Abstract
We describe a case of prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a patient receiving ocrelizumab for multiple sclerosis. Viral RNA shedding, signs, and symptoms persisted for 69 days with resolution after administration of convalescent plasma and antiviral therapy. This case suggests risk for persistent SARS-CoV-2 infection in patients treated with anti-CD-20 monoclonal antibodies and supports a role for humoral immunity in disease resolution.
Keywords: COVID-19, humoral immunity, ocrelizumab, SARS-CoV-2
CASE REPORT
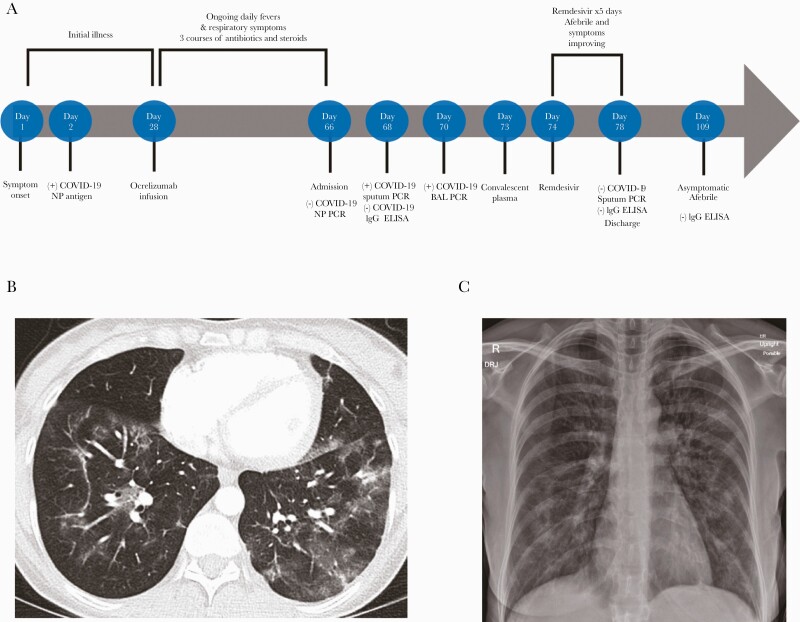
A 46-year-old female with relapsing remitting multiple sclerosis (RRMS) on ocrelizumab presented to the emergency department with an approximate 9-week history of daily fevers and dyspnea. Symptom onset began 65 days earlier with upper respiratory symptoms, fever, and cough. On day 2 of illness, she was diagnosed with coronavirus disease 2019 (COVID-19), as demonstrated by a positive SARS-CoV-2 nasopharyngeal swab antigen immunoassay. She did not require hospitalization, supplemental oxygen, or additional treatment. She continued to have daily fevers and significant dyspnea with minimal exertion after her initial 10-day isolation period. The clinical course is outlined in Figure 1.
Figure 1.
A, Timeline of illness and treatment course. B, Computed tomography of the chest on day 67 of illness with bilateral ground glass opacities. C, Chest radiograph on day 66 of illness with bilateral hazy opacities. Abbreviations: BAL, bronchoalveolar lavage; COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; NP, nasopharyngeal; PCR, polymerase chain reaction.
On day 28, she received a routine infusion of ocrelizumab scheduled every 6 months for RRMS despite remaining symptomatic. Symptoms did not improve over several weeks, and she returned to outpatient clinics for evaluation on 4 occasions. She received 3 empiric courses of antibiotics (azithromycin once and amoxicillin-clavulanate twice) and steroids (2 courses of prednisone 40 mg daily for 5 days) for presumed superimposed bacterial pneumonia following SARS-CoV-2. Pneumonia was diagnosed based on symptoms and radiographic findings. She did not experience clinical improvement with any of these interventions.
On day 66 of illness, she presented to the emergency department with prolonged fevers and dyspnea. Vital signs were notable for an oxygen saturation of 93% on room air, heart rate of 105, temperature of 38.5°C, and blood pressure of 98/60. Her medical history included RRMS, hypothyroidism, celiac disease, and fibromuscular dysplasia. She had no history of underlying lung disease. The patient’s medication list included ocrelizumab infusion every 6 months, levothyroxine, dalfampridine, modafinil, and acetaminophen. She had no known allergies. Her social history was notable for occupation in social services at a school and hospital, no tobacco or alcohol use, and no recent travel. She had no known sick contacts and no relevant family medical history. There were no other known medical risk factors for prolonged SARS-CoV-2 infection.
On exam, she was dyspneic with conversation and minimal exertion. She had tachycardia and a dry cough with diminished bibasilar breath sounds and crackles. Her abdominal exam was tender to palpation throughout. She had normal neurologic, musculoskeletal, and skin exams.
A chest radiograph revealed bilateral patchy opacities (Figure 1). Initial inpatient laboratory testing revealed a hemoglobin of 11.9, a leukocyte count of 9.8 (×103/μL) with a normal differential, thrombocytosis with a platelet count of 470 (×103/μL), and normal renal and hepatic function. A computed tomography pulmonary angiogram identified multifocal patchy ground glass opacities without evidence of a focal pneumonia, pulmonary abscess, or pulmonary embolism (Figure 1). Blood cultures, sputum cultures, respiratory viral polymerase chain reaction (PCR) panel, and serum fungal biomarkers did not reveal any superimposed bacterial, fungal, or viral infection. Serum immunoglobulin A (51 mg/dL), G (445 mg/dL), and M (26 mg/dL) levels were low. A nasopharyngeal swab for SARS-CoV-2 by nucleic acid amplification and COVID-19 serology by enzyme-linked immunoassay (ELISA) were negative. Refer to Supplementary Table 1 for details regarding specific testing and results.
The patient remained febrile with daily fevers to a maximum temperature of 39.4°C. Given concern for prolonged COVID-19 infection, an induced sputum sample was obtained and SARS-CoV-2 RNA was detected by a hologic PCR assay with a cycle threshold (Ct) value of 35.2. On day 70, the patient underwent bronchoscopy. SARS-CoV-2 testing using the same assay was positive in bronchoalveolar lavage fluid with a Ct value of 24.5. Additional bronchoscopy studies did not identify evidence of other bacterial, fungal, or viral infections.
With persistent fevers and testing from lower respiratory fluid providing evidence for active SARS-CoV-2 replication in the setting of humoral immunodeficiency, we administered 1 unit (~250 mL) of COVID-19 convalescent plasma (CCP) on day 73 of illness. Remdesivir 200 mg was administered on day 74 followed by 100 mg daily over the following 4 days. On day 74, her fevers resolved and her respiratory symptoms significantly improved ~48 hours after receiving CCP and 24 hours after starting remdesivir. Serum COVID-19 IgG by ELISA after CCP transfusion remained negative on day 77. On day 78, repeat sputum SARS-CoV-2 testing by PCR was negative and the patient was discharged. She remained asymptomatic and afebrile 1 month later. On day 109, repeat COVID-19 IgG testing by ELISA was negative.
Amplification and sequencing of SARS-CoV-2 from RNA in the original positive sputum specimen were performed. The isolate had 19 missense mutations, which is similar to the number of mutations observed from other members of the same clade, 20G (https://clades.nextstrain.org;version 0.14.0) (Supplementary Table 2). The 20G clade currently comprises 40% of US strains. The patient isolate belongs to the PANGO lineage B.1.2 (pangolin; version 2.3.2) and contains the D614G mutation. None of the mutations recently associated with increased transmissibility or immune escape such as E484K or N501Y were present, and no mutations were present in the receptor-binding domain region of the S gene encoding the spike protein.
DISCUSSION
Understanding potential risks for complicated SARS-CoV-2 illness in patients with humoral immunodeficiency has implications for recognition and management of disease in this population. Although cases of prolonged COVID-19 infection have been reported in patients with malignancy and chemotherapy-related immunosuppression, there are limited data regarding complications of COVID-19 in patients receiving anti-CD20 monoclonal antibodies for autoimmune diseases [1–5].
For most immunocompetent patients, infectious SARS-CoV-2 replication is cleared between 10 and 20 days, with the possibility of prolonged nonviable viral shedding for up to 3 months [6, 7]. However, time to recovery in immunocompromised patients is not as well defined, and infectious viral shedding for >2 months has been demonstrated in patients with severe immunosuppression [2, 8]. Immunocompromised patients may also maintain prolonged viral replication in the lower respiratory tract despite negative results from nasopharyngeal PCR swabs [2].
In this case, COVID-19 antibodies were not identified 10 weeks after initial infection, suggesting an inadequate humoral response to COVID-19 in the setting of ocrelizumab treatment. Anti-CD20 antibodies have been associated with inadequate antibody production to SARS-CoV-2 in patients with multiple sclerosis (MS) [9, 10]. Following treatment, different anti-CD20 antibodies result in variable times to B-cell repletion, with the longest occurring up to 18 months after ocrelizumab [11]. This suggests the need to consider medication-specific effects on antibody production that could have implications in vaccine responsiveness.
The patient improved rapidly after 1 dose of CCP and initiation of remdesivir [12, 13]. As both treatments were initiated as soon as available after admission, either or both may have contributed to the patient’s rapid improvement. As SARS-CoV-2 antibody titers of CCP available at the time were not measured, the negative qualitative test of patient serum after administration may have been due to low anti-SARS-CoV-2 titers in the infused plasma or to in vivo dilution. Remdesivir was started 1 day after CCP administration to avoid confusion about immediate adverse reactions.
A similar prolonged SARS-CoV-2 infection was reported in a patient with B-cell immunodeficiency who temporarily improved after 1 course of CCP but did not clear the virus until treatment with a second course of CCP and remdesivir [5]. Although the patient’s symptoms of active viral infection resolved, repeat testing for SARS-CoV-2 antibodies remained negative, suggesting that she remained incapable of mounting a serological response.
We hypothesize that ocrelizumab-induced immunosuppression contributed to our patient’s prolonged febrile illness from COVID-19. Ocrelizumab, similar to rituximab, is a monoclonal antibody against CD20+ B cells used in the treatment of multiple sclerosis. Early reports of SARS-CoV-2 among patients with MS treated with ocrelizumab or rituximab suggested a potential beneficial effect of B-cell immunosuppression on SARS-CoV-2 disease severity [14–16]. Observational studies have had inconsistent results when evaluating the relationship between disease-modifying therapies (DMTs) for MS, including anti-CD20 antibodies, and SARS-CoV-2 illness severity [11, 17–20]. A small observational study found no relationship between ocrelizumab and SARS-CoV-2 disease severity, while a review of the MS Global Data Sharing Initiative suggested that anti-CD20 DMTs are associated with higher frequencies of hospital admission, intensive care unit admission, and need for artificial ventilation compared with other DMTs [18, 21]. A similar case of prolonged COVID-19 infection in the setting of anti-CD20 DMTs has been reported in a patient on rituximab who had persistent viral shedding on day 151 of illness [4]. Cases of prolonged SARS-CoV-2 infection among patients with humoral immune deficiencies related to CLL, lymphoma, and chemoimmunotherapy have also been described [1, 2, 5]. Together, these reports support the potential importance of humoral responses for SARS-CoV-2 clearance and illness resolution.
Patients treated with therapy that suppresses the humoral immune response may be at particular risk for prolonged illness and viral shedding beyond 20 days and warrant close monitoring if they contract COVID-19. Prophylaxis or treatment soon after COVID-19 diagnosis with long-lasting anti-SARS-CoV-2 monoclonal antibodies, currently under development, may be a consideration for patients on anti-CD20 therapy and at high risk for exposure. In the current case, known mutations associated with increased transmissibility or virulence were not detected despite probable ongoing viral replication for several months. However, significant viral mutation and evolution of SARS-CoV-2 have been reported in cases of prolonged illness due to immunosuppression, both with and without CCP treatment [4, 22]. Although such evolution may occur during prolonged viral replication in the absence of antibody therapy, the possibility that prophylactic or therapeutic use of monoclonal antibodies may facilitate such selection in immunocompromised hosts needs to be considered.
Additional research is needed to further elucidate the role of the humoral immune system in controlling SARS-CoV-2 infection and establish optimal treatment strategies for patients on B-cell-depleting therapies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors thank Salika Shakir, PhD, for her assistance locating laboratory samples and ensuring completion of appropriate testing at ARUP laboratories.
Financial support. S.S. was supported by NIH R01 CA08311.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The patient’s written consent was obtained. The work conforms to standards currently applied in the United States. We have followed procedures in accordance with the Helsinki Declaration.
References
- 1. Helleberg M, Niemann CU, Moestrup KS, et al. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis 2020; 222:1103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020; 183:1901–12.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes R, Pedotti R, Koendgen H. COVID-19 in persons with multiple sclerosis treated with ocrelizumab—a pharmacovigilance case series. Mult Scler Relat Disord 2020; 42:102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020; 383:2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baang JH, Smith C, Mirabelli C, et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis 2021; 223:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh KA, Spillane S, Comber L, et al. The duration of infectiousness of individuals infected with SARS-CoV-2. J Infect 2020; 81:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trypsteen W, Van Cleemput J, Snippenberg WV, et al. On the whereabouts of SARS-CoV-2 in the human body: a systematic review. PLoS Pathog 2020; 16:e1009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 2020; 383:2586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thornton JR, Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in two MS patients treated with ocrelizumab. Mult Scler Relat Disord 2020; 44:102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zabalza A, Cárdenas-Robledo S, Tagliani P, et al. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur J Neurol 2020; doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]
- 11. Bhise V, Dhib-Jalbut S. Potential risks and benefits of multiple sclerosis immune therapies in the COVID-19 era: clinical and immunological perspectives. Neurotherapeutics 2021; 18:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020; 384:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. [published online ahead of print August 12, 2020]. medRxiv. doi:. [DOI] [Google Scholar]
- 14. Montero-Escribano P, Matías-Guiu J, Gómez-Iglesias P, et al. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord 2020; 42:102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rostami Mansoor S, Ghasemi-Kasman M. Impact of disease-modifying drugs on the severity of COVID-19 infection in multiple sclerosis patients. J Med Virol 2021; 93:1314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Novi G, Mikulska M, Briano F, et al. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord 2020; 42:102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Louapre C, Collongues N, Stankoff B, et al. ; Covisep investigators . Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77:1079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes R, Whitley L, Fitovski K, et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord 2020; 49:102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adamczyk-Sowa M, Mado H, Kubicka-Bączyk K, et al. SARS-CoV-2/COVID-19 in multiple sclerosis patients receiving disease-modifying therapy. Clin Neurol Neurosurg 2021; 201:106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sepúlveda M, Llufriu S, Martínez-Hernández E, et al. Incidence and impact of COVID-19 in MS: a survey from a Barcelona MS unit. Neurol Neuroimmunol Neuroinflamm 2021; 8:e954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simpson-Yap S, Kalincik T, Rijke N, et al. First results of the COVID-19 in MS global data sharing initiative suggest anti-CD20 DMTs are associated with worse COVID-19 outcomes. Paper presented at: Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) 2020 (Virtual); 22 September 2020. [Google Scholar]
- 22. Kemp SA, Collier DA, Datir R, et al. Neutralising antibodies in spike mediated SARS-CoV-2 adaptation. Nature 2021; 592:277–82.33545711 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.