-6 plays a crucial role in inducing the systemic inflammatory response in COVID-19, and elevated levels have been associated with worsening renal outcomes [52, 53]. A positive correlation was shown between severity of COVID-19 disease and serum levels of IL-6 and IL-2R [54]. Interestingly, experimental studies demonstrate a role of IL-6 activation and secretion by podocytes, endothelial cells, mesangial cells and tubular epithelial cells in renal inflammatory diseases. IL-6 levels would therefore aid in early diagnosis, serve as a target for therapeutics and prevent AKI worsening [55, 56].
Similarly, a proinflammatory cytokine, IL-8, plays a role in recruiting neutrophils and lymphocytes in the tubular interstitium causing inflammation. Investigational studies demonstrate upregulation of IL-8 in tubular epithelial cells due to elevated albumin and proteinuria leading to AKI [57]. A study on adults reported that 34% of COVID-19-infected individuals acquired proteinuria on the first day of admission, with 63% presenting with proteinuria during their hospital stay [58]. Proteinuria of any degree is associated with poor prognosis and increased in-hospital mortality [59]. In conclusion, it is plausible to hypothesize that these cumulative mechanisms could propel renal function impairment in a hypercytokinemia state of MIS-C.
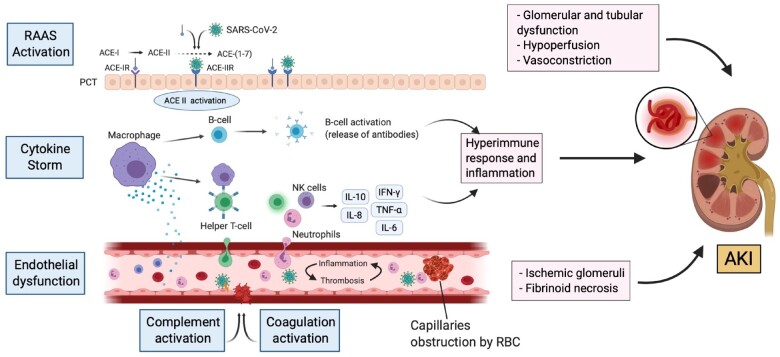
The pathogenesis of post-viral inflammatory response of MIS-C raises the concern for cytokine-mediated hypotension leading to renal hypoperfusion associated with AKI [9]. Deep et al. [28] conducted a multicenter study on 116 PIMS-TS patients, demonstrating 41.4% of children with AKI (any stage) and 27.6% with severe AKI. Nearly half (49%) of patients presented with vasodilatory shock requiring vasopressor support. In the same way, Stewart et al. observed the interaction between hypervolemia and hyperinflammatory shock in majority of their patients with AKI. The renal function improved following fluid resuscitation and use of inotropic support. However, the study was focused on pediatric patients with COVID-19 who suffered renal dysfunction [17]. Other factors, including vomiting and diarrhea, also precipitated dehydration leading to inadequate renal perfusion. These could be contributing to decreasing effective renal perfusion, thereby supporting renal hypoperfusion as a factor causing AKI [60, 61] (Figure 2).
FIGURE 2:
Pathogenesis of renal injury in MIS-C.
ACE-I, angiotensin converting enzyme-I; ACE-IR, angiotensin converting enzyme-I receptor; ACE-II, angiotensin converting enzyme-II; ACE-IIR, angiotensin converting enzyme-II receptor; NK cell, natural killer cell; PCT, proximal convoluted tubule; RAAS, renin–angiotensin–aldosterone system.
Other etiologies that may contribute to AKI development in MIS-C patients include:
(i) imbalanced renin–angiotensin–aldosterone system activation promoting glomerular dysfunction, fibrosis and vasoconstriction [62];
(ii) endothelial dysfunction, complement and coagulation activation, leading to kidney vascular injuries such as ischemic glomeruli and fibrinoid necrosis [45];
(iii) obstruction of the glomerular capillaries by red blood cells similar to the case in thrombotic microangiopathy [63]; and
(iv) drug toxicity and organ cross-talks are non-specific factors relative to critically ill COVID-19 patients management that may aggravate kidney injury [63–65].
Future studies are needed to further highlight the role of COVID-19 in MIS-C and AKI pathogenesis of hypervolemia and hyperinflammatory state. Additionally, constant renal function surveillance in MIS-C patients can be considered early in the disease course, with the help of collaborative multidisciplinary team, while avoiding AKI exacerbating factors and complications.
TREATMENT
There is no conclusive set of guidelines to manage MIS-C. Still, several organizations have published data based on the locally formulated protocol from previous treatment guidelines of similar diseases, including KD or adult protocols of COVID-19 treatment. Dove et al. conducted a multicenter cross-sectional survey on protocols of early hospital evaluation for treating MIS-C patients. The authors discussed various individual institutional protocols. Of the 40 centers, 21 centers required only 1 day of fever to consider MIS-C as a primary diagnosis. The majority of the centers used intravenous immunoglobulin (IVIG) and corticosteroids as primary therapy (98% and 93%, respectively). Additionally, heparin, anakinra and vasopressor agents were used frequently in children with severe illness [66]. The primary goal of the therapy in MIS-C is to reduce systemic inflammation with organ restoration decreasing mortality and reducing the risk of long-term sequelae. There is a need for multispecialty care guiding management and rehabilitation in patients with MIS-C due to its varied nature and presentation.
Categorizing patients at risk to an appropriate level of care
The suitable care setting is determined by the severity of the disease, risk of complications and follow-up ability. Children with mild symptoms, normal vital signs and physical examination can be managed in the outpatient setting with appropriate follow-up. Children with moderate to severe symptomatic MIS-C (Table 7) and those at risk for complications should be admitted to the hospital. Admission to a PICU is considered appropriate for children with significant respiratory compromise, hemodynamic instability and other potentially life-threatening complications. Published studies show the requirement of ICU to be as high as 80% [9, 10, 14, 18, 19].
Supportive care
General supportive care is pivotal in management [9]. The focus should be placed on the patient’s vital signs, hydration status, electrolytes and metabolic status. Special care in early detection of signs of hypoxia with prompt intervention should be taken for better outcomes. Children presenting with shock should be resuscitated according to standard protocols, with early goal-directed volume expansion followed by vasoactive agents [23]. In the event of LV dysfunction, epinephrine is preferable, with the addition of milrinone in case of severe LV dysfunction [23].
MIS-C-specific care
Children meeting the criteria for KD should receive standard therapies, including IVIG, aspirin and, if facing persistent signs of inflammation or coronary artery dilation/aneurysm, glucocorticoids. In instances of fulminant disease, mechanical hemodynamic support could also be necessary within the sort of extracorporeal membrane oxygenation (ECMO) or a ventricular assist device [47].
IVIG 2 g/kg and aspirin 20–25 mg/kg/dose every 6 h (80–100 mg/kg/day) should be used for all patients with KD-like illness, evidence of excessive inflammation (ferritin >700 ng/mL, CRP >30 g/dL or multisystem organ failure) or cardiac involvement. Aspirin dosage may also vary at individual centers. IVIG 2 g/kg as a single infusion with 3-day pulse methylprednisolone should be reserved for patients in high-risk categories (infants, KD shock syndrome, CRP >130 g/dL, admission echo Z-score >2.5 or aneurysms, Asian race) [47]. Patients with persistent inflammation despite IVIG and steroids can be given anakinra, an IL-1R antagonist, tocilizumab, an IL-6 antagonist, and infliximab, TNF-α blocker. Until now, the data on IL-1 and IL-6 blockade in COVID-19 infection and associated MIS-C are limited, but encouraging with multiple clinical trials currently in progress showing a high short-term survival rate in patients receiving IVIG and steroids [55, 56, 67] (Table 8).
Table 8.
Drugs used in the treatment of MISC (relevant to nephrology care)
| Drug | Mechanism | Dose in clinical trial | Dose adjustment | Special consideration |
|---|---|---|---|---|
| Remdesivir | Nucleotide analog: Inhibits viral replication | 3.5 to <40 kg: 5 mg/kg IV loading dose on Day 1, followed by 2.5 mg/kg IV every 24 h for 5–10 days (5 days for those with a rapid clinical response) | Not recommended GFR <30 mL/min/1.73 m2 | The excipient, a cyclodextrine accumulates in GFR <30 mL/min/1.73 m2 |
| ≥40 kg: 200 mg IV loading dose on day 1, followed by 100 mg IV every 24 h for 5–10 days (5 days for those with a rapid clinical response) | FDA approved | |||
| Hydroxychloroquine | Immuno-modulatory effects | 400 mg on day 1, followed by 200 mg twice a day | None | To be avoided in children with underlying QTc abnormalities |
| Lopinavir-ritonavir | Protease inhibitor | 100 mg twice a day | None | Not recommended in children |
| IVIG | Immunomodulatory effects | 2 g/kg | NA | If IVIG is not available or contraindicated, consider using corticosteroids |
| Glucocorticoids | Anti-inflammatory | Low-dose glucocorticoid regimens | None | Pediatric glucocorticoid arm of the RECOVERY trial is ongoing |
| Dexamethasone 0.15 mg/kg orally, IV or via an NG tube once daily (maximum dose 6 mg); | ||||
| prednisolone 1 mg/kg orally or via an NG tube once daily (maximum dose 40 mg); | ||||
| methylprednisolone 0.8 mg/kg IV once daily (maximum dose 32 mg) | ||||
| Tocilizumab | IL-6 antagonist | 8 mg/kg maximum 800 mg | None | In clinical trial only |
| Anakinra | IL-1Ra | 2–6 mg/kg/day IV/SQ, length of therapy to be decided | <30 mL/min GFR—administer every alternate day | Not dialyzable |
| Aspirin | Anti-inflammatory | 30–50 mg/kg/day, decrease to 3–5 mg/kg/day once afebrile 48 h | GFR <10 mL/min/1.73 m2—caution | Dialyzable—50–100% |
FDA, Food and Drug Administration; GFR, glomerular filtration rate; IV, intravenous; NG, nasogastric tube; QTc, corrected QT interval; SQ, subcutaneous.
Treatment of MIS-C with kidney involvement
Kidney replacement therapy (KRT) in the setting of multiorgan disease syndrome should be instituted with expertise. KRT can be utilized non-selectively to clear inflammatory mediators via convection, adsorption and diffusion. Continuous kidney replacement therapy (CKRT) corrects fluid overload and manages solute levels to provide hemodynamic stability in catabolic pediatric patients [68]. Immediate initiation of preemptive KRT in cases with progressive symptomatic respiratory insufficiency improves overall outcomes.
The pediatric continuous renal replacement therapy group advises the early initiation of KRT in critically ill COVID-19-related immune dysregulation syndrome patients since it has been shown to mitigate fluid overload, enhance cytokine clearance, improve PaO2/FiO2 ratios and establish hemodynamic stability earlier, leading to better overall outcomes [69]. No additional benefit has been reported with hybrid therapies such as prolonged intermittent renal replacement therapy (PIRRT) and sustained low efficiency dialysis. Hence, it is suggested to continue using the local institution’s recommended treatment modality. If CKRT/PIRRT is not available, intermittent hemodialysis is acceptable [69].
In terms of CKRT modality, the use of high flow continuous venovenous hemodiafiltration in critically ill COVID-19 pediatric patients is recommended because of its ability to boost the nonspecific removal of the circulatory cytokine mediators [70]. Increased reports of thrombotic complications are coming up in patients with MIS-C. Care should be taken during dialysis to avoid circuit clotting. As per local protocols, after insertion with heparin/citrate, the catheter’s immediate locking may be considered [71].
Some critically ill patients require mechanical ventilation via ECMO. Integration of the ECMO circuit with the CKRT equipment for these patients can provide additional respiratory support while reducing/preventing fluid overload and cytokine clearance [69]. However, the use of ECMO comes with a risk of potentially accentuating the cytokine activation.
CONCLUSION
Although there has been an increasing number of published data, the overall population-specific incidence of MIS-C remains unknown. The causal relationship and pathogenesis of kidney disease and MIS-C are still under debate. Post-viral immunological reaction to COVID-19 remains the best-implicated theory behind this disease’s pathogenesis, although an overall understanding of the immunopathogenesis induced by SARS-CoV-2 remains elusive. A better understanding of the kidney physiology in COVID-19 and treatment modalities available for children with renal dysfunction and MIS-C will help us to provide better care for this subset of children.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data used in this article will be shared on reasonable request to the corresponding author.
Supplementary Material
REFERENCES
- 1.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Geneva, Switzerland: World Health Organization. 2020. https://covid19.who.int/ (21 April 2021, date last accessed)
- 2.Chen YT, Shao SC, Hsu CK. et al. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care 2020; 24: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansrivijit P, Qian C, Boonpheng B. et al. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Invest Med 2020; 68: 1261–1270 [DOI] [PubMed] [Google Scholar]
- 4.Parri N, Lenge M, Buonsenso D.. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med 2020; 383: 187–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei M, Yuan J, Liu Y. et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA 2020; 323: 1313–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riphagen S, Gomez X, Gonzalez-Martinez C. et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395: 1607–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licciardi F, Pruccoli G, Denina M. et al. SARS-CoV-2-Induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics 2020; 146: e20201711. [DOI] [PubMed] [Google Scholar]
- 8.Verdoni L, Mazza A, Gervasoni A. et al. An outbreak of severe Kawasaki-like disease at the Italian epicenter of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395: 1771–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker E, Bamford A, Kenny J. et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324: 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldstein LR, Rose EB, Horwitz SM. et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med 2020; 383: 334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal College of Paediatrics and Child Health. https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance (September 2020, date last accessed)
- 12.New York State of Opportunity. (13 May 2020) Department of Health. Retrieved from https://health.ny.gov/press/releases/2020/docs/2020-05-13_health_advisory.pdf (December 2020, date last accessed)
- 13.Rowley AH.Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 2020; 20: 453–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufort EM, Koumans EH, Chow EJ. et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383: 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González-Dambrauskas S, Vásquez-Hoyos P, Camporesi A et al.; CRITICAL CORONAVIRUS AND KIDS EPIDEMIOLOGY CAKE STUDY. Pediatric critical care and COVID-19. Pediatrics 2020; 146: e20201766. [DOI] [PubMed] [Google Scholar]
- 16.Godfred-Cato S, Bryant B, Leung J. et al. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart DJ, Hartley JC, Johnson M. et al. Renal dysfunction in hospitalized children with COVID-19. Lancet Child Adolesc Health 2020; 4: e28–e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derespina KR, Kaushik S, Plichta A. et al. Clinical manifestations and outcomes of critically ill children and adolescents with COVID-19 in New York City. J Pediatr 2020; 226: 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies P, Evans C, Kanthimathinathan HK. et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolescent Health 2020; 4: 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira MFB, Litvinov N, Farhat SCL. et al. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics 2020; 75: e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee PY, Day-Lewis M, Henderson LA. et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest 2020; 130: 5942–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pouletty M, Borocco C, Ouldali N. et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020; 79: 999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capone CA, Subramony A, Sweberg T. et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 Infection. J Pediatr 2020; 224: 141–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBiasi RL, Song X, Delaney M. et al. Severe coronavirus disease-2019 in children and young adults in the Washington, DC, Metropolitan Region. J Pediatr 2020; 223: 199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dionne A, Mah DY, Son M. et al. Atrioventricular block in children with multisystem inflammatory syndrome. Pediatrics 2020; 146: e2020009704. [DOI] [PubMed] [Google Scholar]
- 26.Mamishi S, Movahedi Z, Mohammadi M. et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect 2020; 148: e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahbaznejad L, Navaeifar M, Abbaskhanian A. et al. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr 2020; 20: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deep A, Upadhyay G, Du Pré P. et al. Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 pandemic: experience from PICUs across United Kingdom. Crit Care Med 2020; 48: 1809–1818 [DOI] [PubMed] [Google Scholar]
- 29.Belhadjer Z, Méot M, Bajolle F. et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142: 429–436 [DOI] [PubMed] [Google Scholar]
- 30.Tullie L, Ford K, Bisharat M. et al. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Health 2020; 4: e19–e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G, Wu D, Guo W. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowley AH, Shulman ST, Arditi M.. Immune pathogenesis of COVID-19-related Multisystem Inflammatory Syndrome in Children (MIS-C). J Clin Invest 2020; 130: 5619–5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee PI, Hu YL, Chen PY. et al. Are children less susceptible to COVID-19? J Microbiol Immunol Infect 2020; 53: 371–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunyavanich S, Do A, Vicencio A.. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 2020; 323: 2427–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Shi L, Wang Y. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AE, Chambers RC.. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol 2014; 306: L217–L230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coronavirus (COVID-19) Statistics and Analysis. https://www.gov.uk/guidance/coronavirus-covid-19-statistics-and-analysis (2 September 2020, date last accessed)
- 38.Jiang L, Tang K, Levin M. et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020; 20: e276–e288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez OM, Bridges ND, Goldmuntz E. et al. The immune roadmap for understanding multi-system inflammatory syndrome in children: opportunities and challenges. Nat Med 2020; 26: 1819–1824. [DOI] [PubMed] [Google Scholar]
- 40.Gruber CN, Patel RS, Trachtman R et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 2020; 183: 982–995.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waggoner JJ, Katzelnick LC, Burger-Calderon R. et al. Antibody-dependent enhancement of severe disease is mediated by serum viral load in pediatric Dengue virus infections. J Infect Dis 2020; 221: 1846–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee WS, Wheatley AK, Kent SJ. et al. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 2020; 5: 1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puelles VG, Lütgehetmann M, Lindenmeyer MT. et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020; 383: 590–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan XW, Xu D, Zhang H. et al. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med 2020; 46: 1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen CP, Bourne TD, Wilson JD. et al. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep 2020; 5: 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakra NA, Blumberg DA, Herrera-Guerra A. et al. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel, Switzerland) 2020; 7: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hennon TR, Penque MD, Abdul-Aziz R. et al. COVID-19 associated Multisystem Inflammatory Syndrome in Children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol 2020; 57: 101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henderson LA, Canna SW, Schulert GS. et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol 2020; 72: 1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aulagnon F, Lapidus N, Canet E. et al. Acute kidney injury in adults with hemophagocytic lymphohistiocytosis. Am J Kidney Dis 2015; 65: 851–859 [DOI] [PubMed] [Google Scholar]
- 50.Esteve-Sole A, Anton J, Pino-Ramírez RM. et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric inflammatory multisystem syndrome and Kawasaki disease. J Clin Invest 2021; 131: e144554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stasi A, Castellano G, Ranieri E. et al. SARS-CoV-2 and viral sepsis: immune dysfunction and implications in kidney failure. J Clin Med 2020; 9: 4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su H, Lei CT, Zhang C.. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front Immunol 2017; 8: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmons EM, Himmelfarb J, Sezer MT. et al.; PICARD Study Group. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 2004; 65: 1357–1365 [DOI] [PubMed] [Google Scholar]
- 54.Chen L, Liu HG, Liu W. et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020; 43: E005 [DOI] [PubMed] [Google Scholar]
- 55.Luo P, Liu Y, Qiu L. et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020; 92: 814–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu X, Han M, Li T. et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 2020; 117: 10970–10975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang S, Leung JC, Abe K. et al. Albumin stimulates interleukin-8 expression in proximal tubular epithelial cells in vitro and in vivo. J Clin Invest 2003; 111: 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z, Wu M, Guo J. et al. Caution on kidney dysfunctions of 2019-nCoV patients. medRxiv 2020; doi: 10.1101/2020.02.08.20021212, preprint: not peer reviewed [Google Scholar]
- 59.Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee M, Hilado M, Sotelo S. et al. Acute kidney injury in multisystem inflammatory syndrome in children (MIS-C): a case report. SN Compr Clin Med 2020; 2: 2899–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McCulloch M, Abugrain K, Mosalakatane T. et al. Peritoneal dialysis for treatment of acute kidney injury in a case of paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. Perit Dial Int 2020; 40: 515–517 [DOI] [PubMed] [Google Scholar]
- 62.Vaduganathan M, Vardeny O, Michel T. et al. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; 382: 1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su H, Yang M, Wan C. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izzedine H, Jhaveri KD, Perazella MA.. COVID-19 therapeutic options for patients with kidney disease. Kidney Int 2020; 97: 1297–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dove ML, Jaggi P, Kelleman M. et al. Multisystem inflammatory syndrome in children: survey of protocols for early hospital evaluation and management. J Pediatr 2021; 229: 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cavalli G, De Luca G, Campochiaro C. et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol 2020; 2: e325–e331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ankawi G, Neri M, Zhang J. et al. Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: the promises and the pitfalls. Crit Care 2018; 22: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raina R, Chakraborty R, Sethi SK, Bunchman T.. Kidney replacement therapy in COVID-19 induced kidney failure and septic shock: a pediatric continuous renal replacement therapy [PCRRT] position on emergency preparedness with resource allocation. Front Pediatr 2020; 8: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ronco C, Tetta C, Mariano F, Wratten ML. et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs 2003; 27: 792–801 [DOI] [PubMed] [Google Scholar]
- 71.Song J-C, Wang G, Zhang W et al.; People’s Liberation Army Professional Committee of Critical Care Medicine, Chinese Society on Thrombosis and Haemostasis. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Military Med Res 2020; 7: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this article will be shared on reasonable request to the corresponding author.