Abstract
Introduction:
The majority of the head and neck squamous cell carcinomas (HNSCC) occur in the oral cavity. Even with advances in cancer therapy only minor improvements in the survival of HNSCC patients have taken place and approximately 350,000 patients die annually of HNSCC worldwide. Tumor budding (TB) is a novel and promising histo-morphological parameter that has been studied in many cancers. The presence of TB is associated with lymph node and distant metastasis as well as poor survival, independently of the applied scoring system. The depth of tumor invasion (D) measured from the surface of the tumor to the deepest point of invasion is also an important prognostic parameter for oral squamous cell carcinoma (OSCC) with a cutoff point of 4 mm. Both taken together constitute BD model and it has also been found to be an independent prognostic factor for patients with OSCC. Therefore, it would be highly beneficial to evaluate TB and BD model in routine histopathological reporting.
Aims and Objectives:
This study aims to compare the detection of TB in hematoxylin-eosin and pan-cytokeratin stained immune-histochemical sections of OSCC and also to evaluate whether BD score can serve as a reliable prognostic indicator for OSCC.
Methodology:
A total of 30 formalin-fixed, paraffin-embedded tissue blocks of clinically and histopathologically diagnosed cases of OSCC were retrieved. One section was stained with hematoxylin and eosin and the other was processed for pancytokeratin immunohistochemistry to evaluate tumor buds. Depth of invasion (D) was also evaluated to achieve the BD score.
Results:
Statistical significance (P < 0.001) was noted between TB score evaluated in hematoxylin and eosin (H&E) and pancytokeratin stained sections. There was no statistical significance between age, gender, site of lesion, clinical staging, survival and BD score.
Conclusion:
Immunohistochemical analysis of TB is superior to H&E staining in detection of tumor buds at the tumor invasive front.
Keywords: BD score, depth of invasion, oral squamous cell carcinoma, tumor budding
INTRODUCTION
Head and neck squamous cell carcinoma is the eighth-most common cancer worldwide and occurs in sinonasal tract, nasopharynx, oral cavity, oropharynx, larynx and hypopharynx. Even with advances in cancer therapy only minor improvements in the survival of head and neck squamous cell carcinomas (HNSCC), patients have taken place and approximately 3,50,000 patients die annually of HNSCC worldwide. This indicates that more reliable prognostic markers as well as more effective treatments are required.[1] Clinical staging of the disease based on TNM classification and location of the tumor are routinely the main criteria for prognostication and treatment of oral squamous cell carcinoma (OSCC). However, variations in the treatment response and prognosis are high for OSCCs; with some patients presenting tumors at the same site and same clinical stage having prolonged survival, while others may die of metastasis rapidly.[2] Many studies have also reported their inability to predict the outcome of patients with early stage OSCC using only TNM staging as base model for treatment planning. The main role of histopathologic prognosticators such as tumors grading, status of surgical margins, depth of invasion and perineural invasion is to complement the shortcomings of the TNM staging system for OSCC. Thus, a need arises to develop a comprehensive predictive model to make the appropriate choices in multimodality treatment of early OSCC.[3]
Tumor budding (TB) is a novel and promising histo-morphological parameter that has been studied in many cancers. The phenomenon of TB was first described in the Japanese medical literature by Imai in 1949, in relation to stomach cancer, followed by other Japanese authors, in the 1950–1960, who found correlations with prognosis in cancers of the tongue, larynx, breast, stomach, colon, rectum and cervix.[4] There is a general agreement on defining TB as the presence of single cancer cell or small clusters of fewer than 5 cancer cells at the invasive front.[1] Budding is a feature of invasive tumor margin, superficial and deep portion of tumor with aggressive behavior along with destruction of basement membrane and loss of inter-cellular adhesion. The presence of TB is associated with lymph node and distant metastasis as well as worse survival, independently of the applied scoring system. TB has been recognized as an adverse prognostic factor in carcinomas of colon, rectum, lung, gastrointestinal tract, anus, larynx and more recently in tongue[5,6] and esophagus[7]; and also in breast cancer[8] and pancreatic adenocarcinomas.[9]
In many studies, the scoring of TB has been carried out on hematoxylin and eosin (H&E) stained sections.[1,10,11,12] TB at the invasive front can often be marked by reactive lymphocytes, cancer associated fibroblasts and other stromal cells[1] which makes it sometimes difficult to detect TB on conventional hematoxylin-eosin stained slides especially when TBs are very small and they present as a small cluster of cells or sometimes even a single cell that has separated from a tumor mass. Therefore, it is necessary to examine TB using pan cytokeratin immune-histochemistry, which makes these buds much easier to detect.[13]
The depth of tumor invasion measured from the surface of the tumor to the deepest point of invasion is an important prognostic parameter for OSCCs and the cutoff point of 4 mm showed an association with lymph node metastasis, the most important marker for therapy and prognosis of OSCC.[14] A simple prognostic model was proposed by Almangush et al. in 2015,[3] wherein they utilized parameters of TB and depth of invasion together as the BD model and found that the model was strongly associated with patient survival and locoregional recurrence for patients with oral tongue squamous cell carcinoma (OTSCC). BD model was also found to be an independent prognostic factor for patients with OSCC[2] and early stage OTSCC.
Therefore, the present study was undertaken to compare the detection of TB in hematoxylin-eosin and pan-cytokeratin stained IHC slides of OSCC. Further, the BD score was evaluated to determine whether it serves as a reliable prognostic indicator for OSCC.
Study design
The present retrospective cross-sectional study was carried out in the Department of Oral Pathology and Microbiology of the institution. Institutional Ethics Committee approval was taken prior to undertaking the study (vpdc/659/2018-2019 dated October 12, 2018). A total of 30 formalin-fixed, paraffin-embedded tissue blocks of clinically and histopathologically diagnosed cases of OSCC were retrieved from registry of the department. Two tissue sections from each paraffin embedded block were cut. One was stained with hematoxylin and eosin and the other was processed for pan cytokeratin immunohistochemistry. Diagnosed cases of conventional primary OSCC were included and histopathological variants of OSCC, malignancies other than SCC, recurrent or secondary OSCC and metastatic lesions of oral cavity were excluded from the study.
METHODOLOGY
Demographic and clinical history details of selected cases were retrieved from the archival records of department registry (January 2018–August 2019). Demographic details such as age, gender, socio-economic status and history of habits such as tobacco chewing and/or alcoholism were included. Clinical history details like site of lesion and TNM staging were also recorded. H&E stained sections of OSCC were evaluated for degree of differentiation according to the criteria given by the WHO histological grading system.[15] Pattern of invasion (POI) and lymphocytic host response were assessed at tumor invasive front according to criteria of Brandwein-Gensler et al. in 2005.[16]
Tumor buds were evaluated according to scoring criteria of Almangush et al.[3] To eliminate bias, each section was evaluated by two observers and then jointly by consensus. Scoring criteria for the evaluation of tumor buds in H&E stained and pancytokeratin stained sections were the same. TB was evaluated at the invasive front of the tumor. TB is defined as the presence of a single cancer cell or small cluster of <5 cancer cells at the invasive front. During the scoring of TB 'B', the whole tumor area was scanned at low magnification (×4) then the highest number of TB was counted at a higher magnification (×20) and was used as the score for budding. The cut off point for TB was set at 5 buds (low <5; high ≥5). Depth of invasion 'D' was measured from the tumor surface to the deepest point of invasion. The cut off point for depth of invasion was set at 4 mm (low <4 mm; high ≥4 mm). TB 'B' and depth of invasion 'D' were combined in one model, the BD model. The scoring criteria for TB and DI are taken together as BD score according to Almangush et al.[3]
In accordance with the BD model, each case was assigned a score between 0 and 2. Cases with score 0 were classified as low risk, those with score 1 as inter-mediate risk and those with score 2 as high risk. Pancytokeratin was used as IHC marker for tumor buds. The anti-CK antibody cocktail was chosen to target a full spectrum of keratin polypeptides both acidic and basic to enhance the detection level provided by Biogenex AE1+ AE3, Fremont, CA 94538, USA. Adjacent non-cancerous epithelium served as internal positive control in the slides stained with pancytokeratin.
Statistical analysis
Statistical analysis was performed using IBM SPSS software, version 20, Armonk, New York, USA. Descriptive analysis was employed to summarize patient's clinical data such as age, gender, site of lesion, TNM staging and histopathological grading. Categorical data were measured using frequency counts and percentages. Pearson Chi-Square test was used to test possible relationship between various clinicopathological variables and BD score. Cox Regression model was performed for the assessment of survival to evaluate independent prognostic strength. The endpoints for survival analyses were the time to last follow-up (23 months) or the time to death. Significance level was set at P < 0.05.
RESULTS
The clinicopathological characteristics of patient's are summarized in Table 1. The study consisted of 30 diagnosed cases of OSCC, who were treated with primary resection and/or RT or CT. Patient's age ranged from 21 to 72 years. There were 24 males and 6 female patients among 30 cases. The most common site of lesion was buccal mucosa (n = 10), followed by tongue and alveolus (n = 7), retromolar trigone (n = 4), gingivobuccal sulcus and floor of mouth (n = 1). As far as TNM staging is considered, there were 14 cases in clinical stage IV, 8 cases in stage III and 4 cases each in stage I and II. There was no statistical significance between age, gender, site of lesion, clinical staging and BD score [Tables 2 and 3].
Table 1.
Clinicopathological characteristics of 30 oral squamous cell carcinoma cases
| Characteristics (clinical and histopathological) | Number of patients, n (%) |
|---|---|
| Age | |
| 21-40 | 8 (26.6) |
| 41-60 | 17 (56.6) |
| 61 and above | 5 (16.6) |
| Gender | |
| Male | 24 (80) |
| Female | 6 (20) |
| Site of lesion | |
| Buccal mucosa | 10 (33.3) |
| Tongue | 7 (23.3) |
| Alveolus | 7 (23.3) |
| Retromolar trigone | 4 (13.3) |
| Gingivobuccal sulcus | 1 (3.3) |
| Floor of mouth | 1 (3.3) |
| Clinical stage | |
| Stage I | 4 (13.3) |
| Stage II | 4 (13.3) |
| Stage III | 8 (26.7) |
| Stage IV | 14 (46.7) |
| Degree of differentiation | |
| Well differentiated | 28 (93.3) |
| Moderately differentiated | 1 (3.3) |
| Poorly differentiated | 1 (3.3) |
| Pattern of invasion | |
| Type 1 | 7 (23.3) |
| Type 2 | 5 (16.7) |
| Type 3 | 10 (33.3) |
| Type 4 | 4 (13.3) |
| Type 5 | 4 (13.3) |
| Lymphocytic host response | |
| Strong | 18 (60) |
| Interim | 5 (16.6) |
| Weak | 7 (23.3) |
| Tumour budding | |
| Score 0 | 2 (6.6) |
| Score 1 | 3 (10) |
| Score 2 | 25 (83.3) |
| Depth of invasion | |
| Score 1 | 1 (3.3) |
| Score 2 | 29 (96.7) |
| BD score | |
| Score 0-low risk | 0 (0) |
| Score 1- intermediate risk | 6 (20) |
| Score 2- high risk | 24 (80) |
| Treatment modalities | |
| Surgery only | 13 (43.3) |
| Surgery + RT | 10 (33.3) |
| Surgery + RT + CT | 5 (16.7) |
| Surgery + CT | 2 (6.7) |
| Follow-up | |
| Alive | 17 (56.7) |
| Dead | 7 (23.3) |
| Lost to follow up | 6 (20) |
CT: Chemotherapy, RT: Radiotherapy, BD: Budding and Depth of Invasion
Table 2.
Age and gender wise correlation with BD score
| Crosstab | ||||
|---|---|---|---|---|
| Age | Counts and percentages (%) within BD score | BD score | Total | |
| Score 1 | Score 2 | |||
| <40 | Count | 1 | 7 | 8 |
| Percentage within BD score | 16.7 | 29.2 | 26.7 | |
| 41-50 | Count | 2 | 7 | 9 |
| Percentage within BD score | 33.3 | 29.2 | 30.0 | |
| 51-60 | Count | 2 | 6 | 8 |
| Percentage within BD score | 33.3 | 25.0 | 26.7 | |
| >60 | Count | 1 | 4 | 5 |
| Percentage within BD score | 16.7 | 16.7 | 16.7 | |
| Total | Count | 6 | 24 | 30 |
| Percentage within BD score | 100.0 | 100.0 | 100.0 | |
| Chi-square tests | ||||
| Value | df | Asymptotic significant (two-sided) | ||
| Pearson χ2 | 0.434 | 3 | 0.933 | |
| Crosstab | ||||
| Gender | Counts and percentages (%) within BD score | BD score | Total | |
| Score 1 | Score 2 | |||
| Gender | ||||
| Female | Count | 1 | 5 | 6 |
| Percentage within BD score | 16.7 | 20.8 | 20.0 | |
| Male | Count | 5 | 19 | 24 |
| Percentage within BD score | 83.3 | 79.2 | 80.0 | |
| Total | Count | 6 | 24 | 30 |
| Percentage within BD score | 100.0 | 100.0 | 100.0 | |
| Chi-square tests | ||||
| Value | df | P<0.05 is significant | ||
| Pearson χ2 | 0.052 | 1 | 0.819 | |
DF: Degrees of freedom, BD: Tumour budding (B) and Depth of invasion (D)
Table 3.
Site wise and tumor, nodes, metastases clinical staging correlation with BD score
| Crosstab | ||||
|---|---|---|---|---|
| Site | Counts and percentages (%) within BD score | BD score | Total | |
| Score 1 | Score 2 | |||
| Site | ||||
| Buccal mucosa | Count | 4 | 6 | 10 |
| Percentage within BD score | 66.7 | 25.0 | 33.3 | |
| Tongue | Count | 1 | 6 | 7 |
| Percentage within BD score | 16.7 | 25.0 | 23.3 | |
| Alveolus | Count | 1 | 6 | 7 |
| Percentage within BD score | 16.7 | 25.0 | 23.3 | |
| Retromolar trigone | Count | 0 | 4 | 4 |
| Percentage within BD score | 0.0 | 16.7 | 13.3 | |
| Gingivo-buccal sulcus | Count | 0 | 1 | 1 |
| Percentage within BD score | 0.0 | 4.2 | 3.3 | |
| Floor of mouth | Count | 0 | 1 | 1 |
| Percentage within BD score | 0.0 | 4.2 | 3.3 | |
| Total | Count | 6 | 24 | 30 |
| Percentage within BD score | 100.0 | 100.0 | 100.0 | |
| Chi-square tests | ||||
| Value | df | P<0.05 is significant | ||
| Pearson χ2 | 4.286 | 5 | 0.509 | |
| Crosstab | ||||
| TNM Clinical Staging | Counts and percentages (%) within BD score | BD score | Total | |
| Score 1 | Score 2 | |||
| TNM clinical staging | ||||
| I | Count | 1 | 3 | 4 |
| Percentage within BD score | 16.7 | 12.5 | 13.3 | |
| II | Count | 2 | 2 | 4 |
| Percentage within BD score | 33.3 | 8.3 | 13.3 | |
| III | Count | 2 | 6 | 8 |
| Percentage within BD score | 33.3 | 25.0 | 26.7 | |
| IV A | Count | 0 | 2 | 2 |
| Percentage within BD score | 0.0 | 8.3 | 6.7 | |
| IV C | Count | 1 | 11 | 12 |
| Percentage within BD score | 16.7 | 45.8 | 40.0 | |
| Total | Count | 6 | 24 | 30 |
| Percentage within BD score | 100.0 | 100.0 | 100.0 | |
| Chi-square tests | ||||
| Value | df | P<0.05 is significant | ||
| Pearson χ2 | 3.958 | 4 | 0.412 | |
TNM: Tumor, nodes, metastases DF: Degrees of freedom, BD: Tumour budding (B) and depth of invasion (D)
Histopathological parameters
In H&E stained sections, out of 30 cases, 18 cases (60%) had score 2 [Figure 1a], eight cases (26.6%) failed to detect tumor buds and 4 cases (13.3%) had score 1. In pancytokeratin stained sections, 25 cases (83.3%) showed score 2 tumor buds [Figure 1b]; followed by 3 cases (10%) with score 1, and 2 cases (6.6%) did not show any buds. Statistical significance was noted on comparison of TB scores in H&E and pancytokeratin stained sections (P = 0.001, kappa value 0.431) [Table 4]. Moderate agreement was seen between the TB assessed in H&E and Pancytokeratin. Pancytokeratin score for TB was 2 in 5 cases where H&E scores was 0 [Figure 2a] and in 1 case the score was 1 [Figure 2b].
Figure 1.
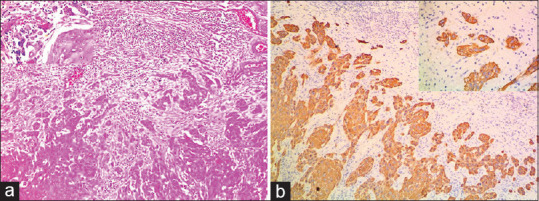
(a) Hematoxylin and eosin stained section showing score 2 tumour buds (×10) Inset (×40). (b) Pan cytokeratin stained section showing score 2 tumour buds ((×10) (Inset ×40)
Table 4.
Kappa statistics: Tumour budding-H and E and pancytokeratin (score)
| Tumour Budding H&E (Score) | Counts and percentages (%) within BD score | Tumour budding pancytokeratin (score) | Total | ||
|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | |||
| Tumour budding H and E (score) | |||||
| Score 0 | Count | 2 | 1 | 5 | 8 |
| Percentage within tumour budding pancytokeratin (score) | 100.0 | 33.3 | 20.0 | 26.7 | |
| Score 1 | Count | 0 | 2 | 2 | 4 |
| Percentage within tumour budding pancytokeratin (score) | 0.0 | 66.7 | 8.0 | 13.3 | |
| Score 2 | Count | 0 | 0 | 18 | 18 |
| Percentage within tumour budding pancytokeratin (score) | 0.0 | 0.0 | 72.0 | 60.0 | |
| Total | Tumour budding score in H and E stained sections | 2 | 3 | 25 | 30 |
| Percentage within tumour budding pancytokeratin (score) | 100.0 | 100.0 | 100.0 | 100.0 | |
| Symmetric measures | |||||
| Value | Asymptotic SEa | Approximatetb | Approximate significant | P | |
| Measure of agreement | |||||
| κ | 0.431 | 0.142 | 3.745 | <0.001 | 0.001 |
| Number of valid cases | 30 | ||||
aNot assuming the null hypothesis, bUsing the asymptotic standard error assuming the null hypothesis. SE: Standard error
Figure 2.
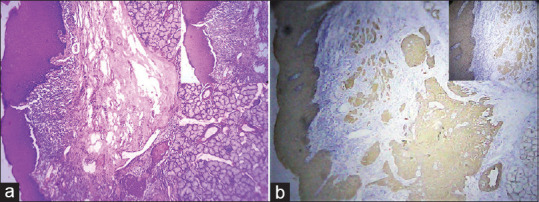
(a) Hematoxylin and eosinstained section showing Score 0 tumour buds (×4) Inset (×10). (b) Pan cytokeratin stained section showing Score 2 tumour buds (×4) (Inset 10)
Twenty nine cases (96.7%) showed score 2 depth of invasion and only one case (3.3%) had score 1. As far as BD model is concerned, there were 24 cases (80%) with high risk score (score 2) [Figure 3a] and 6 cases (20%) with intermediate risk score (score 1) [Figure 3b]. Statistical significance was noted between BD score and TB score of H&E and pan cytokeratin staining. However, it was highly significant in association with pan cytokeratin IHC staining [Table 5]. Most of the tumors were well differentiated OSCC, (N = 28, 93.3%), followed by 1 case (3.3%) each of moderate and poorly differentiated OSCC. Lymphocytic host response was strong in 18 cases (60%) followed by interim in 5 cases (16.6%) and weak in 7 cases (23.3%). There was no statistical significance between BD score and variables such as degree of differentiation and lymphocytic host response [Table 6].
Figure 3.
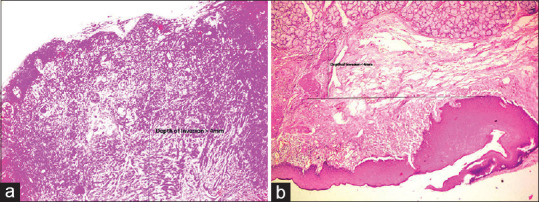
(a) Hematoxylin and eosinstained section showing BD score 2 (×4). (b) Hematoxylin and eosinstained section showing BD score 1 (×4)
Table 5.
Tumour budding score in H and E and pancytokeratin correlation with BD score
| Crosstab | |||||
|---|---|---|---|---|---|
| Tumour Budding H&E (Score) | Counts and percentages (%) within BD score | BD score | Total | ||
| Score 1 | Score 2 | ||||
| Tumour budding H and E (score) | |||||
| Score 0 | Count | 4 | 4 | 8 | |
| Percentage within BD score | 66.7 | 16.7 | 26.7 | ||
| Score 1 | Count | 2 | 2 | 4 | |
| Percentage within BD score | 33.3 | 8.3 | 13.3 | ||
| Score 2 | Count | 0 | 18 | 18 | |
| Percentage within BD score | 0.0 | 75.0 | 60.0 | ||
| Total | Count | 6 | 24 | 30 | |
| Percentage within BD score | 100.0 | 100.0 | 100.0 | ||
| Chi-square tests | |||||
| Value | df | P<0.05 is significant | |||
| Pearson χ2 | 11.250 | 2 | 0.004 | ||
| Crosstab | |||||
| Tumour Budding Pancytokeratin (Score) | Counts and percentages (%) within BD score | BD score | Total | ||
| Score 1 | Score 2 | ||||
| Tumour budding pancytokeratin (score) | |||||
| Score 0 | Count | 2 | 0 | 2 | |
| Percentage within BD score | 33.3 | 0.0 | 6.7 | ||
| Score 1 | Count | 3 | 0 | 3 | |
| Percentage within BD score | 50.0 | 0.0 | 10.0 | ||
| Score 2 | Count | 1 | 24 | 25 | |
| Percentage within BD score | 16.7 | 100.0 | 83.3 | ||
| Total | Count | 6 | 24 | 30 | |
| Percentage within BD score | 100.0 | 100.0 | 100.0 | ||
| Chi-square tests | |||||
| Value | df | P<0.05 is significant | |||
| Pearson χ2 | 24.000 | 2 | <0.001 | ||
DF: Degrees of freedom, BD: Tumour budding (B) and depth of invasion (D)
Table 6.
Degree of differentiation and LHR × BD score
| Crosstab | |||||
|---|---|---|---|---|---|
| Degree Of Differentiation | Counts and percentages (%) within BD score | BD score | Total | ||
| Score 1 | Score 2 | ||||
| Degree of differentiation | |||||
| WDSCC | Count | 5 | 23 | 28 | |
| Percentage within BD score | 83.3 | 95.8 | 93.3 | ||
| MDSCC | Count | 0 | 1 | 1 | |
| Percentage within BD score | 0.0 | 4.2 | 3.3 | ||
| PDSCC | Count | 1 | 0 | 1 | |
| Percentage within BD score | 16.7 | 0.0 | 3.3 | ||
| Total | Count | 6 | 24 | 30 | |
| Percentage within BD score | 100.0 | 100.0 | 100.0 | ||
| Chi-square tests | |||||
| Value | df | P <0.05 is significant | |||
| Pearson χ2 | 4.330 | 2 | 0.115 | ||
| LHR | Counts and percentages (%) within BD score | BD score | Total | ||
| Score 1 | Score 2 | ||||
| LHR | 3 | 15 | 18 | ||
| Strong | Count | 50.0 | 62.5 | 60.0 | |
| Percentage within BD score | 2 | 3 | 5 | ||
| Interim | Count | 33.3 | 12.5 | 16.7 | |
| Percentage within BD score | 1 | 6 | 7 | ||
| Weak | Count | 16.7 | 25.0 | 23.3 | |
| Percentage within BD score | 6 | 24 | 30 | ||
| Total | Count | 100.0 | 100.0 | 100.0 | |
| Percentage within BD score | |||||
| Chi-square tests | |||||
| Value | df | P<0.05 is significant | |||
| Pearson χ2 | 1.518 | 2 | 0.468 | ||
DF: Degrees of freedom, BD: Tumour budding (B) and depth of invasion (D), LHR: Lymphocytic host response
The most predominant POI (PPOI) noted was Type 3, (n = 10, 33.3%) [Figure 4a]. Out of these 10 cases, 9 cases had BD score 2 and only 1 case had BD score 1. There were 4 cases each (13.3%) in Type 4 [Figure 4b], Type 5 (worst POI [WPOI]) [Figure 4c] and all the cases had BD score 2. However, this result was not statistically significant with P = 0.129 [Table 7].
Figure 4.
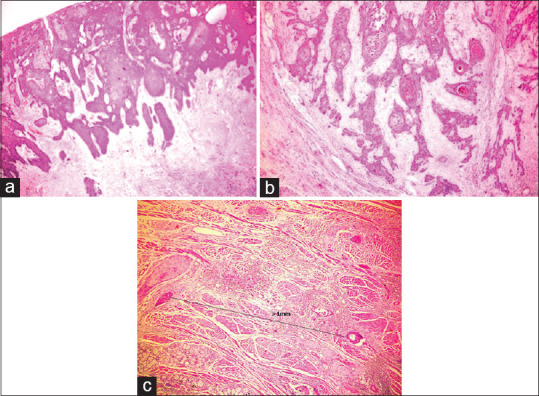
(a) Hematoxylin and eosinstained section showing Type 3 predominant pattern of invasion (×4). (b) Hematoxylin and eosinstained section showing Type 4 predominant pattern of invasion (×4). (c) Hematoxylin and eosinstained section showing Type 5 worst pattern of invasion (×4)
Table 7.
Predominant pattern of invasion × BD score
| PPOI × BD score crosstabulation | ||||
|---|---|---|---|---|
| PPOI | Counts and percentages (%) within BD score | BD score | Total | |
| Score 1 | Score 2 | |||
| PPOI | ||||
| Type 1 | Count | 0 | 1 | 1 |
| Percentage within BD score | 0.0 | 4.2 | 3.3 | |
| Type 1 | Count | 2 | 4 | 6 |
| Percentage within BD score | 33.3 | 16.7 | 20.0 | |
| Type 2 | Count | 3 | 2 | 5 |
| Percentage within BD score | 50.0 | 8.3 | 16.7 | |
| Type 3 | Count | 1 | 9 | 10 |
| Percentage within BD score | 16.7 | 37.5 | 33.3 | |
| Type 4 | Count | 0 | 4 | 4 |
| Percentage within BD Score | 0.0 | 16.7 | 13.3 | |
| Type 5 | Count | 0 | 4 | 4 |
| Percentage within BD score | 0.0 | 16.7 | 13.3 | |
| Total | Count | 6 | 24 | 30 |
| Percentage within BD score | 100.0 | 100.0 | 100.0 | |
| Chi-square tests | ||||
| Value | df | P<0.05 is significant | ||
| Pearson χ2 | 8.542 | 5 | 0.129 | |
PPOI: Predominant pattern of invasion, BD: Tumour budding (B) and depth of invasion (D)
The follow-up period was 23 months. Ten patients (33.33%) died, out of which 2 (20%) died due to loco-regional recurrence; 5 (50%) died of distant metastasis; and 2 (20%) died of immediate postoperative complications and one (10%) due to myocardial infarction. Twenty patients (66.66%) were alive at end of follow-up, out of which only one was alive with recurrence and nineteen cases were alive without recurrence. Out of 24 cases with BD score 2, 16 were alive at end of follow-up period and no statistically significance was noted between BD score and survival [Table 8 and Graph 1].
Table 8.
Cox regression analysis
| Variables in the equation | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | Wald | df | Significant | Odds ratio | 95.0% CI for odds ratio | ||
| Lower | Upper | |||||||
| BD score | 0.919 | 0.637 | 2.083 | 1 | 0.149 | 2.507 | 0.720 | 8.735 |
SE: Standard error, DF: Degrees of freedom, CI: Confidence interval, BD: Tumour budding (B) and Depth of invasion (D)
Graph 1.
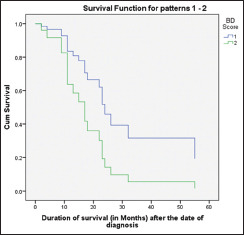
Cox regression analysis for survival of patients with BD score 1 and 2. (B-Tumour Budding and D- Depth of invasion)
DISCUSSION
Invasion is one of the hallmarks of cancer which is instrumental in progression and metastasis facets of malignant tumors. Brandwein and Gensler et al 16 have put forth the concept of worst patten of invasion (WPOI) type 5 in carcinomas which contain dispersed tumor satellites. By definition, there is at least 1mm of intervening normal tissue between at least 2 tumor masses. An important refinement of POI known as TB, defined as the presence of single cancer cells or small clusters of cells (<5 cells) at the IF, was first introduced in HNSCC prognostication during 2010.[11]
Tumor buds are defined as the presence of single tumor cells or small clusters of up to five tumor cells at the peritumoral invasive front (peritumoral buds) or within the main tumor body (intratumoral buds). TB has been strongly linked to adverse clinicopathological features and poor overall survival and disease free patient survival. They are thought to represent the morphological correlate of cancer cells having undergone epithelial-mesenchymal transition (EMT), an important mechanism for progression of epithelial cancers.[17]
Literature search reveals few studies which have evaluated TB in HNSCC and OSCC particularly.[1,11,18,19] Studies have shown that tumor buds as a whole show a loss of epithelial markers and a gain of mesenchymal markers as compared to the main tumor mass. These features are compatible with EMT, which is why budding is considered to be the histological expression of the same.[4] It is noteworthy that routine pathological reporting does not include histopathological parameters for the evaluation of EMT even though scientific evidence shows strong connection of EMT with poor prognosis.
TB is not a static, histological feature rather it represents a snap-shot of a dynamic process undertaken by an aggressive tumor with the potential to disseminate and metastasize. Strong, consistent evidence shows that TB is a predictor of lymph node metastasis, distant metastatic disease, local recurrence, worse overall and disease-free survival time and an independent prognostic factor. Extensive studies on TB in colorectal cancers can be dated back to 1989 where strong association between budding and the presence of lymphovascular invasion and lymph node metastases had been noted by Morodomi et al.[20] Moreover, the International Union against Cancer (UICC) recognized TB as a highly relevant, additional prognostic parameter in cancer. Immunohistochemical studies have been crucial for improving our understanding of TB.[21] Shinto et al.[22] used immunohistochemistry with anti-cytokeratin antibodies to highlight another feature of buds, termed cytoplasmic pseudo fragmentation in colorectal cancers. These cytoplasmic fragments, visible only by immunohistochemistry, are seen in the immediate vicinity of tumor buds. When examined on serial sections, some of the fragments were shown to be connected to the buds so called pseudo fragments. The presence of pseudo fragments was associated with high-grade budding, but was shown to be an independent prognostic factor on multivariate analysis, suggesting that its presence signifies an aggressive budding phenotype.
We found statistical significance (P < 0.001) in TB score evaluated on H&E stained sections. Similar results are also noted in various studies.[23,24,25,26,27,28] High TB score was noted as an independent prognostic factor in oral tongue SCC[10] and OSCC,[18] laryngeal carcinoma[11] and nasopharyngeal carcinoma[19] to predict invasiveness, disease-specific survival (DSS), distant metastasis. In squamous cell carcinomas of the tongue which are the most common and aggressive OSCC, Wang et al.[23] have reported TB as an independent prognostic marker. In addition, it had a strong correlation with tumor differentiation, tumor size, clinical stage and lymph node metastasis. TB may represent cells undergoing EMT, and such an interpretation has also been suggested by Wang et al.[23] based on low expression of the cell adhesion molecule E-cadherin at the budding site.
We noted a statistical significance on comparison of TB scores in H&E and pancytokeratin stained sections (P = 0.001). A high TB score has been noted in studies wherein TB was evaluated using only pancytokeratin instead of H&E.[5,13,29,30,31,32] We noted a moderate agreement between the TB assessed in H&E and pancytokeratin. H&E score was 0 when pancytokeratin score was 2 in 5 cases and in 1 case the score was 1. Attramadal et al.[5] have also found pancytokeratin to be more sensitive and easy to score than H&E. Similarly, several studies which were considered in the meta-analysis conducted by Almangush et al[33] on tumour budding in OSCC also showed a high tumour budding score. Zlobec et al.[34], Lugli et al.[35], Ohtsuki K et al.[36] and Koelzer V H et al.[37] have also identified significantly higher counts with immunohistochemistry than those obtained on H&E staining in their studies on colorectal cancers. Few studies that have attempted to define an optimal budding threshold on anti-cytokeratin-stained slides, have reported higher cutoffs than those found to be prognostically significant on H&E. H&E staining tends to underestimate TB while pan-cytokeratin immunostaining frequently helps to visualize numerous buds intermingled with stromal fibroblasts, improves the reproducibility and helps in easy categorization of cases.[29,36,38] However, Van Wyk et al.[39] have reported that pan-keratin is neither more reproducible nor gives a higher prognostic value than HE scoring in colorectal cancer.
There are some qualitative considerations contributing to subjectivity in assessment of TB like stromal cells or histiocytes masquerading as buds, marked inflammation obscuring buds and difficulties in determining whether a small cluster of cells represents a true bud or the mechanical fragmentation of a larger gland. These considerations lead to difficulties that unexperienced pathologists have in discriminating small buds from surrounding stromal or inflammatory cells on H&E stained slides.[40,41] Immunohistochemistry for anticytokeratin helps to highlight tumor buds in this setting and may also improve interobserver agreement.[37,40] Therefore, it is highly recommended that TB assessment by using pan-cytokeratin immunohistochemistry is mandatory in cases where identification of buds becomes difficult in H&E stained slides especially for young and aspiring pathologists.
Our other objective was to co-relate the BD score and risk stratification with various clinical and histopathological prognosticators. The two elected features of BD model (TB and Depth of invasion) have been individually described as predictors of prognosis for OSCC patients.[5,42,43,44] Sawazaki et al.[2] have noted the BD model to be significantly associated with disease outcome as an independent prognostic marker. In contrary, we did not find any statistical significance between various clinical variables such as age, gender, site of lesion, clinical staging and BD score. Neither did we notice any statistical significance between BD score and histo-pathological variables like degree of differentiation and lymphocytic host response. Our results are comparable to the results of study conducted by Almangush et al.[3] in 2015, on early stage oral tongue cancer where they did not find statistically significant association between BD score and age, gender or histological differentiation (tumor grade). However, on multivariate analysis, they noted that high risk score (BD score 2) correlated significantly with loco-regional recurrence and death due to SCC. They also noted that among all the histopathological parameters evaluated, only the worst POI and TB were associated with both disease-free survival (DFS) and DSS in the unadjusted univariate analysis. Depth of invasion was related to DSS, but not to DFS. We had an interesting finding that 4 cases each of Type 4 (PPOI) and Type 5 (WPOI) had BD score 2, i.e., high risk grade. Although there was no statistical significance between BD score and PPOI, this result is clinically and pathologically very relevant. No statistically significance was noted between BD score and survival. This result seems contradictory and may be due to lower number of cases in score 1. The only limitation noted in our study is less number of cases and shorter follow-up period. Hence, we recommend further studies with more number of cases and longer follow-up to assess 5-year survival.
Currently, the role for immunohistochemistry in the evaluation of TB is unclear, and further studies are needed to assess the relationship between bud counts obtained on H&E versus those obtained with anti-cytokeratin antibodies, and to determine the most prognostically useful cutoff for bud counting with both H&E and anti-cytokeratin antibodies. Even with all the limitations in mind, based on our findings, we recommend that TB evaluation should be included in routine histopathology reporting of OSCC. This might serve as a useful prognostic tool in multimodality treatment decisions for OSCC.
CONCLUSION
We can state that immunohistochemical analysis of TB is superior to H&E staining in detection of tumor buds at the tumor invasive front and evaluation of TB and BD score with risk stratification definitely serves as a promising histopathologic prognostic parameter in OSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We acknowledge the help and support of Director and Oncopathology laboratory of Mahatma Gandhi Cancer Hospital, Miraj, Maharashtra.
REFERENCES
- 1.Almangush A, Salo T, Hagström J, Leivo I. Tumour budding in head and neck squamous cell carcinoma-a systematic review. Histopathology. 2014;65:587–94. doi: 10.1111/his.12471. [DOI] [PubMed] [Google Scholar]
- 2.Sawazaki-Calone I, Rangel A, Bueno AG, Morais CF, Nagai HM, Kunz RP, et al. The prognostic value of histopathological grading systems in oral squamous cell carcinomas. Oral Dis. 2015;21:755–61. doi: 10.1111/odi.12343. [DOI] [PubMed] [Google Scholar]
- 3.Almangush A, Coletta RD, Bello IO, Bitu C, Keski-Säntti H, Mäkinen LK, et al. A simple novel prognostic model for early stage oral tongue cancer. Int J Oral Maxillofac Surg. 2015;44:143–50. doi: 10.1016/j.ijom.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H. Tumor budding: The name is EMT. Partial EMT. J Clin Med. 2016;5:51. doi: 10.3390/jcm5050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attramadal CG, Kumar S, Boysen ME, Dhakal HP, Nesland JM, Bryne M. Tumor budding, EMT and cancer stem cells in T1-2/N0 oral squamous cell carcinomas. Anticancer Res. 2015;35:6111–20. [PubMed] [Google Scholar]
- 6.Cho SJ, Kakar S. Tumor budding in colorectal carcinoma: Translating a morphologic score into clinically meaningful results. Arch Pathol Lab Med. 2018;142:952–7. doi: 10.5858/arpa.2018-0082-RA. [DOI] [PubMed] [Google Scholar]
- 7.Roh MS, Lee JI, Choi PJ. Tumor budding as a useful prognostic marker in esophageal squamous cell carcinoma. Dis Esophagus. 2004;17:333–7. doi: 10.1111/j.1442-2050.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- 8.Gujam FJ, McMillan DC, Mohammed ZM, Edwards J, Going JJ. The relationship between tumour budding, the tumour microenvironment and survival in patients with invasive ductal breast cancer. Br J Cancer. 2015;113:1066–74. doi: 10.1038/bjc.2015.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor RT, Veronese N, Nottegar A, Malleo G, Smith L, Demurtas J, et al. Prognostic Role of High-Grade Tumor Budding in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis with a Focus on Epithelial to Mesenchymal Transition. Cancers (Basel) 2019;11:113–20. doi: 10.3390/cancers11010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almangush A, Bello IO, Keski–S€antti H, M€akinen LK, Kauppila JH, Pukkila M, et al. Depth of invasion, tumor budding, and worst pattern of invasion: Prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36(6):811–8. doi: 10.1002/hed.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarioglu S, Acara C, Akman FC, Dag N, Ecevit C, Ikiz AO, et al. Tumor budding as a prognostic marker in laryngeal carcinoma. Pathol Res Pract. 2010;206:88–92. doi: 10.1016/j.prp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Teramoto H, Koike M, Tanaka C, Yamada S, Nakayama G, Fujii T, et al. Tumor budding as a useful prognostic marker in T1-stage squamous cell carcinoma of the esophagus. J Surg Oncol. 2013;108:42–6. doi: 10.1002/jso.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seki M, Sano T, Yokoo S, Oyama T. Tumour budding evaluated in biopsy specimens is a useful predictor of prognosis in patients with cN0 early stage oral squamous cell carcinoma. Histopathology. 2017;70:869–79. doi: 10.1111/his.13144. [DOI] [PubMed] [Google Scholar]
- 14.Huang SH, Hwang D, Lockwood G, Goldstein DP, O’Sullivan B. Predictive value of tumor thickness for cervical lymph-node involvement in squamous cell carcinoma of the oral cavity. Cancer. 2009;15(7):1489–97. doi: 10.1002/cncr.24161. [DOI] [PubMed] [Google Scholar]
- 15.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. WHO Classification of Tumors: Pathology and Genetics of Head and Neck Tumors. Lyon, France: IARC Press; 2005. pp. 118–31. [Google Scholar]
- 16.Brandwein-Gensler M, Smith RV, Wang B, Penner C, Theilken A, Broughel D, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676–88. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- 17.Dawson H, Lugli A. Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front Med (Lausanne) 2015;2:11. doi: 10.3389/fmed.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marangon Junior H, Rocha VN, Leite CF, de Aguiar MC, Souza PE, Horta MC. Laminin-5 gamma 2 chain expression is associated with intensity of tumor budding and density of stromal myofibroblasts in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43:199–204. doi: 10.1111/jop.12121. [DOI] [PubMed] [Google Scholar]
- 19.Luo WR, Gao F, Li SY, Yao KT. Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology. 2012;61:1072–81. doi: 10.1111/j.1365-2559.2012.04350.x. [DOI] [PubMed] [Google Scholar]
- 20.Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An lndex for estimating the probability of lymph node metastasis in rectal cancers - Lymph node metastasis and the histopathology of actively lnvasive regions of cancer. Cancer. 1989;63:539–43. doi: 10.1002/1097-0142(19890201)63:3<539::aid-cncr2820630323>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: Tumor budding as oncotarget. Oncotarget. 2010;1:651–61. doi: 10.18632/oncotarget.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinto E, Mochizuki H, Ueno H, Matsubara O, Jass JR. A novel classification of tumour budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology. 2005;47:25–31. doi: 10.1111/j.1365-2559.2005.02162.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Huang H, Huang Z, Wang A, Chen X, Huang L, et al. Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2011;40:545–51. doi: 10.1111/j.1600-0714.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angadi PV, Patil PV, Hallikeri K, Mallapur MD, Hallikerimath S, Kale AD. Tumor budding is an independent prognostic factor for prediction of lymph node metastasis in oral squamous cell carcinoma. Int J Surg Pathol. 2015;23:102–10. doi: 10.1177/1066896914565022. [DOI] [PubMed] [Google Scholar]
- 25.Nandita KP, Boaz K, Srikant N, Lewis AJ, Manaktala N. Tumour budding: A promising parameter in oral squamous cell carcinoma. Res J Pharm Biol and Chem Sci. 2016;7:2059–63. [Google Scholar]
- 26.Boxberg M, Jesinghaus M, Dorfner C, Mogler C, Drecoll E, Warth A, et al. Tumour budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma: Proposal for an adjusted grading system. Histopathology. 2017;70:1125–37. doi: 10.1111/his.13173. [DOI] [PubMed] [Google Scholar]
- 27.Hori Y, Kubota A, Yokose T, Furukawa M, Matsushita T, Takita M, et al. Predictive significance of tumor depth and budding for late lymph node metastases in patients with clinical N0 early oral tongue carcinoma. Head Neck Pathol. 2017;11:477–86. doi: 10.1007/s12105-017-0814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arora A, Husain N, Bansal A, Neyaz A, Jaiswal R, Jain K, et al. Development of a new outcome prediction model in early-stage squamous cell carcinoma of the oral cavity based on histopathologic parameters with multivariate analysis: The Aditi-Nuzhat lymph-node prediction score (ANLPS) system. Am J Surg Pathol. 2017;41:950–60. doi: 10.1097/PAS.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 29.Jensen DH, Dabelsteen E, Specht L, Fiehn AM, Therkildsen MH, Jønson L, et al. Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236:505–16. doi: 10.1002/path.4550. [DOI] [PubMed] [Google Scholar]
- 30.Xie N, Wang C, Liu X, Li R, Hou J, Chen X, et al. Tumor budding correlates with occult cervical lymph node metastasis and poor prognosis in clinical early-stage tongue squamous cell carcinoma. J Oral Pathol Med. 2015;44:266–72. doi: 10.1111/jop.12242. [DOI] [PubMed] [Google Scholar]
- 31.Seki M, Sano T, Yokoo S, Oyama T. Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck. 2016;38(Suppl 1):E1582–90. doi: 10.1002/hed.24282. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen NJ, Jensen DH, Lelkaitis G, Kiss K, Charabi B, Specht L, et al. Construction of a pathological risk model of occult lymph node metastases for prognostication by semi-automated image analysis of tumor budding in early-stage oral squamous cell carcinoma. Oncotarget. 2017;8:18227–37. doi: 10.18632/oncotarget.15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almangush A, Pirinen M, Heikkinen I, Mäkitie AA, Salo T, Leivo I. Tumour budding in oral squamous cell carcinoma: A meta-analysis. Br J Cancer. 2018;118:577–86. doi: 10.1038/bjc.2017.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zlobec I, Hädrich M, Dawson H, Koelzer VH, Borner M, Mallaev M, et al. Intratumoural budding (ITB) in preoperative biopsies predicts the presence of lymph node and distant metastases in colon and rectal cancer patients. Br J Cancer. 2014;110:1008–13. doi: 10.1038/bjc.2013.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lugli A, Vlajnic T, Giger O, Karamitopoulou E, Patsouris ES, Peros G, et al. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum Pathol. 2011;42:1833–40. doi: 10.1016/j.humpath.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Ohtsuki K, Koyama F, Tamura T, Enomoto Y, Fujii H, Mukogawa T, et al. Prognostic value of immunohistochemical analysis of tumor budding in colorectal carcinoma. Anticancer Res. 2008;28:1831–6. [PubMed] [Google Scholar]
- 37.Koelzer VH, Zlobec I, Berger MD, Cathomas G, Dawson H, Dirschmid K, et al. Tumor budding in colorectal cancer revisited: Results of a multicenter interobserver study. Virchows Arch. 2015;466:485–93. doi: 10.1007/s00428-015-1740-9. [DOI] [PubMed] [Google Scholar]
- 38.Kai K, Aishima S, Aoki S, Takase Y, Uchihashi K, Masuda M, et al. Cytokeratin immunohistochemistry improves interobserver variability between unskilled pathologists in the evaluation of tumor budding in T1 colorectal cancer. Pathol Int. 2016;66:75–82. doi: 10.1111/pin.12374. [DOI] [PubMed] [Google Scholar]
- 39.Van Wyk HC, Park J, Roxburgh C, Horgan P, Foulis A, MacMillan DC. The role of tumour budding in predicting survival in patients with primary operable colorectal cancer: A systematic review. Cancer Treat Rev. 2015;41(2):151–9. doi: 10.1016/j.ctrv.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: Time to take notice. Mod Pathol. 2012;25:1315–25. doi: 10.1038/modpathol.2012.94. [DOI] [PubMed] [Google Scholar]
- 41.Max N, Harbaum L, Pollheimer MJ, Lindtner RA, Kornprat P, Langner C. Tumour budding with and without admixed inflammation: Two different sides of the same coin? Br J Cancer. 2016;114:368–71. doi: 10.1038/bjc.2015.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu K, Wei J, Liu Z, Yu B, Yang X, Zhang C, et al. Can pattern and depth of invasion predict lymph node relapse and prognosis in tongue squamous cell carcinoma. BMC Cancer. 2019;19:714. doi: 10.1186/s12885-019-5859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faisal M, Abu Bakar M, Sarwar A, Adeel M, Batool F, Malik KI, et al. Depth of invasion (DOI) as a predictor of cervical nodal metastasis and local recurrence in early stage squamous cell carcinoma of oral tongue (ESSCOT) PLoS One. 2018;13:e0202632. doi: 10.1371/journal.pone.0202632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang WC, Chang CF, Li YH, Yang CY, Su RY, Lin CK, et al. A histopathological evaluation and potential prognostic implications of oral squamous cell carcinoma with adverse features. Oral Oncol. 2019;95:65–73. doi: 10.1016/j.oraloncology.2019.06.012. [DOI] [PubMed] [Google Scholar]


