Abstract
We report the unprecedented complete absence of pediatric enteroviral meningitis in 2020 in the area of Bern, Switzerland. Presumably an unintended effect of coronavirus disease 2019 public health measures, this finding highlights the potential of community-wide nonpharmaceutical interventions for controlling the circulation of a major pediatric pathogen, which is mainly transmitted by the fecal–oral route.
Keywords: child, COVID-19, Enterovirus, epidemiology, meningitis, SARS-CoV-2
We report an 11-year time-series analysis from a Swiss University Hospital demonstrating the unprecedented complete absence of pediatric enteroviral meningitis in 2020. This observation emphasizes that non-pharmaceutical interventions designed to prevent COVID-19 also suppress the mainly fecal-to-oral transmission of enteroviruses.
In temperate climates, infections caused by nonpoliovirus enteroviruses (EVs) in children peak between June and October [1–3]. Outbreaks recur each year despite cycling changes of the predominant EV types [1, 4]. Human-to-human transmission occurs primarily via the fecal–oral route, and less commonly by respiratory secretions [5]. EVs cause various nonspecific febrile syndromes. They are also the most commonly identified etiology of acute central nervous system (CNS) infection in children [6], with the greatest age-specific incidence in infants [4, 6].
At our institution, EV meningitis case counts peak each summer, and testing cerebrospinal fluid (CSF) for EV by polymerase chain reaction (PCR) is part of the standard diagnostic algorithm for all infants <90 days of age undergoing lumbar puncture for sepsis workup and for children of any age evaluated for possible meningitis.
Unexpectedly, we observed no case in 2020. Here we describe the occurrence of EV meningitis over 11 consecutive years and correlate the epidemic curve with the timing of various nonpharmaceutical interventions (NPIs) introduced to halt the coronavirus disease 2019 (COVID-19) pandemic in 2020. As the NPIs were primarily designed to interrupt respiratory transmissions and EVs may also, albeit less commonly, be transmitted by the respiratory route, we also calculated the epidemic curve for respiratory rhinovirus/enterovirus detections.
METHODS
This is a retrospective single-center time series analysis of the occurrence of EV meningitis at an institution, which is the only provider of advanced pediatric emergency services (20 000 consultations per year) and inpatient services (100 beds; 6000 admissions per year) for a catchment area population of 1.5 million. We defined EV meningitis as the detection of enteroviral RNA by CSF PCR, which was introduced in 2010/2011 to the in-house diagnostic algorithm for infants <90 days of age undergoing sepsis workup and pediatric patients of any age evaluated for meningitis. Using the electronic hospital database, we analyzed 3 separate data sets from January 1, 2010, to December 31, 2020: (1) CSF real-time EV PCR test results were used to capture the number of patients tested and the number of EV meningitis cases; (2) hospital admissions coded for EV meningitis (ICD-10 code A87.0) were obtained for validation of PCR results; (3) nasopharyngeal aspirate (NPA) immunofluorescence (IF) tests for rhinovirus/enterovirus [7] were obtained to monitor overall respiratory rhinovirus/enterovirus activity (IF assay does not distinguish between rhinoviruses and enteroviruses). For each case, the date and patient age were recorded. We plotted counts by month, quarter, or year and performed a time series analysis comparing the frequencies in 2020 with those in 2010–2019. We performed separate analyses for patients <1 year of age and patients 1 to 16 years of age.
We calculated the average epidemic curve from January 1, 2010, to December 31, 2019, using the method recommended by the World Health Organization Global Epidemiological Standards for Influenza (https://www.who.int/influenza/resources/documents/influenza_surveillance_manual/en/). We used a generalized linear model with a Poisson distribution to predict expected numbers for 2020 based on annual counts of EV CSF PCR tests, NPA IF tests, and ICD-10 code counts for the years 2010 to 2019. Statistical analysis was performed using the R software package, version 4.0.3 (Vienna, Austria; https://www.R-project.org/). Start and end dates of NPIs implemented in Switzerland during the first wave of the COVID-19 pandemic were provided by the Swiss Federal Office of Public Health and are detailed in the Supplementary Table 1.
RESULTS
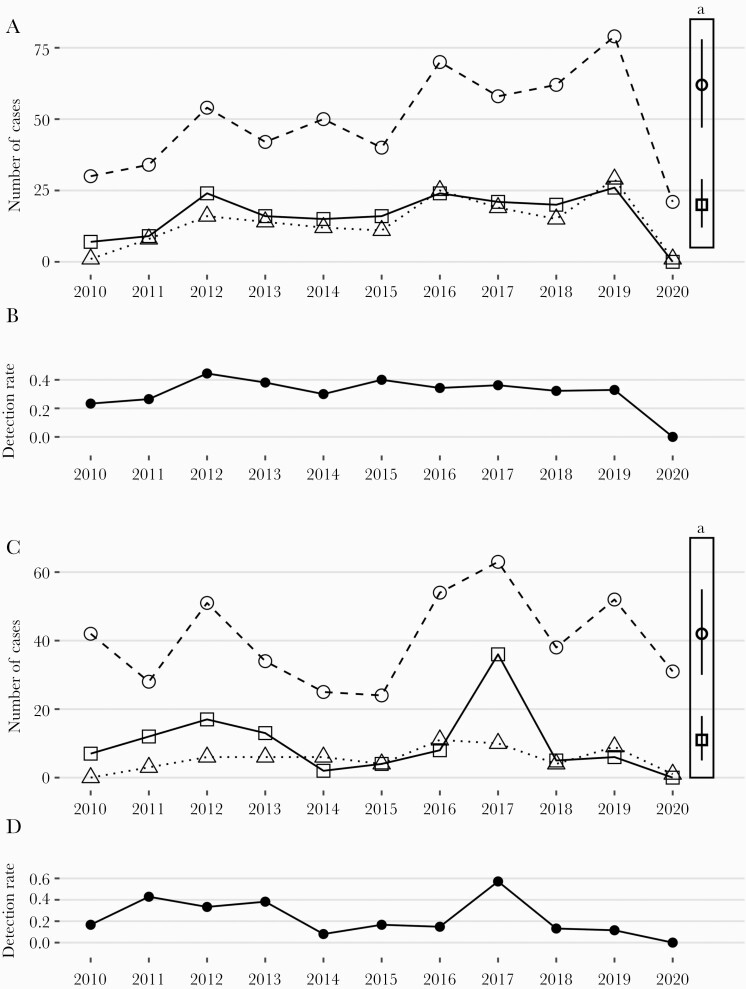
During 11 consecutive years from 2010 to 2020, we observed 178 and 110 cases of EV meningitis among children 0–1 and 1–16 years of age. Both the annual frequencies and proportions of positive CSF EV PCR tests are shown in Figure 1. The counts of hospitalizations coded for EV meningitis (ICD-10 code A87.0) are also provided. The Supplementary Data provides the raw data for all patients (Table 2).
Figure 1.
Timeline of Enterovirus meningitis in infants (A and B) and children 1–16 years of age (C and D) at the Bern University Hospital. A and C, Open circles indicate the annual number of EV CSF PCR tests performed, open squares indicate the annual number of positive EV detections in CSF, and open triangles indicate the annual number of patients in whom a discharge diagnosis of EV meningitis was coded. B and D, Detection rates of EV RNA in CSF in infants (B) and children 1–16 years of age (D). aPredicted annual numbers in 2020 of infants (A) and children 1–16 years of age (C) with EV CSF PCR tests performed (open circle) and positive (open square) based on a time series analysis (2010–2019). Vertical bars indicate the 95% CI. Abbreviations: CSF, cerebrospinal fluid; EV, Enterovirus; PCR, polymerase chain reaction.
The predicted counts for 2020 for CSF EV PCR tests performed and positive (ie, EV meningitis cases), respectively, based on the observed cases in infants from 2010 to 2019 were 62 (95% CI, 47–78) and 20 (95% CI, 12–29), respectively. However, only 21 patients were tested for EV, and none was positive (Figure 1 A). A similar discrepancy was found for the 1–16-year-olds (Figure 1 C; Supplementary Table 3).
The seasonal distribution of cases shown in Figure 2 demonstrates that from 2010 to 2019 most cases clustered in the third quarter (115 of 178 cases [65%] were <1 year old; 66 of 110 cases [60%] were 1–16 years old). In 2020, no cases were diagnosed even though most NPIs had been lifted at that time. In particular, primary and secondary schools had been opened by May 11, 2020, after 2 months of closure, and day care facilities had never been closed.
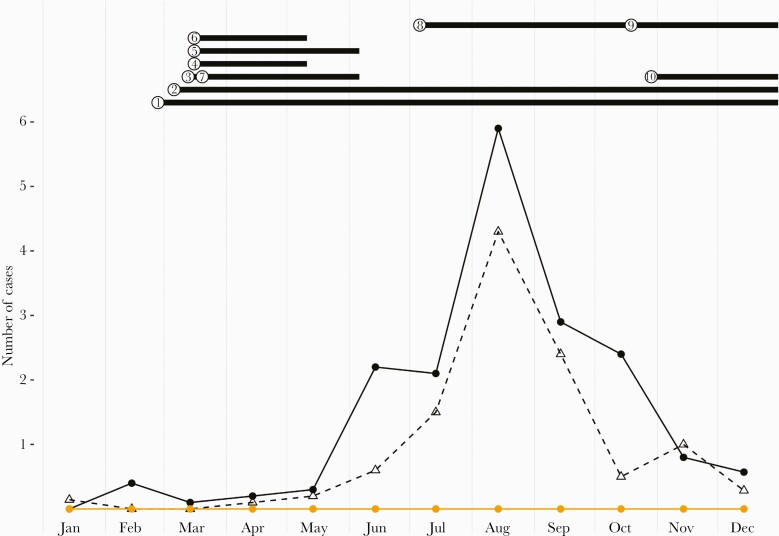
Figure 2.
Average epidemic curve of EV meningitis at the Bern University Hospital. Black dots indicate the mean number of EV meningitis cases in infants, and open triangles indicate the average number of EV meningitis cases in children 1–16 years of age based on data from 2010 to 2019. Red dots indicate the number of children 0–16 years of age with CSF EV RNA detection in 2020. NPIs in 2020 are indicated by numbers: ① hygiene recommendations in educational setting, 02/27/2020–ongoing; ②social distancing >1.5 m, 03/06/2020–ongoing; ③ gatherings >100 persons banned, 03/13/2020–06/06/2020; ④ public venues closed, 03/06/2020–05/11/2020; ⑤ tertiary schools and universities closed (age >16 years), 03/16/2020–06/06/2020; ⑥ primary and secondary schools closed (age 6–12 years), 03/16/2020–05/11/2020; ⑦ gatherings >5 persons banned, 03/20/2020–06/06/2020; ⑧face masks in public transport (age >12 years), 07/05/2020–ongoing; ⑨ face masks (outdoors and public indoor venues, age >12 years), 10/19/2020–ongoing; ⑩ private gatherings >15 persons banned, 10/29/2020–ongoing. Abbreviations: CSF, cerebrospinal fluid; EV, enterovirus; NPI, nonpharmaceutical intervention.
Analysis of the time trends for rhinovirus/enterovirus detections by IF in NPA revealed that detections occurred throughout the year and peaked either in the second or fourth quarter, but never in the third quarter (data not shown). While both the number of tests performed and positive detections in 2020 were significantly lower than predicted (Supplementary Table 3), the reduction was only ~50% compared with 100% for EV CSF PCR.
DISCUSSION
NPIs to prevent the spread of COVID-19 have been associated with markedly decreased community activity of major respiratory viruses other than severe acute respiratory disease coronavirus 2 (SARS-CoV-2) [8–11]. Here we report the complete absence in 2020 of the summer outbreak of enteroviral meningitis, which previously peaked in the third quarter of each year since at least 2010. This finding strongly suggests that community-wide COVID-19 control measures intended to prevent respiratory transmissions also dramatically impacted enteroviral transmission, which most commonly occurs by direct human-to-human contact [5].
This striking disruption of enteroviral epidemic cycles was first reported for the second quarter of 2020 from Korea [9] and Taiwan [12–14] by analysis of a national health insurance database [12], ICD codes for hand, foot, and mouth disease and herpangina [13], and national disease notification systems [9, 14]. More recently, 2 clinical microbiology reports from Southeast France [15] and the United States [16] describe the near absence in EV CSF PCR detections during the third quarter of 2020.
The starting point of our study was the clinical observation of absent EV meningitis cases in children in 2020. Infants appear to be a suitable sentinel population for enteroviral circulation in the community, because their febrile presentation is usually brought to medical attention and the workup for fever without source or meningitis commonly includes EV PCR in CSF and/or blood [17]. Conversely, for older children such data may be less reliable, because EV disease is mostly mild, nonspecific, and rarely confirmed virologically. Also, health care avoidance during lockdown periods [18, 19] may have negatively affected the case counts for such minor and self-limited illnesses. Thus, our clinical observation on infant EV meningitis corroborates what public health authorities and clinical microbiologists have recently reported.
Correlating the epidemic curve with various NPIs (Figure 2) reveals several important insights. Hand hygiene and social distancing recommendations were the only NPIs in place in Switzerland throughout the entire observation period and appear to be the most likely drivers of reduced enteroviral circulation. Day care centers were never closed [11]. While horizontal spread of EV in this setting is well established [20], our findings suggest that effective control of community transmission is possible without day care closure. Similarly, schools were reopened well before the expected onset of the EV season [11]. Thus, school closure between March and May 2020 was either irrelevant for subsequent EV suppression in the third quarter or exerted a critical delay by reducing early reproduction in spring. Finally, the use of face masks in public was not enforced until later in the year and, consistent with primarily fecal–oral transmission, is unlikely to have contributed substantially. The reduction of combined rhinovirus/enterovirus detection in NPA in 2020 was significant, but substantially less pronounced (Supplementary Table 3) than EV detection in CSF. This suggests that there was ongoing circulation of respiratory types of the genus Enterovirus, that is, mainly rhinoviruses [21], while nonrespiratory enterovirus genotypes, which cause the majority of CNS infections, were largely suppressed. This further supports the notion that fecal–oral transmission was disrupted in 2020.
The strengths of this study include the long observation period and the hospital setting, which affords greater precision in associating EV detection with specific clinical disease than public health or clinical microbiology data. Limitations include the following: First, our single-site approach may not be representative of other geographic areas. Second, we did not routinely search for parechoviral infections in CSF, which in Europe occur with biannual peaks in even-numbered years [3]. However, EV and parechoviruses co-circulate with no apparent mutual interference [2]. Third, we may have underestimated the true number of EV meningitis cases as CSF PCR is less sensitive for detection of EV than PCR of stool or respiratory specimens [22]. Fourth, as with all retrospective studies, factors unaccounted for may have contributed to the effects observed.
CONCLUSIONS
Evidence from 3 continents demonstrates that enteroviral summer activity was nearly abolished during the COVID-19 pandemic year. This unintended effect of NPI to control COVID-19 presumably had a major protective effect against the burden of pediatric EV CNS summer disease and the utilization of health care resources. Further studies are needed to dissect the specific set of NPIs responsible for this collateral effect. This could be beneficial for postpandemic pediatric disease prevention.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. C.A., M.B., K.K., and M.V.K. contributed to conceptualization. L.S., P.K.A.A., M.B., and C.A. contributed to the methodology. L.S. and P.K.A.A. performed the data analysis. L.S., M.V.K., and C.A. wrote the manuscript, which was edited by all authors. All authors have read and approved the published version of the manuscript. C.A. takes responsibility for the integrity of the work as a whole.
Patient consent. The research protocol was approved by the Ethics Committee of the Canton of Bern (project ID #2020–02770, December 24, 2020). The requirement for informed consent was waived because of the retrospective design, large number of data sets, and coded nature of the data.
References
- 1. Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA; Centers for Disease Contol and Prevention . Enterovirus surveillance—United States, 1970–2005. MMWR Morb Mortal Wkly Rep 2006; 55(8):1–20. [PubMed] [Google Scholar]
- 2. de Jong EP, van den Beuken MGA, van Elzakker EPM, et al. Epidemiology of sepsis-like illness in young infants: major role of enterovirus and human parechovirus. Pediatr Infect Dis J 2018; 37:113–8. [DOI] [PubMed] [Google Scholar]
- 3. van der Sanden SM, Koopmans MP, van der Avoort HG. Detection of human enteroviruses and parechoviruses as part of the national enterovirus surveillance in the Netherlands, 1996–2011. Eur J Clin Microbiol Infect Dis 2013; 32:1525–31. [DOI] [PubMed] [Google Scholar]
- 4. Kang HJ, Yoon Y, Lee YP, et al. A different epidemiology of enterovirus A and enterovirus B co-circulating in Korea, 2012–2019. J Pediatr Infect Dis. 2021; 10:398–407. [DOI] [PubMed] [Google Scholar]
- 5. Baggen J, Thibaut HJ, Strating JRPM, van Kuppeveld FJM. The life cycle of non-polio enteroviruses and how to target it. Nat Rev Microbiol 2018; 16:368–81. [DOI] [PubMed] [Google Scholar]
- 6. Hasbun R, Wootton SH, Rosenthal N, et al. Epidemiology of meningitis and encephalitis in infants and children in the United States, 2011–2014. Pediatr Infect Dis J 2019; 38:37–41. [DOI] [PubMed] [Google Scholar]
- 7. Barbani MT, Gorgievski-Hrisoho M. Rapid detection of respiratory picornaviruses in nasopharyngeal aspirates by immunofluorescence assay. J Clin Virol 2009; 45:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee H, Lee H, Song KH, et al. Impact of public health interventions on seasonal influenza activity during the SARS-CoV-2 outbreak in Korea. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huh K, Jung J, Hong J, et al. Impact of non-pharmaceutical interventions on the incidence of respiratory infections during the COVID-19 outbreak in Korea: a nationwide surveillance study. Clin Infect Dis. 2021; 72:e184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased influenza activity during the COVID-19 pandemic—United States, Australia, Chile, and South Africa, 2020. Am J Transplant 2020; 20:3681–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kohns Vasconcelos M, Meyer Sauteur PM, Keitel K, et al. Strikingly decreased community-acquired pneumonia admissions in children despite open schools and day-care facilities in Switzerland. Pediatr Infect Dis J. 2021; 40:e171–2. [DOI] [PubMed] [Google Scholar]
- 12. Lee HH, Lin SH. Effects of COVID-19 prevention measures on other common infections, Taiwan. Emerg Infect Dis 2020; 26:2509–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuo SC, Tsou HH, Wu HY, et al. Nonpolio enterovirus activity during the COVID-19 pandemic, Taiwan, 2020. Emerg Infect Dis 2021; 27:306–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiu NC, Chi H, Tai YL, et al. Impact of wearing masks, hand hygiene, and social distancing on influenza, enterovirus, and all-cause pneumonia during the coronavirus pandemic: retrospective national epidemiological surveillance study. J Med Internet Res 2020; 22:e21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luciani L, Ninove L, Zandotti C, Nougairede A. COVID-19 pandemic and its consequences disrupt epidemiology of enterovirus meningitis, South-East France. J Med Virol 2021; 93:1929–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kies KD, Thomas AS, Binnicker MJ, Bashynski KL, Patel R. Decrease in enteroviral meningitis - an unexpected benefit of COVID-19 mitigation? Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lafolie J, Labbé A, L’Honneur AS, et al. ; Blood Enterovirus Diagnosis Infection (BLEDI) in paediatric population study team . Assessment of blood enterovirus PCR testing in paediatric populations with fever without source, sepsis-like disease, or suspected meningitis: a prospective, multicentre, observational cohort study. Lancet Infect Dis 2018; 18:1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dean P, Zhang Y, Frey M, et al. The impact of public health interventions on critical illness in the pediatric emergency department during the SARS-CoV-2 pandemic. J Am Coll Emerg Physicians Open 2020; 1:1542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams TC, MacRae C, Swann OV, et al. Indirect effects of the COVID-19 pandemic on paediatric healthcare use and severe disease: a retrospective national cohort study. Arch Dis Child. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan JH, Law CK, Hamblion E, et al. Best practices to prevent transmission and control outbreaks of hand, foot, and mouth disease in childcare facilities: a systematic review. Hong Kong Med J 2017; 23:177–90. [DOI] [PubMed] [Google Scholar]
- 21. Poole S, Brendish NJ, Tanner AR, Clark TW. Physical distancing in schools for SARS-CoV-2 and the resurgence of rhinovirus. Lancet Respir Med 2020; 8:e92–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harvala H, Broberg E, Benschop K, et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J Clin Virol 2018; 101:11–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.