Abstract
Background
Early in the coronavirus disease 2019 (COVID-19) pandemic, there was a concern over possible increase in antibiotic use due to coinfections among COVID-19 patients in the community. Here, we evaluate the changes in nationwide use of broad-spectrum antibiotics during the COVID-19 epidemic in South Korea.
Methods
We obtained national reimbursement data on the prescription of antibiotics, including penicillin with β-lactamase inhibitors, cephalosporins, fluoroquinolones, and macrolides. We examined the number of antibiotic prescriptions compared with the previous 3 years in the same period from August to July. To quantify the impact of the COVID-19 epidemic on antibiotic use, we developed a regression model adjusting for changes of viral acute respiratory tract infections (ARTIs), which are an important factor driving antibiotic use.
Results
During the COVID-19 epidemic in South Korea, the broad-spectrum antibiotic use dropped by 15%–55% compared to the previous 3 years. Overall reduction in antibiotic use adjusting for ARTIs was estimated to be 14%–30%, with a larger impact in children.
Conclusions
Our study found that broad-spectrum antibiotic use was substantially reduced during the COVID-19 epidemic in South Korea. This reduction can be in part due to reduced ARTIs as a result of stringent public health interventions including social distancing measures.
Keywords: antibiotic, COVID-19, respiratory virus, Korea, stewardship, public health measure
During the COVID-19 epidemic in South Korea, broad-spectrum antibiotic use was reduced by 14%–30%, with a larger impact on children.
The spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), has had an enormous health impact worldwide. Beyond the direct health impacts of infection and hospitalization with COVID-19, there is also the possibility of impact on other health conditions, and on changes in the use of medications such as antibiotics in the community [1]. Widespread changes in antibiotic use would have knock-on effects on antimicrobial resistance (AMR), which is a global health problem [2–4]. However, to date, there has been limited assessment of the impact of the COVID-19 pandemic on antibiotic use.
In South Korea, the first COVID-19 case was identified on 20 January 2020, and the national public health alert was raised to the highest level on 23 February 2020 shortly after a localized outbreak in Daegu city [5]. Combined public health measures were implemented to control the spread of COVID-19 [5], and strict social distancing measures were implemented on 22 March 2020 and relaxed on 20 April 2020 [6].
As public health measures were implemented, reduced respiratory virus activity was observed globally, which could lead to a slowdown in antibiotic use [7] and potentially AMR in the community [8]. Monitoring nationwide patterns of antibiotic use is thus crucial to evaluate antimicrobial stewardship during the COVID-19 pandemic [9]. Here, we aimed to quantify the impact of the COVID-19 epidemic on antibiotic use in South Korea in 2020. To achieve this, we analyzed the data on usage of broad-spectrum antibiotics that are commonly used to treat acute respiratory tract infections (ARTIs), which include bronchitis, rhinitis, and sinusitis, and their sequelae.
METHODS
Antibiotic Prescription Data
We obtained nationwide antibiotic prescribing data between August 2016 and July 2020 from the Korean Health Insurance Review and Assessment Service based on reimbursement data from >80 000 healthcare providers in South Korea, covering around 95% of the South Korean population. The monthly antibiotic prescription data were classified by the Anatomic Therapeutic Chemical classification system for penicillin with β-lactamase inhibitors (J01CR), cephalosporins (J01D), fluoroquinolones (J01MA), and macrolides (J01FA). Data were obtained for different age groups (<5, 5–19, 20–49, 50–65, and ≥65 years) and types of medical institutions (primary clinics, secondary and tertiary hospitals). Primary clinics were defined as institutions with <30 beds that mainly provided outpatient care, and secondary hospitals were defined as medical institutions with >30 beds. Tertiary hospitals were defined as hospitals commonly conducting complicated treatment for severe illnesses with >100 beds designated by the Korean Ministry of Health and Welfare [10].
Data of Hospitalization for Acute Respiratory Virus Infections
The number of patients hospitalized for common viral ARTIs was used as a proxy measure for the overall activity of ARTIs, which is known as a major driver of antibiotic use in the community [11]. Data were collected from weekly sentinel surveillance reports by the Korea Disease Control and Prevention Agency obtained from 196 sentinel hospitals [12]. The clinical samples acquired from hospitalized patients were tested according to a consistent protocol, using multiplex polymerase chain reaction (PCR) or real-time reverse transcription PCR. Respiratory viruses including human adenovirus, bocavirus, parainfluenza virus, respiratory syncytial virus, rhinovirus, metapneumovirus, coronavirus, and influenza virus were tested. Using the extracted hospitalization data, we estimated the nationwide rate of patients hospitalized for viral ARTIs per 100 000 individuals.
Statistical Methods
To examine the effect of the COVID-19 epidemic on antibiotic use, we first compared the pattern of antibiotic use during 2019/2020 with the preceding 3 years (2016/2017, 2017/2018, and 2018/2019). We computed weekly antibiotic use from monthly data by interpolating the spline functions keeping the monthly counts the same [13]. We then quantified the change in the weekly use of antibiotics comparing the pattern during pre- and post-timing of the highest public health alert (23 February 2020) for COVID-19 in South Korea [5, 14]. To compare the changes in antibiotic use, we used Welch 2-sample t test on the weekly antibiotic use for before and after periods of the highest public health alert. Furthermore, to identify the excess number of reductions on weekly antibiotic use, we measured the weekly difference of the use between 2016/2017–2018/2019 and 2019/2020, and compared the difference for the before and after periods of the highest public health alert. We also used a paired t test on the weekly antibiotic use during the months March through July in 2020 and the preceding 3 years.
To quantify the impact of the COVID-19 epidemic on antibiotic use accounting for potential change in ARTIs, we estimated the overall reduction in antibiotic use during the COVID-19 epidemic by estimating the difference of each fitted regression model for weekly antibiotic use, adjusted for viral ARTI hospitalizations.
As antibiotic use is highly correlated with the activity of viral ARTIs (ie, the number of hospitalizations for viral ARTIs at time t) [15] and affected by other extrinsic factors such as the COVID-19 epidemic (ie, an indicator variable defined as for the weeks after declaration of the national public health alert (23 February–31 July 2020) and 0 otherwise), the regression model was defined as . The decreases (/increases) over time as the decreases (/increases) and further modified by accounting for the effects of the public health measures on COVID-19. and are the regression coefficients that quantify the effect of COVID-19 epidemic on antibiotic use (). Simplifying, we constructed a log-linear multivariable regression model as , where and were estimated, and the overall reduction in was estimated using these parameters.
We conducted similar analyses for different age groups and types of medical institutions. All analyses were done in R version 3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
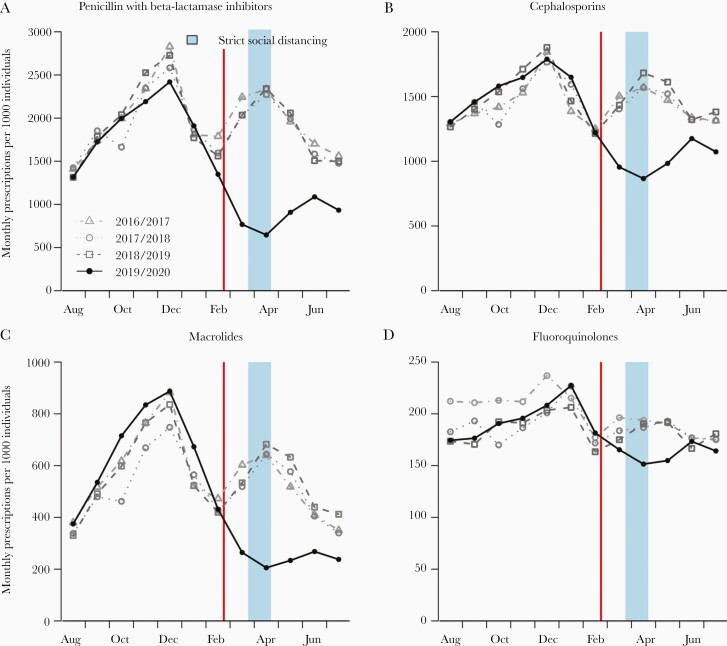
We identified a clear seasonal pattern in broad-spectrum antibiotic use in South Korea from 2016 to 2019, with a large peak around December and a second peak around April (Figure 1). In 2019/2020, the pattern was similar until emergence of the COVID-19 epidemic, after which the use of all broad-spectrum antibiotics dropped substantially compared with the previous 3 years (all P < .001, Table 1). The estimated reduction on weekly antibiotic use in 2019/2020 was 178 per 1000 persons for penicillin with β-lactamase inhibitors, 106 per 1000 persons for cephalosporins, 72 per 1000 persons for macrolides, and 35 per 1000 persons for fluoroquinolones (Table 1). Compared to March–July in the previous 3 years, the use of penicillin with β-lactamase inhibitors, cephalosporins, macrolides, and fluoroquinolones in 2020 were reduced by 54%, 32%, 55%, and 15%, respectively (Figure 1 and Table 1). Antibiotic use rebounded after strict social distancing measures were relaxed in April, but still remained below mean use in the prior years for all broad-spectrum antibiotics except fluoroquinolones (Figure 1).
Figure 1.
Monthly antibiotic use, including penicillin with β-lactamase inhibitors (A), cephalosporins (B), macrolides (C), and fluoroquinolones (D) in South Korea. Points indicate monthly use by the years 2016/2017, 2017/2018, 2018/2019, and 2019/2020. The red vertical lines indicate declaration of the highest public health alert for coronavirus disease 2019 (23 February 2020), and the blue vertical area depicts the period of strict social distancing measures.
Table 1.
Reduction of Weekly Number of Antibiotic Prescriptions per 1000 Persons During 2019/2020 Compared to the Corresponding Period in the Previous 3 Years, South Korea
| Characteristic | Estimated Reduction in Antibiotic Use, During Pre-and Post-timing of COVID-19 epidemic | Estimated Reduction (%) in Antibiotic Use, During COVID-19 Epidemic vs Corresponding Period in 2017–2019, With P Valuec | Estimated Overall Reduction in Antibiotic Use Associated With COVID-19 Epidemic, % (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Broad-Spectrum Antibiotics | Years | Before 23 Feb, Median, (IQR) | After 23 Feb, Median, (IQR) | Change (%) With P Valueb | Excess Number of Reduction with P Valueb | Unadjusted (Univariate Regression Model) | Adjustedd (Multivariable Regression Model) | |
| Penicillin with β-lactamase inhibitors | 2016/2017–2018/2019a | 426.9 (395.0–492.8) | 399.9 (360.1–510.3) | –5.7 (P = .311) | … | … | … | … |
| 2019/2020 | 421.5 (311.3–481.7) | 205.5 (156.6–239.9) | –51.0 (P < .001) | 178.0 (P < .001) | 54.1 (P < .001) | 52.7 (46.6–58.1) | 29.5 (7.8–55.5) | |
| Cephalosporins | 2016/2017–2018/2019a | 325.0 (309.9–355.4) | 322.7 (305.6–361.7) | –1.2 (P = .678) | … | … | … | … |
| 2019/2020 | 352.2 (296.1–361.7) | 219.7 (209.9–262.1) | –32.8 (P < .001) | 106.3 (P < .001) | 31.9 (P < .001) | 33.1 (29.5–38.2) | 14.2 (3.5–26.1) | |
| Macrolides | 2016/2017–2018/2019a | 120.2 (108.5–148.4) | 111.1 (89.1–143.2) | –10.4 (P = .144) | … | … | … | … |
| 2019/2020 | 139.1 (94.5–179.6) | 52.4 (48.1–59.5) | –62.0 (P < .001) | 71.9 (P < .001) | 54.6 (P < .001) | 60.1 (54.0–65.5) | 24.3 (–2.5 to 58.5) | |
| Fluoroquinolones | 2016/2017–2018/2019a | 44.4 (42.9–46.7) | 41.7 (40.1–43.9) | –6.3 (P < .001) | … | … | … | … |
| 2019/2020 | 43.4 (40.1–46.0) | 35.9 (34.7–39.1) | –18.3 (P < .001) | 34.7 (P = .002) | 14.8 (P = .001) | 16.2 (12.4–19.8) | 13.9 (3.9–24.8) |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; IQR, interquartile range.
aMean of antibiotic use during 2017–2019.
bWelch 2-sample t test.
cPaired t test.
dEstimated from the regression model adjusted on changes of viral acute respiratory tract infection hospitalization.
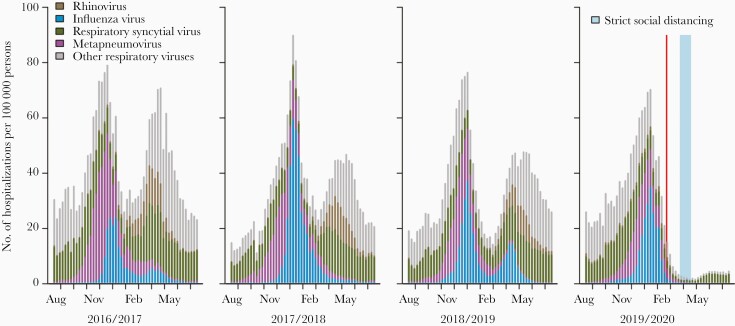
Hospitalizations for viral ARTIs in 2016/2017–2018/2019 had a similar seasonal pattern as antibiotic use (Figure 2). However, the pattern in 2019/2020 was significantly reduced compared with the previous 3 years (P < .001, Supplementary Table). In the regression analysis, the COVID-19 epidemic in South Korea was associated with a reduction of 30% (95% confidence interval [CI], 8%–56%) for penicillin with β-lactamase inhibitor use, 14% (95% CI, 4%–26%) for cephalosporin use, and 14% (95% CI, 4%–25%) for fluoroquinolone use. For macrolides, the reduction was 24% (95% CI, –3% to 59%), but this was not statistically significant.
Figure 2.
The estimated number of hospitalizations for acute respiratory tract infections in South Korea, 2016–2020. The red vertical bar indicates declaration of the highest public health alert for coronavirus disease 2019 (23 February 2020), and the blue vertical bar indicates the period with strict social distancing measures.
For the use of penicillin with β-lactamase inhibitors, cephalosporins, and macrolides, similar effects were estimated across different age groups, including preschoolers (0–5 years: 31%–51%), school-aged children (6–19 years: 23%–51%), young adults (20–49 years: 16%–36%), and the elderly (>65 years: 15%–30%) (Table 2). However, the effects on the use of fluoroquinolone were not statistically significant for children and adults. For the medical institutions, primary clinics and the secondary hospital similarly had reduced antibiotic use of around 16%–46% and 10%–45%, respectively. However, the decline was lower at tertiary hospitals (7%–16%) and the changes in fluoroquinolone use were not statistically significant at tertiary hospitals (Table 3).
Table 2.
Estimated Overall Reduction in the Weekly Number of Antibiotic Prescriptions per 1000 Persons Associated With the Coronavirus Disease 2019 Epidemic by Different Age Groups in South Korea
| Broad-Spectrum Antibiotics | Reduction in Antibiotic Use per 1000 Persons, % (95% CI)a | ||||
|---|---|---|---|---|---|
| 0–5 y | 6–19 y | 20–49 y | 50–64 y | ≥65 y | |
| Penicillin with β-lactamase inhibitors | 39.8 (43.1–63.6) | 39.2 (24.1–54.3) | 30.8 (15.3–46.3) | 27.4 (11.8–43.0) | 20.6 (5.7–35.5) |
| Cephalosporins | 31.4 (15.4–47.5) | 22.9 (8.8–36.9) | 15.9 (2.5–29.3) | 16.4 (2.6–30.1) | 14.5 (.8–28.2) |
| Macrolides | 50.5 (30.6–70.4) | 51.0 (30.5–71.4) | 35.7 (19.2–52.2) | 35.5 (17.8–53.2) | 29.9 (12.4–47.4) |
| Fluoroquinolones | NAb | 5.8 (–7.4 to 19.1) | 11.0 (–2.7 to 24.7) | 12.3 (–1.6 to 26.2) | 14.1 (.1–28.1) |
Abbreviations: CI, confidence interval; NA, not available.
aEstimated from the regression model adjusting for viral acute respiratory tract infection hospitalization.
bStable estimate was not available due to the low number of antibiotic use.
Table 3.
Estimated Overall Reduction in the Weekly Number of Antibiotic Prescriptions per 1000 Persons Associated With the Coronavirus Disease 2019 Epidemic in Different Types of Medical Institutions in South Korea
| Broad-Spectrum Antibiotics | Reduction in Antibiotic Use per 1000 Persons, % (95% CI)a | ||
|---|---|---|---|
| Primary Clinics | Secondary Hospitals | Tertiary Hospitals | |
| Penicillin with β-lactamase inhibitors | 41.7 (31.6–51.8) | 31.7 (27.6–47.8) | 12.1 (5.8–18.5) |
| Cephalosporins | 25.7 (19.4–32.0) | 17.9 (11.4–24.4) | 7.1 (1.6–12.6) |
| Macrolides | 45.7 (33.8–57.6) | 45.3 (31.3–59.3) | 16.3 (7.7–24.9) |
| Fluoroquinolones | 15.9 (10.0–21.9) | 9.5 (4.0–15.1) | 5.7 (–.001 to 11.4) |
Abbreviation: CI, confidence interval.
aEstimated from the regression model adjusting for viral acute respiratory tract infection hospitalization.
Discussion
After declaration of the highest public health alert on COVID-19 in South Korea, use of penicillin with β-lactamase inhibitors, cephalosporins, macrolides, and fluoroquinolones dropped by 54%, 32%, 55%, and 15% compared to prior years. Accounting for reduced ARTI hospitalizations during the COVID-19 epidemic in South Korea, the estimated overall reduction on antibiotic use was 14%–30%. This result was significant across all broad-spectrum antibiotics except macrolides. However, in the different age groups and different types of medical institutions, the overall reduction on macrolides was significantly associated with the COVID-19 epidemic, when adjusted for viral ARTI hospitalizations. This suggests that macrolides may be used in a more targeted manner and thus were less affected by broad reductions in overall use.
For the overall reduction adjusting for viral ARTI hospitalizations by the different age groups, the changes in the use of fluoroquinolones were not significantly associated with the COVID-19 epidemic. This is likely to be affected by the narrow indication (eg, genitourinary infections or hospital-acquired infections) on the fluoroquinolone prescription for the children as well as adults. This also explains the nonsignificance of changes in fluoroquinolone use at the tertiary hospitals.
We also observed a clear rebound in antibiotic use after relaxing strict social distancing measures in each of the antibiotics analyzed. This may have been because strict social distancing measures reduced use of medical services resulting in fewer prescriptions for viral ARTIs, which are typically unnecessary. However, as we estimated hospitalizations with the ARTIs, and these are unlikely to be affected by medical-seeking behavior, it is more likely that social distancing measures reduced the spread of respiratory viruses, and that relaxing restrictions led to an increase in viral transmission, ARTIs and subsequently antibiotic use [16].
Consistent with the latter theory, in which tertiary hospitals generally have a more stringent prescription on broad-spectrum antibiotics, the level of reduction of antibiotic use was lower in tertiary hospitals than in other types of medical institutions.
Our findings are similar to a previous report that found reductions in the risk of acquiring and spreading respiratory infections during the COVID-19 pandemic through implementation of nonpharmaceutical measures including hand-washing, face masks, physical distancing, and travel restrictions [17]. Our findings also suggested that the implementation of public health measures during the COVID-19 pandemic could lead to reductions in AMR in the community [18]. Because patients were less likely to seek care in the outpatient setting (eg, primary physicians), there were fewer opportunities for antibiotics to be prescribed, which should reduce the selection pressure that drives AMR. Furthermore, there was no observed increase in the prescribing rate per ARTI patient visit, suggesting that the pandemic did not erode antimicrobial stewardship efforts for ARTIs (Supplementary Figure 1).
Our findings have several limitations. First, since February 2020, the Korean government has recommended that all ARTI patients be screened for COVID-19 without cost [19]. Furthermore, prescribing requirements were loosened to allow prescriptions to be written through telephone consultations without the need for patients to visit a medical facility [20]. However, changes in health-seeking behavior during the outbreak in South Korea may have reduced the propensity of individuals to seek care for symptoms consistent with upper respiratory symptoms that were not COVID-19. Therefore, we used admission data as an explanatory variable in our model, which should be somewhat resilient to this effect. The nationwide use of chronic-disease medications remained stable during the COVID-19 epidemic (Supplementary Figure 2), suggesting that transmission of ARTIs, rather than care-seeking, was the most likely factor in reduced antibiotic use. Second, we used weekly data on antibiotic use, estimated by spline approximation, which may affect the accuracy of our weekly estimates. Third, population-level data may not represent the individual level. However, a previous cohort study showed a significant association between antibiotic prescribing and respiratory virus activity in the population and individual levels [21]. Furthermore, a previous study conducted in a single hospital demonstrated that overall antibiotic prescriptions decreased by an average of 6.1% early in the COVID-19 pandemic [22], which is consistent with our results. Finally, social distancing measures were continuously adjusted based on the evolving situations of COVID-19 by Korean public health authorities in different administrative regions. In this study, we only assessed the overall impact of these interventions on antibiotic use, but were not able to assess the impact of their change over the course of the epidemic.
Conclusions
In conclusion, we found a substantial decline in the use of broad-spectrum antibiotics after declaration of the highest public alert on COVID-19 in South Korea. Our findings suggested that public health control measures including social distancing measures for the COVID-19 pandemic could have contributed to disrupting the transmission of all respiratory viruses, reducing the number of viral ARTIs as well as care-seeking behavior and hence reducing the use of broad-spectrum antibiotics.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funding bodies had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Financial support. This work was supported by the Basic Science Research Program through the National Research Foundation of Korea by the Ministry of Education (grant number NRF-2020R1I1A3066471).
Potential conflicts of interest. B. J. C. received honoraria from Roche and Sanofi Pasteur. E. Y. K. was supported by the United States Centers for Disease Control and Prevention MInD-Healthcare Program (grant numbers U01CK000589 and 1U01CK000536, and contract number 75D30120P07912). All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gonzalez-Zorn B. Antibiotic use in the COVID-19 crisis in Spain. Clin Microbiol Infect 2021; 27:646–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clancy CJ, Nguyen MH. COVID-19, superinfections and antimicrobial development: what can we expect? Clin Infect Dis 2020; 71:2736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monnet DL, Harbarth S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Euro Surveill 2020; 25:2001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clancy CJ, Buehrle DJ, Nguyen MH. PRO: the COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC-Antimicrob Resis 2020; 2. doi:10.1093/jacamr/dlaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryu S, Ali ST, Jang C, Kim B, Cowling BJ. Effect of nonpharmaceutical interventions on transmission of severe acute respiratory syndrome coronavirus 2, South Korea, 2020. Emerg Infect Dis 2020; 26:2406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park IN, Yum HK. Stepwise strategy of social distancing in Korea. J Korean Med Sci 2020; 35:e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein EY, Schueller E, Tseng KK, Morgan DJ, Laxminarayan R, Nandi A. The impact of influenza vaccination on antibiotic use in the United States, 2010–2017. Open Forum Infect Dis 2020; 7:ofaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawson TM, Moore LSP, Castro-Sanchez E, et al. . COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother 2020; 75: 1681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staub MB, Beaulieu RM, Graves J, Nelson GE. Changes in antimicrobial utilization during the COVID-19 pandemic after implementation of a multispecialty clinical guidance team [manuscript published online ahead of print 26 October 2020]. Infect Control Hosp Epidemiol 2020. doi:10.1017/ice.2020/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korea Centers for Disease Control and Prevention. Korea antimicrobial resistance monitoring system 2016. Osong, South Korea: Korea Centers for Disease Control and Prevention, 2018. [Google Scholar]
- 11.Rebnord IK, Sandvik H, Mjelle AB, Hunskaar S. Factors predicting antibiotic prescription and referral to hospital for children with respiratory symptoms: secondary analysis of a randomised controlled study at out-of-hours services in primary care. BMJ Open 2017; 7:e012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korea Disease Control and Prevention Agency. Weekly sentinel surveillance report. Available at: http://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=2&pblctDtaSn=2276. Accessed 4 January 2021.
- 13.Ali ST, Cowling BJ, Lau EHY, Fang VJ, Leung GM. Mitigation of influenza B epidemic with school closures, Hong Kong, 2018. Emerg Infect Dis 2018; 24:2071–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim I, Lee J, Lee J, Shin E, Chu C, Lee SK. KCDC risk assessments on the initial phase of the COVID-19 outbreak in Korea. Osong Public Health Res Perspect 2020; 11: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu S, Kim S, Kim BI, Klein EY, Yoon YK, Chun BC. Temporal relationship between antibiotic use and respiratory virus activities in the Republic of Korea: a time-series analysis. Antimicrob Resist Infect Control 2018; 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu S, Ali ST, Cowling BJ, Lau EHY. Effects of school holidays on seasonal influenza in South Korea, 2014–2016. J Infect Dis 2020; 222:832–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieuwlaat R, Mbuagbaw L, Mertz D, et al. . COVID-19 and antimicrobial resistance: parallel and interacting health emergencies [manuscript published online ahead of print 16 June 2020]. Clin Infect Dis 2020. doi:10.1093/cid/ciaa773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knight GM, Glover RE, McQuaid CF, et al. . Antimicrobial resistance and COVID-19: intersections and implications. Elife 2021; 10:e64139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korean Ministry of Health and Welfare. COVID-19 update (7 February 2020). Available at: http://www.mohw.go.kr/react/al/sal0301vw.jsp?PAR_MENU_ID=04&MENU_ID=0403&SEARCHKEY=&SEARCHVALUE=&page=1&CONT_SEQ=352757. Accessed 2 April 2021.
- 20.Korean Ministry of Health and Welfare. Information on temporary allowance of phone consultation and prescription. Available at: http://www.mohw.go.kr/react/al/sal0101vw.jsp?PAR_MENU_ID=04&MENU_ID=040101&page=1&CONT_SEQ=353269. Accessed 2 April 2021.
- 21.Nitsch-Osuch A, Gyrczuk E, Wardyn A, Życinska K, Brydak L. Antibiotic prescription practices among children with influenza. Adv Exp Med Biol 2016; 905:25–31. [DOI] [PubMed] [Google Scholar]
- 22.Buehrle DJ, Decker BK, Wagener MM, et al. . Antibiotic consumption and stewardship at a hospital outside of an early coronavirus disease 2019 epicenter. Antimicrob Agents Chemother 2020; 64:e01011-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.