Abstract
Objective
To assess the risk of colorectal cancer (CRC) in first degree relatives (parents and full siblings) of patients with precursor lesions (polyps) for CRC.
Design
Case-control study.
Setting
Linkage to the multi-generation register and gastrointestinal ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden) histopathology cohort in Sweden.
Participants
68 060 patients with CRC and 333 753 matched controls.
Main outcome measures
Multivariable adjusted odds ratios of CRC according to the number of first degree relatives with a colorectal polyp and the histology of polyps and age at diagnosis in first degree relatives. Subgroup analysis was also performed according to age at CRC diagnosis and evaluated the joint association of family history of colorectal polyps and family history of CRC.
Results
After adjusting for family history of CRC and other covariates, having a first degree relative with a colorectal polyp (8.4% (5742/68 060) in cases and 5.7% (18 860/333 753) in controls) was associated with a higher risk of CRC (odds ratio 1.40, 95% confidence interval 1.35 to 1.45). The odds ratios ranged from 1.23 for those with hyperplastic polyps to 1.44 for those with tubulovillous adenomas. To better put this risk in perspective, the age specific absolute risk of colon and rectal cancers was estimated according to family history of polyps based on the 2018 national CRC incidence in Sweden. For example, the absolute risk of colon cancer in individuals aged 60-64 years with and without a family history of colorectal polyp was, respectively, 94.3 and 67.9 per 100 000 for men and 89.1 and 64.1 per 100 000 for women. The association between family history of polyps and CRC risk was strengthened by the increasing number of first degree relatives with polyps (≥2 first degree relatives: 1.70, 1.52 to 1.90, P<0.001 for trend) and decreasing age at polyp diagnosis (<50 years: 1.77, 1.57 to 1.99, P<0.001 for trend). A particularly strong association was found for early onset CRC diagnosed before age 50 years (≥2 first degree relatives: 3.34, 2.05 to 5.43, P=0.002 for heterogeneity by age of CRC diagnosis). In the joint analysis, the odds ratio of CRC for individuals with two or more first degree relatives with polyps but no CRC was 1.79 (1.52 to 2.10), with one first degree relative with CRC but no polyps was 1.70 (1.65 to 1.76), and with two or more first degree relatives with both polyps and CRC was 5.00 (3.77 to 6.63) (P<0.001 for interaction).
Conclusions
After adjusting for family history of CRC, the siblings and children of patients with colorectal polyps are still at higher risk of CRC, particularly early onset CRC. Early screening for CRC might be considered for first degree relatives of patients with polyps.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer related deaths worldwide.1 Endoscopic screening reduces the incidence of and mortality from CRC by reoval of precursor lesions, namely colorectal polyps.2 3 4 Identifying high risk individuals for tailored screening has important public health and economic implications for improving the prevention of CRC.5 In contrast with the established increased risk associated with a family history of CRC, it remains unclear whether those with a family history of colorectal polyps have an increased risk of CRC.6 As a result, available screening recommendations are discrepant for individuals with a family history of polyps. The US Multi-Society Task Force on Colorectal Cancer recommends earlier screening in people with a family history of advanced polyps in the same manner as for those with a family history of CRC,7 whereas the British Society of Gastroenterology only considers family history of CRC but not polyps in screening recommendations.8 Given the higher prevalence of polyps than CRC associated with the increasing uptake of endoscopic screening, a better understanding about the influence of family history of polyps on CRC risk is critical to improve current screening recommendations.
Existing data on the association between family history of colorectal polyps and CRC risk are inconclusive. Whereas some studies reported an increased risk of CRC in first degree relatives of patients with a history of colorectal polyps,9 10 11 12 others indicated that the risk varied by polyp type and was restricted to large or advanced adenomas.13 14 Moreover, these studies had limited ability to inform screening recommendations owing to several limitations. Firstly, the most important unanswered question is whether the first degree relatives of someone with a colorectal polyp are still at greater risk of CRC after adjusting for family history of CRC. If the risk of CRC is increased, the next question is whether the first degree relatives of individuals with polyps are specifically at risk of early onset CRC that develops before the age of 50 years, the commonly recommended age for starting CRC screening in adults at average risk.7 8 This is a particularly important question because of the global increase in the incidence of early onset CRC.15 In addition, previous studies have focused on conventional adenomas only, and the influence of family history of serrated polyps, a newly recognised precursor lesion for CRC, remains to be determined. Finally, past studies were limited by small sample size (<300 participants with CRC),9 11 12 13 14 cross sectional design,11 12 13 and reliance on self-reported family history data.9 11 12 13 14
In our nationwide case-control study, we used information from several national registers in Sweden to assess the relation between family history of colorectal polyps and risk of CRC.
Methods
Study population
We extracted data from several national registries in Sweden using the unique personal identity number assigned to all residents for the universal public healthcare system.16 Patients with CRC were obtained from the nationwide ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden) cohort, which included information on gastrointestinal biopsy samples from all 28 pathology departments in Sweden between 1965 and 2017.17 In ESPRESSO, histopathological findings were defined by morphology codes (a Swedish modification of the Systematized Nomenclature of Medicine (SNOMED) coding system) and topography codes. To identify patients with CRC, we used topography codes T67x (for colon) and T68x (for rectum) in combination with SNOMED codes (supplementary table 1). For each of the patients in ESPRESSO, we selected up to five controls from the general population matched on age at biopsy (in years), sex, year of birth, and county of residence.17
Supplementary figure 1 shows the flowchart of participant selection. For both cases and matched controls, we excluded people younger than 18 years, those with hereditary syndromes of CRC, and those with a history of inflammatory bowel disease and colectomy before CRC diagnosis. Because the multi-generation register is confined to individuals born after 1932, we further excluded those born before 1 January 1932. In total, 68 060 CRC cases and 333 753 matched controls were included in the study. Informed consent was waived by the Stockholm ethics review board because the study was strictly register based.18
Assessment of family history of colorectal polyps and CRC
From the family relationship data in the Swedish multi-generation register,19 we retrieved information on family history and identified the parents and full siblings (first degree relatives) of our study participants. Then we assessed the history of colorectal polyps in the first degree relatives through linkage to the ESPRESSO cohort. Details of polyp assessment have been described previously.20 Briefly, we used topography codes T67/T68 in combination with SNOMED codes to identify the first diagnosis of colorectal polyps in first degree relatives. For conventional adenomas, SNOMED code M82100 was used for tubular adenomas, M82630 for tubulovillous adenomas, and M82611 for villous adenomas. Serrated polyps included hyperplastic polyps (M72040) and sessile serrated polyps. Given the evolving nature of the diagnosis criteria for sessile serrated polyps, we used a combination of SNOMED codes and free text search to identify such polyps. This approach has been validated with a positive predictive value of 93%.21
For each participant, we counted the number of first degree relatives with a polyp diagnosed before the index date, which was defined as the diagnosis date of CRC in cases and their matched controls. We also assessed the youngest age at polyp diagnosis and the histological subtypes of polyps among first degree relatives. Advanced polyps included tubulovillous or villous adenomas and sessile serrated polyps.
We assessed the history of CRC in first degree relatives based on the Cancer Register, which has recorded incident malignancies in Sweden since 1958 and has an estimated completeness of 96.3%.22 CRC was identified using ICD-7 (international classification of diseases, seventh revision) codes 153 and 154.
Statistical analysis
Means (standard deviations) were calculated for continuous variables and percentages for categorical variables among cases and controls. We used conditional logistic regression to calculate the odds ratio and corresponding 95% confidence interval of CRC according to the history of colorectal polyps in first degree relatives (yes or no), the number of first degree relatives with a polyp (0, 1, ≥2), and the youngest age at polyp diagnosis in first degree relatives (<50, 50-59, 60-69, and ≥70 years). P values for trend were calculated by treating the number of relatives and age of diagnosis as a continuous variable. We considered three models: model 1 was conditional on the matching factors, and model 2 was further adjusted for other potential confounding factors (see supplementary methods for details of covariate assessment), including year of birth (continuous), family size (continuous), income levels (fifths), education (≤9 years, 10-12 years, >12 years, missing), total number of previous clinic visits (fifths), number of previous endoscopic examinations (0, 1, 2, and >2), Charlson comorbidity index score (continuous), and major comorbidities with a prevalence of at least 1% (all binary, including diabetes, cardiovascular disease, non-CRC, liver disease, chronic pulmonary disease, connective tissue disease, and peptic ulcer disease). To assess the effect of family history of polyps independent of family history of CRC, we further adjusted for the number of first degree relatives with a history of CRC (continuous) in the multivariable model (model 3).
To evaluate whether first degree relatives are specifically at risk for early onset CRC, we performed a subgroup analysis according to age at diagnosis of CRC (<50, 50-59, 60-69, and ≥70 years). We estimated the subgroup specific odds ratios using a fully unconstrained approach in which the confounder effects are allowed to be different among the CRC subgroups, and we used the contrast test method to calculate the P value for heterogeneity for the difference in the odds ratios across different age groups.23 The associations were also assessed according to specific subsites of CRC. Cancers from the caecum to transverse colon were classified as proximal colon cancer (topography codes T671-674), cancers from the splenic flexure to the sigmoid colon as distal colon cancer (T675-677), and those in the rectum or rectosigmoid junction as rectal cancer (T68x).
Furthermore, we performed stratified analysis according to sex, proband identity (parents, siblings), period of birth (<1940, 1940-49, ≥1950), and period of CRC diagnosis (<2008, 2008-11, ≥2012). In addition, we assessed the joint association of family history of polyps and family history of CRC with risk of overall CRC and early onset CRC. The P value for interaction was calculated using the Wald test for the product terms between the two joint factors.
To assess the influence of family history on absolute risk of CRC, we calculated the age specific CRC incidence in individuals with and without a family history of polyps based on the 2018 national CRC incidence in Sweden24 using the Greenland method for multivariate estimation of group specific incidence.25 26 Because the national data are provided for colon and rectal cancer separately according to sex, our estimates also included each cancer subsite and sex.
Finally, to assess the robustness of our findings to ascertainment bias that might result from the earlier diagnosis of CRC in family members whose relatives had a history of polyps, we conducted a sensitivity analysis by using death from incident CRC as the outcome (that is, restricted to CRC cases who died from CRC). Furthermore, to better capture polyps diagnosed during routine screening, we also performed a sensitivity analysis by restricting to cases with CRC diagnosed after 2008, when organised screening started in the Stockholm region in Sweden among those aged 60-69 years using faecal occult blood testing,27 and by restricting the timeframe of family history assessment to the post-2008 period in Stockholm only. In addition, we assessed the number of diagnostic investigations performed in those with and without a family history of polyps by calculating the prevalence of common diseases diagnosed in the two groups. We used SAS 9.4 for the analyses. All statistical tests were two sided, with a P value of 0.05 considered to be significant.
Patient and public involvement
Because we used national registers, patients and the public were not involved in the design or conduct of the study, or in the interpretation of the study results.
Results
Table 1 shows the characteristics of the study participants. The mean age was 63 (SD 10) years and 46% of participants were women. Among cases, 7.7% (n=5220) and 0.8% (n=522) had one and two or more first degree relatives with a history of polyps, respectively, compared with 5.3% (n=17 627) and 0.4% (n=1233) in controls. The mean youngest age at polyp diagnosis in first degree relatives was 66.7 years for cases and 67.5 years for controls. Tubular adenomas were the most common polyp types in first degree relatives of both cases and controls, with a prevalence of 3.6% (n=2458) and 2.3% ((n=7783), respectively. Compared with controls, cases had a higher number of previous clinic visits and comorbidities.
Table 1.
Characteristics of study participants. Values are numbers (percentages) unless stated otherwise
| Characteristics | Cases (n=68 060) | Controls (n=333 753) |
|---|---|---|
| Women | 31 464 (46.2) | 154 526 (46.3) |
| Mean (SD) age (years) | 63.4 (10.5) | 63.3 (10.5) |
| Age groups (years): | ||
| <50 | 7372 (10.8) | 36 668 (11.0) |
| 50-59 | 15 143 (22.3) | 75 151 (22.5) |
| 60-69 | 25 937 (38.1) | 127 781 (38.3) |
| ≥70 | 19 608 (28.8) | 94 153 (28.2) |
| Mean (SD) year of birth | 1943.3 (9.2) | 1943.4 (9.2) |
| Mean (SD) No of FDRs | 4.2 (2.6) | 4.6 (3.0) |
| No of FDRs with polyps: | ||
| 0 | 62 318 (91.6) | 314 893 (94.4) |
| 1 | 5220 (7.7) | 17 627 (5.3) |
| ≥2 | 522 (0.8) | 1233 (0.4) |
| No of FDRs with CRC: | ||
| 0 | 60 472 (88.9) | 311 400 (93.3) |
| 1 | 7059 (10.4) | 21 446 (6.4) |
| ≥2 | 529 (0.8) | 907 (0.3) |
| Mean (SD) youngest age at polyp diagnosis in FDRs (years)* | 66.7 (11.6) | 67.5 (11.3) |
| Youngest age at polyp diagnosis in FDRs (years): | ||
| <50 | 438 (0.1) | 1170 (0.1) |
| 50-59 | 1085 (0.2) | 3464 (0.2) |
| 60-69 | 1753 (0.3) | 5724 (0.3) |
| ≥70 | 2466 (0.4) | 8502 (0.5) |
| Types of lesions in at least one FDR: | ||
| Hyperplastic polyps | 1667 (2.5) | 6114 (1.8) |
| Sessile serrated polyps | 123 (0.2) | 437 (0.1) |
| Tubular adenomas | 2458 (3.6) | 7783 (2.3) |
| Tubulovillous adenomas | 1856 (2.7) | 5437 (1.6) |
| Villous adenomas | 252 (0.4) | 697 (0.2) |
| Advanced polyps | 2194 (3.2) | 6477 (1.9) |
| Mean (SD) total No of past clinic visits | 10.4 (12.1) | 7.4 (10.2) |
| Mean (SD) Charlson comorbidity index score | 0.5 (0.9) | 0.4 (0.8) |
| Comorbidities: | ||
| Myocardial infarction | 3178 (4.7) | 13 694 (4.1) |
| Congestive heart failure | 1926 (2.8) | 6660 (2.0) |
| Peripheral vascular disease | 1462 (2.2) | 5371 (1.6) |
| Cerebrovascular disease | 3952 (5.8) | 16 936 (5.1) |
| Dementia | 347 (0.5) | 2283 (0.7) |
| Chronic pulmonary disease | 4127 (6.1) | 15 750 (4.7) |
| Connective tissue disease-rheumatic disease | 1339 (2.0) | 5842 (1.8) |
| Peptic ulcer disease | 1872 (2.8) | 3121 (0.9) |
| Mild liver disease | 808 (1.2) | 1798 (0.5) |
| Diabetes without complications | 4663 (6.9) | 14 414 (4.3) |
| Diabetes with complications | 1654 (2.4) | 5367 (1.6) |
| Paraplegia and hemiplegia | 328 (0.5) | 1262 (0.4) |
| Renal disease | 629 (0.9) | 2027 (0.6) |
| Cancer, excluding CRC | 7422 (10.9) | 25 802 (7.7) |
| Moderate or severe liver disease | 213 (0.3) | 534 (0.2) |
| Metastatic carcinoma of unspecified sites | 1864 (2.7) | 2098 (0.6) |
| AIDS/HIV | 36 (0.1) | 138 (0.0) |
CRC=colorectal cancer; FDR=first degree relative (parents and full siblings).
All variables were assessed at time of CRC diagnosis for cases and their matched controls.
Among FDRs with a history of polyps.
A history of polyps in first degree relatives was associated with a higher risk of CRC (multivariable odds ratio 1.62, 95% confidence interval 1.57 to 1.68; table 2). Further adjustment for family history of CRC led to a modest attenuation in the association (1.40, 1.35 to 1.45). For the rest of the results section, the odds ratios adjusted for family history of CRC are given. The odds ratios varied by polyp subtypes in first degree relatives, ranging from 1.23 (1.16 to 1.31) for hyperplastic polyps to 1.44 (1.36 to 1.53) for tubulovillous adenomas.
Table 2.
Association between family history of polyps in first degree relatives (FDRs, parents and siblings) and risk of colorectal cancer (CRC). Values are numbers (percentages) unless stated otherwise
| Polyp types in FDRs | Cases (n=68 060) | Controls (n=333 753) | Age adjusted odds ratio (95% CI)* | Multivariable adjusted odds ratio (95% CI)† | Multivariable+family history of CRC adjusted odds ratio (95% CI)‡ |
|---|---|---|---|---|---|
| No polyps | 62 318 (91.6) | 314 893 (94.3) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Any polyp | 5742 (8.4) | 18 860 (5.7) | 1.55 (1.50 to 1.60) | 1.62 (1.57 to 1.68) | 1.40 (1.35 to 1.45) |
| Advanced polyps | 2194 (3.2) | 6477 (1.9) | 1.68 (1.60 to 1.77) | 1.76 (1.67 to 1.86) | 1.44 (1.36 to 1.51) |
| Serrated polyps: | |||||
| Hyperplastic | 1667 (2.4) | 6114 (1.8) | 1.34 (1.27 to 1.42) | 1.38 (1.30 to 1.46) | 1.23 (1.16 to 1.31) |
| Sessile serrated | 123 (0.2) | 437 (0.1) | 1.37 (1.12 to 1.67) | 1.43 (1.16 to 1.77) | 1.27 (1.03 to 1.57) |
| Conventional adenomas: | |||||
| Tubular | 2458 (3.6) | 7783 (2.3) | 1.57 (1.50 to 1.64) | 1.62 (1.54 to 1.70) | 1.39 (1.32 to 1.46) |
| Tubulovillous | 1856 (2.7) | 5437 (1.6) | 1.69 (1.60 to 1.79) | 1.77 (1.67 to 1.87) | 1.44 (1.36 to 1.53) |
| Villous | 252 (0.4) | 697 (0.2) | 1.77 (1.54 to 2.05) | 1.82 (1.57 to 2.12) | 1.40 (1.20 to 1.63) |
Conditional logistic regression was used to account for matching on age, sex, year of birth, and county of residence.
Multivariable model was further adjusted for year of birth (continuous), family size (continuous), income levels (fifths), education (≤9 years, 10-12 years, >12 years, missing), total number of previous clinic visits (fifths), number of previous endoscopies (0, 1, 2, ≥3), Charlson comorbidity index score (continuous), and major comorbidities (all binary, including diabetes, cardiovascular disease, non-colorectal cancer, liver disease, chronic pulmonary disease, connective tissue disease, and peptic ulcer disease).
Further adjusted for family history of CRC in FDRs.
The association between family history of polyps and risk of CRC strengthened with the increasing number of first degree relatives with polyps (odds ratio for ≥2 first degree relatives with polyps: 1.70, 1.52 to 1.90, P<0.001 for trend) and decreasing age at polyp diagnosis (<50 years, 1.77, 1.57 to 1.99, P<0.001 for trend; table 3). Similar patterns were found for individual polyp subtypes (see supplementary tables 2 and 3). When the joint association of the number of first degree relatives with a history of polyps and youngest age at polyp diagnosis was examined (table 4), the odds ratios ranged from 1.33 (1.28 to 1.39) for one first degree relative and youngest age at polyp diagnosis 60 years or older, to 1.82 (1.49 to 2.22) for two or more first degree relatives and youngest age at polyp diagnosis less than 60 years.
Table 3.
Risk of colorectal cancer (CRC) according to number of first degree relatives (FDRs, parents and siblings) with a family history of polyps. Values are numbers (percentages) unless stated otherwise
| Polyp types in FDRs | Cases (n=68 060) | Controls (n=333 753) | Multivariable adjusted odds ratio (95% CI)* | Multivariable+family history of CRC adjusted odds ratio (95% CI)† |
|---|---|---|---|---|
| Any polyps | ||||
| No of FDRs with any polyps: | ||||
| 0 | 62 318 (91.6) | 314 893 (94.3) | 1 (Ref) | 1 (Ref) |
| 1 | 5220 (7.7) | 17 627 (5.3) | 1.58 (1.53 to 1.63) | 1.38 (1.33 to 1.43) |
| ≥2 | 522 (0.8) | 1233 (0.4) | 2.27 (2.03 to 2.53) | 1.70 (1.52 to 1.90) |
| P for trend | — | — | <0.001 | <0.001 |
| Youngest age at diagnosis of any polyps (years): | ||||
| ≥70 | 2466 (3.6) | 8502 (2.5) | 1.52 (1.45 to 1.60) | 1.29 (1.23 to 1.36) |
| 60-69 | 1753 (2.6) | 5724 (1.7) | 1.66 (1.57 to 1.76) | 1.44 (1.35 to 1.52) |
| 50-59 | 1085 (1.6) | 3464 (1.0) | 1.67 (1.56 to 1.80) | 1.48 (1.37 to 1.59) |
| <50 | 438 (0.6) | 1170 (0.4) | 2.00 (1.78 to 2.24) | 1.77 (1.57 to 1.99) |
| P for trend | — | — | <0.001 | <0.001 |
| Advanced polyps | ||||
| No of FDRs with advanced polyps: | ||||
| 0 | 65 866 (96.8) | 327 276 (98.1) | 1 (Ref) | 1 (Ref) |
| 1 | 2137 (3.1) | 6326 (1.9) | 1.76 (1.67 to 1.85) | 1.44 (1.36 to 1.52) |
| ≥2 | 57 (0.1) | 151 (0.0) | 1.93 (1.41 to 2.66) | 1.26 (0.91 to 1.75) |
| P for trend | — | — | <0.001 | <0.001 |
| Youngest diagnosis age of advanced polyps (years): | ||||
| ≥70 | 1067 (1.6) | 3451 (1.0) | 1.59 (1.48 to 1.71) | 1.29 (1.20 to 1.39) |
| 60-69 | 638 (0.9) | 1785 (0.5) | 1.87 (1.70 to 2.06) | 1.51 (1.37 to 1.67) |
| 50-59 | 371 (0.5) | 989 (0.3) | 1.98 (1.74 to 2.24) | 1.64 (1.45 to 1.87) |
| <50 | 118 (0.2) | 252 (0.1) | 2.57 (2.04 to 3.23) | 2.12 (1.68 to 2.68) |
| P for trend | — | — | <0.001 | <0.001 |
Multivariable conditional logistic regression was used to account for matching on age, sex, year of birth, and county of residence, and was further adjusted for year of birth (continuous), family size (continuous), income levels (fifths), education (≤9 years, 10-12 years, >12 years, missing), total number of previous clinic visits (fifths), number of previous endoscopies (0, 1, 2, ≥3), Charlson comorbidity index score (continuous), and major comorbidities (all binary, including diabetes, cardiovascular disease, non-colorectal cancer, liver disease, chronic pulmonary disease, connective tissue disease, and peptic ulcer disease).
Further adjusted for number of FDRs with a history of CRC (continuous).
Table 4.
Risk of total and age specific colorectal cancer (CRC) according to number of first degree relatives (FDRs, parents and siblings) with polyps and youngest age at polyp diagnosis. Vales are numbers (percentages) unless stated otherwise
| Family history of polyps | Cases (n=68 060) | Controls (n=333 753) | Multivariable adjusted odds ratio (95% CI)* | Multivariable+family history of CRC adjusted odds ratio (95% CI)† | P value |
|---|---|---|---|---|---|
| Any polyps | |||||
| Any FDR, youngest age at diagnosis <60 years | 1523 (2.4) | 4634 (1.5) | 1.75 (1.64 to 1.86) | 1.53 (1.44 to 1.64) | <0.001 |
| 1 FDR, youngest age at diagnosis ≥60 years | 3880 (5.9) | 13 367 (4.1) | 1.54 (1.48 to 1.61) | 1.33 (1.28 to 1.39) | <0.001 |
| 1 FDR, youngest age at diagnosis <60 years | 1340 (2.1) | 4260 (1.3) | 1.68 (1.57 to 1.80) | 1.51 (1.41 to 1.61) | <0.001 |
| ≥2 FDRs, any age | 522 (0.8) | 1233 (0.4) | 2.23 (1.99 to 2.49) | 1.64 (1.46 to 1.84) | <0.001 |
| ≥2 FDRs, youngest age at diagnosis <60 years | 183 (0.3) | 374 (0.1) | 2.51 (2.07 to 3.04) | 1.82 (1.49 to 2.22) | <0.001 |
| Advanced polyps | |||||
| Any FDR, youngest age at diagnosis <60 years | 489 (0.7) | 1241 (0.4) | 2.10 (1.88 to 2.35) | 1.73 (1.54 to 1.94) | <0.001 |
| 1 FDR, youngest age at diagnosis ≥60 years | 1660 (2.5) | 5129 (1.5) | 1.67 (1.57 to 1.77) | 1.36 (1.28 to 1.45) | <0.001 |
| 1 FDR, youngest age at diagnosis <60 years | 477 (0.7) | 1197 (0.4) | 2.14 (1.91 to 2.39) | 1.77 (1.58 to 1.99) | <0.001 |
| ≥2 FDRs, any age | 57 (0.1) | 151 (0.0) | 1.92 (1.39 to 2.65) | 1.22 (0.88 to 1.70) | 0.24 |
| ≥2 FDRs, youngest age at diagnosis <60 years | 12 (0.0) | 44 (0.0) | 1.21 (0.62 to 2.38) | 0.73 (0.36 to 1.47) | 0.38 |
Multivariable conditional logistic regression was used to account for the matching on age, sex, year of birth, and county of residence, and was further adjusted for year of birth (continuous), family size (continuous), income levels (fifths), education (≤9, 10-12 years, >12 years, missing), total number of previous clinic visits (fifths), number of previous endoscopies (0, 1, 2, ≥3), Charlson comorbidity index score (continuous), and major comorbidities (all binary, including diabetes, cardiovascular disease, non-colorectal cancer, liver disease, chronic pulmonary disease, connective tissue disease, and peptic ulcer disease).
Further adjusted for number of FDRs with a history of CRC (continuous).
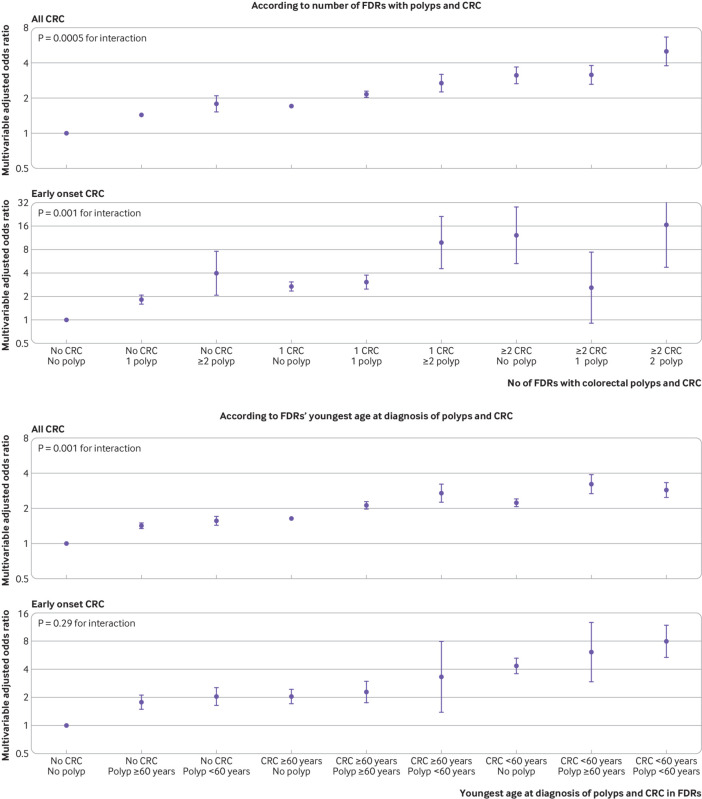
When the risk of CRC was investigated according to age at diagnosis (fig 1), the association between family history of polyps and risk of CRC declined with age (P<0.01 for heterogeneity). A particularly strong association was found for early onset CRC diagnosed before age 50 years, with an odds ratio of 3.34 (2.05 to 5.43) for two or more first degree relatives with polyps, 2.03 (1.69 to 2.43) for the youngest age at polyp diagnosis in first degree relatives of less than 60 years, and 5.27 (2.54 to 10.91) for having two or more first degree relatives with polyps along with youngest age at polyp diagnosis of less than 60 years.
Fig 1.
Risk of colorectal cancer (CRC) according to age at diagnosis in relation to number of first degree relatives (FDRs) with any polyps and youngest age at polyp diagnosis. Odds ratios are presented on the log(2) scale and were calculated using the fully adjusted conditional logistic regression model that included family history of CRC
When assessed by CRC subsites, no statistically significant difference was found for a family history of any polyps, conventional adenomas, or serrated polyps, although a family history of serrated polyps seemed to be strongly associated with risk of proximal colon cancer (odds ratio for ≥2 first degree relatives: 2.53, 1.06 to 6.03) but not distal colon cancer (0.96, 0.37 to 2.46) or rectal cancer (1.14, 0.68 to 1.92; see supplementary table 4).
Stratified analysis did not reveal any substantial difference in the association of family history of polyps with risk of CRC according to sex or proband identity, whereas a stronger association was found for more recent birth cohort (P=0.01 for interaction) and for CRCs diagnosed before 2008 (P=0.003 for heterogeneity; see supplementary table 5).
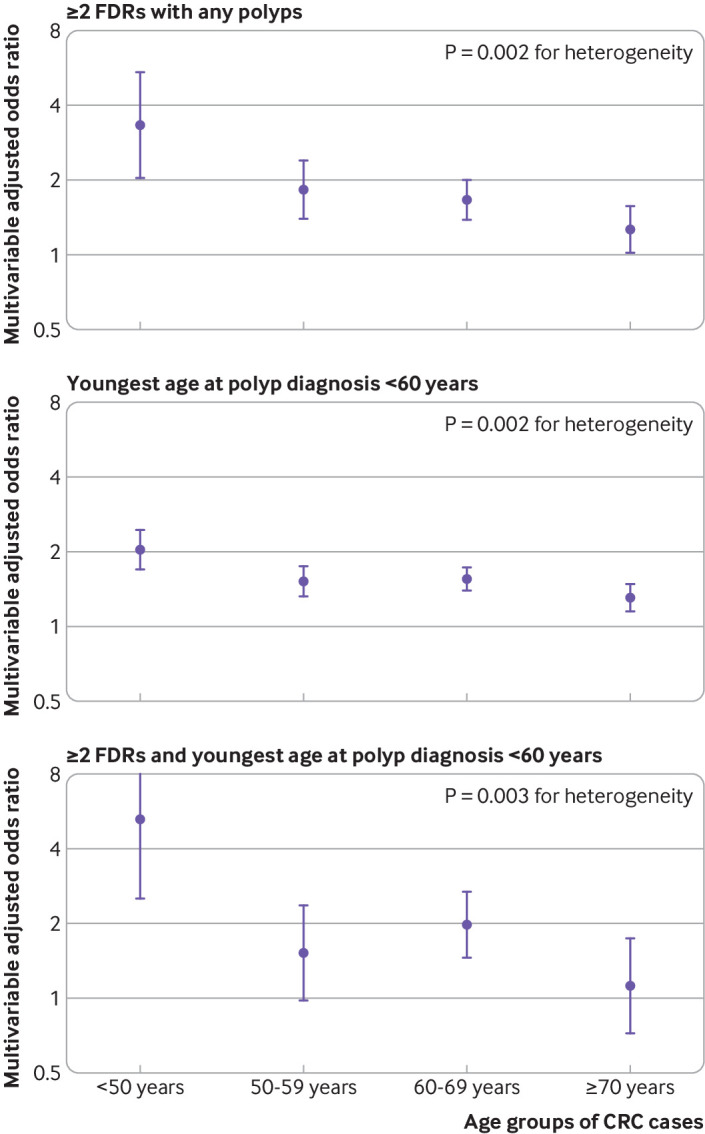
Figure 2 presents the joint association of family history of polyps and family history of CRC. Even among participants without a family history of CRC (cases: n=60 472 (88.9%); controls: n=311 400 (93.3%)), those with a family history of polyps (cases: n=3475 (5.7%); controls: n=13 303 (4.3%)) still showed a higher risk of CRC. Compared with individuals with no first degree relatives with either polyps or CRC, the odds ratio for those having two or more first degree relatives with polyps but no CRC was 1.79 (1.52 to 2.10), for those having one first degree relative with CRC but no polyps was 1.70 (1.65 to 1.76), and for those having two or more first degree relatives with both polyps and CRC was 5.00 (3.77 to 6.63) (P<0.001 for interaction). A similar synergistic association was found when individuals were classified based on age at diagnosis of polyps and CRC in their first degree relatives (P=0.001 for interaction); and those with first degree relatives in whom both polyps and CRC had been diagnosed before age 60 years had an OR of 2.85 (2.48 to 3.29). A more noticeable association was found for early onset CRC, with an odds ratio of 16.57 (4.81 to 57.13) for individuals having two or more first degree relatives with both polyps and CRC (see supplementary table 6 for detailed results).
Fig 2.
Joint association of history of polyps and colorectal cancer (CRC) in first degree relatives (FDRs) with risk of overall CRC and early onset CRC (diagnosed before age 50 years). Odds ratios are presented on the log(2) scale and were calculated using the fully adjusted conditional logistic regression model. See supplementary table 6 for detailed results
Figure 3 shows the age specific absolute incidence of CRC according to family history of polyps. As CRC incidence increases with age, the risk difference between those with and those without a family history of polyps also showed a substantial increase. For example, the risk difference of colon cancer in men showed an increase from 5.6 per 100 000 in those aged 45-49 years to 124.2 per 100 000 in those aged 80-84 years.
Fig 3.
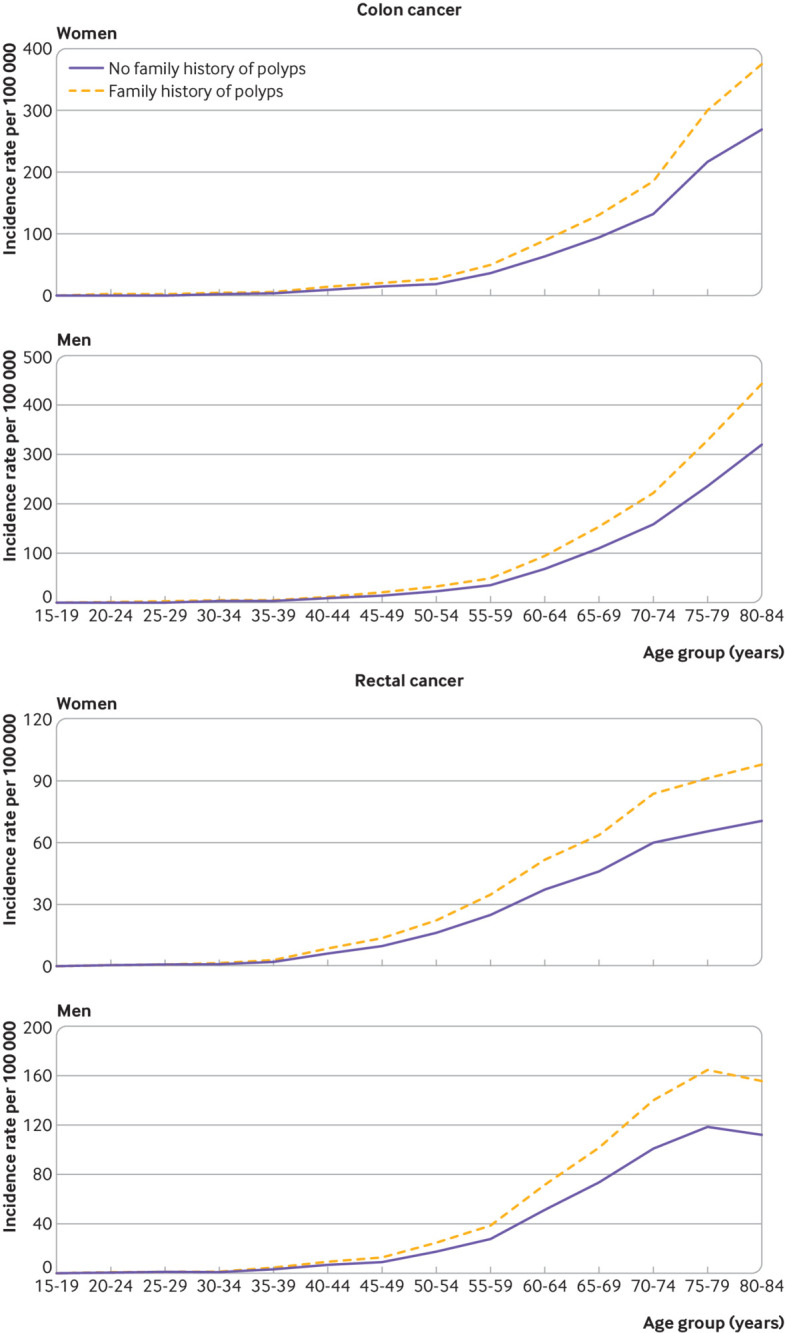
Estimated absolute incidence of colon cancer and rectal cancer in women and men in those with and without a family history of polyps, according to age
To investigate the potential influence of ascertainment bias, the mortality from incident CRC was assessed as the outcome in a sensitivity analysis. A family history of polyps was found to be similarly associated with mortality of incident CRC (multivariable odds ratio 1.40, 95% confidence interval 1.30 to 1.51; see supplementary table 7). The sensitivity analyses restricted to cases of CRC diagnosed after 2008 also showed similar results (see supplementary table 7). When the prevalence of common diseases was compared, no substantial differences were observed in those with and those without a family history of polyps (see supplementary table 8). Taken together, these results indicate little influence of ascertainment bias.
Discussion
In this nationwide case-control study, using data from established national registers in Sweden, individuals with a history of CRC precursor lesions (ie, polyps) in first degree relatives were shown to have a 62% increased risk of CRC compared with those with no family history of polyps. The increase in risk was attenuated to 40% after adjusting for family history of CRC but increased to 70-77% when more than one first degree relative had a polyp or when a polyp was first diagnosed in a first degree relative before age 50 years. A particularly strong association was found for early onset CRC. In the joint analysis of family history of polyps and CRC, individuals who had two or more first degree relatives with polyps but no CRC were found to be at a slightly higher risk of CRC compared with those who had one first degree relative with CRC but no polyps; and those who had two or more first degree relatives with both polyps and CRC had a fivefold increased risk of overall CRC and more than a 16-fold increased risk of early onset CRC, compared with individuals with no first degree relatives with polyps or CRC. These findings provide robust evidence for the impact of family history of polyps on risk of CRC and have important implications for CRC screening.
Comparison with other studies and implications
Our study extends knowledge in several respects. Firstly, we specifically examined the independent and joint associations of history of polyps and CRC in first degree relatives with risk of CRC. Without mutual adjustment, our effect estimates are consistent with those in previous studies, with the odds ratio of 1.62 for one first degree relative or more with polyps (range 1.35-1.78 in past studies)9 10 11 14 and 1.83 for one first degree relative or more with CRC (range 1.80-2.25 in past studies).6 After mutual adjustment, a family history of polyps was still associated with a 40% increased risk of CRC. Moreover, in a joint analysis we found a synergistic association between a family history of polyps and CRC. First degree relatives with a history of both conditions had a substantially increased risk of CRC compared with those without either. A higher risk was found in individuals with two more first degree relatives with polyps but no CRC (odds ratio 1.79) than in those with one first degree relative with CRC but no polyps (odds ratio 1.70). Taken together, these findings indicate that a family history of polyps might provide critical additional information beyond a family history of CRC for risk assessment in first degree relatives. Individuals with at least two first degree relatives with polyps, most of whom are not yet recommended for early screening according to existing recommendations,7 8 might benefit from and be considered for early CRC screening as those with one first degree relative with CRC.
Secondly, we addressed another yet unanswered critical question about how a family history of polyps might be associated with risk of CRC according to age of onset in first degree relatives. We found that the association tended to increase with decreasing age at CRC diagnosis, with a particularly strong association observed for early onset CRC. These data have critical implications for refining the current screening recommendations. Although screening in the US has contributed substantially to the decreasing incidence of CRC in older adults,28 incidence has increased by 0.5-2.4% annually since the mid-1980s in adults younger than 50 years, for whom routine screening has not been recommended.29 A similar increase has been documented in many other regions worldwide.15 This trend led the American Cancer Society to lower its recommended age for starting CRC screening from 50 to 45 years.30 This revision has led to intense debate about the potential benefits, harms, liabilities, and costs.31 32 33 34 35 36 Recently, the US Preventive Services Task Force issued a draft recommendation that also lowered the starting age of CRC screening to 45 years. Since the incidence of early onset CRC remains much lower than that of late onset CRC, a risk based approach for screening might be particularly appealing for younger adults. Our observation for a strong association between family history of polyps and early onset CRC suggests that an assessment of family history might be considered to tailor screening for better prevention of early onset CRC.
Thirdly, we performed detailed analysis according to histological subtype of polyps in first degree relatives. In line with the increasing recognition of the role of serrated polyps in CRC, we found that first degree relatives of patients with serrated polyps had an increased risk of CRC and that the increased risk was similar to that of patients with conventional adenomas. Moreover, in support of the predominant effect of the serrated pathway on development of proximal colon cancer,37 we observed a stronger association of family history of serrated polyps with proximal colon cancer. These findings suggest a familial clustering of neoplastic pathways in CRC and have implications for studying hereditary risk of CRC. Interestingly, we found that a family history of hyperplastic polyps was also associated with a moderately increased risk of CRC. This is consistent with our previous findings in the ESPRESSO cohort for an increased risk of CRC associated with hyperplastic polyps, possibly as a result of the misdiagnosis of sessile serrated polyps as hyperplastic polyps.20 However, given the evolving diagnosis criteria of serrated polyps,38 our findings should be interpreted with caution. In addition, compared with first degree relatives of patients with any polyps, the increase in risk of CRC in those with polyps of advanced histology was only slightly higher. In contrast, the increasing number of first degree relatives with polyps, and, to a lesser extent, the younger age at polyp diagnosis seemed to have a stronger influence on risk of CRC. These results are not unexpected because compared with polyp histology in first degree relatives, a higher number of relatives with polyps and the early onset of polyps might better reflect the familial risk associated with genetic susceptibility and early environmental factors. These findings suggest that the information on polyp histology in first degree relatives might not be as critical as currently perceived for CRC risk assessment. As a result, the concern about lack of accurate recall or documentation of polyp histology could be eased when making screening recommendations based on family history of polyps.39
Finally, we found that proband identity (parents and siblings) did not affect the risk of CRC associated with family history of polyps. This is consistent with previous data indicating a similar risk of colorectal neoplasia among individuals with different first degree relatives affected with CRC.40
Strengths and limitations of this study
Our study has several strengths, including the nationwide population based design; large sample size; high validity of the cancer and polyp registers; comprehensive assessment of the number of first degree relatives, their age at diagnosis, and histological subtypes of polyps; and detailed subgroup analysis according to CRC subsites and age of onset. In particular, our use of objective register based data minimises measurement error in family history assessment and represents a major advantage over previous studies that relied on patients’ recall.9 11 12 13 14
Several limitations of our study also need to be noted. Firstly, we lacked information on other factors that might influence the risk of CRC, including the size and multiplicity of polyps in first degree relatives and lifestyle risk factors. However, we have established the predictivity of different histological polyp subtypes for CRC risk in the study population.20 Moreover, we adjusted for the Charlson comorbidity index score and several major individual comorbidities that are strongly associated with CRC risk factors (eg, smoking and obesity). Secondly, the nationwide polyp data in ESPRESSO are confined to 1965 and onwards, with limited coverage before 1990. As a result, we might have missed some first degree relatives with polyps diagnosed in earlier years, leading to misclassification of some individuals with a family history of polyps that might have biased our results towards the null. Thirdly, we lacked information on indications of endoscopic examination for polyp detection in first degree relatives. As organised screening did not start regionally in Sweden until 2008, the polyps in first degree relatives of our study participants are likely to be clinically detected (ie, based on symptoms) with a low prevalence and our findings might not be generalisable to populations in which screening endoscopy is common. However, any misclassification in the polyp history in first degree relatives is unlikely to differ between the index cases and controls and thus could only have attenuated our effect estimates. Indeed, we observed a stronger association among individuals in the more recent birth cohort, for whom misclassification was reduced and who had a greater family history of polyps. Also, the sensitivity analysis of restricting the timeframe of family history assessment to the post-2008 period in Stockholm and CRC cases diagnosed after 2008 revealed similar results, indicating the robustness of our findings.
Conclusions
After adjusting for family history of CRC, siblings and children of patients with colorectal polyps were found to be at increased risk of CRC, particularly when polyps were diagnosed in more than one first degree relative or before age 60 years. The increased risk is more prominent for early onset CRC and heightened in association with a family history of CRC. Our findings suggest that to better prevent early onset CRC, early screening, if proved effective, might be tailored for first degree relatives of individuals with polyps, particularly those with multiple first degree relatives with a history of polyps and when polyps are diagnosed in first degree relatives at a younger age.
What is already known on this topic
Endoscopic screening reduces the incidence of and mortality from colorectal cancer (CRC) by removal of precursor lesions—namely, colorectal polyps
A family history of CRC is an established risk factor for CRC
Individuals with a history of advanced colorectal polyps are at higher risk of developing CRC
What this study adds
Individuals with at least two first degree relatives with polyps or a first degree relative with polyps diagnosed at a young age, most of whom are not yet recommended for early screening according to existing guidelines, are at an increased risk of CRC, particularly early onset disease, and they might benefit from early screening
Compared with the advanced histology of polyps, the higher number of first degree relatives with polyps and younger age at polyp diagnosis seemed to be more predictive of CRC risk in family members
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: MS and LE conceived and designed the study. JFL acquired the data. MS had access to all the data, performed all analyses, and designed the figures. MS and JFL obtained funding. MS wrote the first draft of the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final version. JFL supervised the study. MS is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by the National Institutes of Health (R00 CA215314 to MS) and American Cancer Society (MRSG-17-220-01-NEC to MS). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the US National Institutes of Health and American Cancer Society for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. JFL coordinates a study unrelated to the present study on behalf of the Swedish IBD Quality Register (SWIBREG). That study has received funding from Janssen.
Ethical approval: This study was approved by the Stockholm ethical review board (2014/1287-31/4).
Data sharing: No additional data available.
The lead author (MS) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We plan to disseminate the results of our research to the public and relevant patient community through the current publication and its related press release as well as presentations at scientific conferences.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687-96. 10.1056/NEJMoa1100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095-105. 10.1056/NEJMoa1301969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holme Ø, Løberg M, Kalager M, et al. NORCCAP Study Group† . Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A Randomized Trial. Ann Intern Med 2018;168:775-82. 10.7326/M17-1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robertson DJ, Ladabaum U. Opportunities and Challenges in Moving From Current Guidelines to Personalized Colorectal Cancer Screening. Gastroenterology 2019;156:904-17. 10.1053/j.gastro.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 6. Lowery JT, Ahnen DJ, Schroy PC, 3rd, et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: A state-of-the-science review. Cancer 2016;122:2633-45. 10.1002/cncr.30080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;153:307-23. 10.1053/j.gastro.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 8. Cairns SR, Scholefield JH, Steele RJ, et al. British Society of Gastroenterology. Association of Coloproctology for Great Britain and Ireland . Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. 10.1136/gut.2009.179804 [DOI] [PubMed] [Google Scholar]
- 9. Winawer SJ, Zauber AG, Gerdes H, et al. National Polyp Study Workgroup . Risk of colorectal cancer in the families of patients with adenomatous polyps. N Engl J Med 1996;334:82-7. 10.1056/NEJM199601113340204 [DOI] [PubMed] [Google Scholar]
- 10. Tuohy TM, Rowe KG, Mineau GP, Pimentel R, Burt RW, Samadder NJ. Risk of colorectal cancer and adenomas in the families of patients with adenomas: a population-based study in Utah. Cancer 2014;120:35-42. 10.1002/cncr.28227 [DOI] [PubMed] [Google Scholar]
- 11. Ahsan H, Neugut AI, Garbowski GC, et al. Family history of colorectal adenomatous polyps and increased risk for colorectal cancer. Ann Intern Med 1998;128:900-5. 10.7326/0003-4819-128-11-199806010-00006 [DOI] [PubMed] [Google Scholar]
- 12. Nakama H, Zhang B, Fukazawa K, Abdul Fattah AS. Family history of colorectal adenomatous polyps as a risk factor for colorectal cancer. Eur J Cancer 2000;36:2111-4. 10.1016/S0959-8049(00)00293-8 [DOI] [PubMed] [Google Scholar]
- 13. Lynch KL, Ahnen DJ, Byers T, Weiss DG, Lieberman DA. First-degree relatives of patients with advanced colorectal adenomas have an increased prevalence of colorectal cancer. Clin Gastroenterol Hepatol 2003;1:96-102. 10.1053/cgh.2003.50018 [DOI] [PubMed] [Google Scholar]
- 14. Cottet V, Pariente A, Nalet B, et al. ANGH Group . Colonoscopic screening of first-degree relatives of patients with large adenomas: increased risk of colorectal tumors. Gastroenterology 2007;133:1086-92. 10.1053/j.gastro.2007.07.023 [DOI] [PubMed] [Google Scholar]
- 15. Lui RN, Tsoi KKF, Ho JMW, et al. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol Biomarkers Prev 2019;28:1275-82. 10.1158/1055-9965.EPI-18-1111 [DOI] [PubMed] [Google Scholar]
- 16. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659-67. 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol 2019;11:101-14. 10.2147/CLEP.S191914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Håberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol 2015;7:491-508. 10.2147/CLEP.S90589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ekbom A. The Swedish Multi-generation Register. Methods Mol Biol 2011;675:215-20. 10.1007/978-1-59745-423-0_10 [DOI] [PubMed] [Google Scholar]
- 20. Song M, Emilsson L, Bozorg SR, et al. Risk of colorectal cancer incidence and mortality after polypectomy: a Swedish record-linkage study. Lancet Gastroenterol Hepatol 2020;5:537-47. 10.1016/S2468-1253(20)30009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bozorg SR, Song M, Emilsson L, Ludvigsson JF. Validation of serrated polyps (SPs) in Swedish pathology registers. BMC Gastroenterol 2019;20:3. 10.1186/s12876-019-1134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 2009;48:27-33. 10.1080/02841860802247664 [DOI] [PubMed] [Google Scholar]
- 23. Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med 2016;35:782-800. 10.1002/sim.6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. The National Board of Health and Welfare . Statistics on Cancer Incidence 2018 – tables. The National Board of Health and Welfare, 2018. [Google Scholar]
- 25. Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol 2004;160:301-5. 10.1093/aje/kwh221 [DOI] [PubMed] [Google Scholar]
- 26. Greenland S. Multivariate estimation of exposure-specific incidence from case-control studies. J Chronic Dis 1981;34:445-53. 10.1016/0021-9681(81)90004-7 [DOI] [PubMed] [Google Scholar]
- 27. Senore C, Basu P, Anttila A, et al. Performance of colorectal cancer screening in the European Union Member States: data from the second European screening report. Gut 2019;68:1232-44. 10.1136/gutjnl-2018-317293 [DOI] [PubMed] [Google Scholar]
- 28. Vogelaar I, van Ballegooijen M, Schrag D, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer 2006;107:1624-33. 10.1002/cncr.22115 [DOI] [PubMed] [Google Scholar]
- 29. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 30. Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250-81. 10.3322/caac.21457 [DOI] [PubMed] [Google Scholar]
- 31. Corley DA, Peek RM, Jr. When Should Guidelines Change? A Clarion Call for Evidence Regarding the Benefits and Risks of Screening for Colorectal Cancer at Earlier Ages. Gastroenterology 2018;155:947-9. 10.1053/j.gastro.2018.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Braillon A. Screening for Colorectal Cancer at Earlier Ages: Putting the Cart before the Horse. Gastroenterology 2019;156:1532. 10.1053/j.gastro.2018.12.034 [DOI] [PubMed] [Google Scholar]
- 33. Ladabaum U, Mannalithara A, Meester RGS, Gupta S, Schoen RE. Cost-Effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-Risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology 2019;157:137-48. 10.1053/j.gastro.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imperiale TF, Kahi CJ, Rex DK. Lowering the Starting Age for Colorectal Cancer Screening to 45 Years: Who Will Come…and Should They?. Clin Gastroenterol Hepatol 2018;16:1541-4. 10.1016/j.cgh.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 35. Liang PS, Allison J, Ladabaum U, et al. Potential Intended and Unintended Consequences of Recommending Initiation of Colorectal Cancer Screening at Age 45 Years. Gastroenterology 2018;155:950-4. 10.1053/j.gastro.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 36. Bretthauer M, Kalager M, Weinberg DS. From Colorectal Cancer Screening Guidelines to Headlines: Beware! Ann Intern Med 2018;169:405-6. 10.7326/M18-1720 [DOI] [PubMed] [Google Scholar]
- 37. Meester RGS, van Herk MMAGC, Lansdorp-Vogelaar I, Ladabaum U. Prevalence and Clinical Features of Sessile Serrated Polyps: A Systematic Review. Gastroenterology 2020;159:105-118.e25. 10.1053/j.gastro.2020.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niv Y. Changing pathological diagnosis from hyperplastic polyp to sessile serrated adenoma: systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2017;29:1327-31. 10.1097/MEG.0000000000000994 [DOI] [PubMed] [Google Scholar]
- 39. Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315-29, quiz 1314, 1330. 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong MC, Ching JY, Chiu HM, et al. Risk of Colorectal Neoplasia in Individuals With Self-Reported Family History: A Prospective Colonoscopy Study from 16 Asia-Pacific Regions. Am J Gastroenterol 2016;111:1621-9. 10.1038/ajg.2016.52 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials