ABSTRACT
We describe a patient with subclinical coccidioidomycosis who experienced rapid disease dissemination shortly after SARS-CoV-2 infection, suggesting host immune response dysregulation to coccidioidomycosis by SARS-CoV-2. We hypothesize that disrupted cell-mediated signaling may result after SARS-CoV-2 infection leading to functional exhaustion and CD8+ T-cell senescence with impairment in host cellular response to Coccidioides infection.
INTRODUCTION
Host Factors and Related Mechanisms of Immunity Against Coccidioides Infection
Coccidioidomycosis is a disease caused by the soil fungi Coccidioides immitis and C. posadasii, which are endemic to the southwestern desert regions of the USA, as well as parts of Central and South America.1 Predisposition to disseminated disease has been well-described among military personnel of African and Asian-Pacific Islander descent.2
Innate and adaptive immune responses are crucial for immunity to Coccidioides. Tumor necrosis factor-alpha signaling contributes to functional adaptive responses, and both CD4+ T-helper 1 (Th1) and cytotoxic CD8+ T-cells appear to be essential in facilitating a possible component of humoral immunity to Coccidioides.3 Impaired cytokine signaling from CD4+ Th1 and cytotoxic CD8+ T-cells among patients with refractory disseminated coccidioidomycosis has informed decisions to treat with IFN-gamma, resulting in positive clinical responses.4,5 In a murine model deficient in CD4+ Th1 cells, immunity to Coccidioides is maintained, thus elevating the implied relevance of CD8+ T-cells in protection against infection.6
Host Immunology and Cellular Signaling in SARS-CoV-2 Infection
SARS-CoV-2 is the novel zoonotic beta-coronavirus responsible for the ongoing COVID-19 pandemic, and our related understanding of host immunology is rapidly developing. In COVID-19, both impairment and hyperactivation of the immune system may occur7 with a wide spectrum of host responses. SARS-CoV-2 infects the human respiratory tract by its spike (S) protein binding to the host human angiotensin-converting enzyme receptor 2 (h-ACE2).8 Following endocytosis, inflammatory pathways, including the expression of NF-kappa B, are activated, leading to numerous cellular responses.9 Th1 and cytotoxic T-cells are activated by macrophages with varied degrees of cytokine release which may be predictive of the degree of disease incurred after SARS-CoV-2 infection.10 Among activated CD8+ T-cells in COVID-19, there is increased expression CD57, a described marker of cellular senescence, as well as decreased potential for proliferation.11
CASE
A previously healthy 23-year-old African American man, originally from New York City, had been serving in the U.S. Navy at Naval Air Station Lemoore (NASL) in the San Joaquin Valley of California. He was deployed onboard the USS Theodore Roosevelt during that ship’s COVID - 19 outbreak.12 During the outbreak, day 0, he developed a mild febrile illness and tested positive for SARS-CoV-2 by nasopharyngeal swab polymerase chain reaction testing; he had no associated respiratory symptoms, and his infection resolved without need for further medical care. During this time, he was ashore on the island of Guam and had been away from NASL at sea for 3 months.
On day 30, he was hospitalized in Guam following an episode of presyncope. Upon admission, he reported 21 days of drenching night sweats, 14 kg of unintentional weight loss, bony pain, and non-tender ulcers over his right shoulder. Physical examination revealed generalized lymphadenopathy; laboratory testing demonstrated leukocytosis (17 x 109 cells/L) with marked eosinophilia (33%). Computed tomography (CT) of his chest demonstrated diffuse sub-centimeter ground-glass opacities with mediastinal, hilar, and right axillary lymphadenopathy and centrilobular nodules along with widespread lesions throughout the axial and appendicular skeleton. Because of concern for malignancy, he was transported from Guam to San Diego for further radiographic staging and evaluation on day 33.
In San Diego, positron emission tomography re-demonstrated the initial CT findings with avid fluorodeoxyglucose uptake in the associated areas (Fig. 1A). A core needle biopsy of a right axillary lymph node demonstrated granulomatous lymphadenitis with fungal yeast elements. Skin biopsies of his right shoulder lesions demonstrated rare, thick-walled spherules with multinucleated giant cells and eosinophils consistent with Coccidioides (Fig. 1B–C). Serologic testing for anti-Coccidioides IgG and IgM was positive, with a complement fixation titer of 1:64. There was no evidence of central nervous system involvement based on cerebral spinal fluid analysis. Given extensive axial skeletal involvement, the patient was started on induction therapy with intravenous liposomal amphotericin B 5 mg/kg daily, but it was stopped after 14 days (54 days since initial COVID-19 diagnosis) because of drug-induced liver injury. He was transitioned to therapy with oral itraconazole 200 mg twice daily and has had continued clinical improvement.
FIGURE 1.
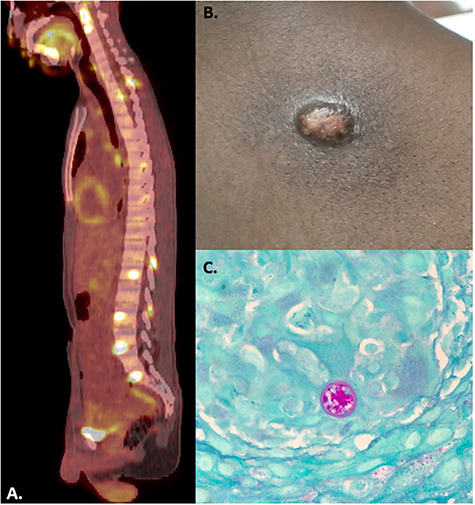
(A) Positron emission tomography with avid fluorodeoxyglucose uptake demonstrated at multiple vertebral levels and in the sacrum. (B) Cutaneous coccidioidomycosis on right upper back. (C) A spherule of coccidioides from skin biopsy, 400x magnification.
DISCUSSION
Cellular immunity to Coccidioides infection requires both coordinated and sustained Th1 and cytotoxic T-cell response,3 possibly with emphasis on the later.6 In host defense against SARS-CoV-2, CD8+ T-cells are described as central for clearance and recovery.13
SARS-CoV-2 undergoes endocytosis upon binding to the h-ACE2 receptors in the respiratory tract, with subsequent mast cell production of IL-49 which is necessary for activation of cellular immune response. As of present, Th1 and cytotoxic T-cell responses are believed to be essential for host defense in COVID-19.14 However, an impairment and reduction of cytotoxic CD8+ T-cells are also observed11 and described to undergo “functional exhaustion”11,15 after SARS-CoV-2 infection.
Our patient had no significant symptoms because of Coccidioides during the months at sea preceding the COVID-19 outbreak. This is common, as a majority of cases of coccidioidomycosis are subclinical with spontaneous resolution.1 We posit that his initially subclinical infection was worsened by SARS-CoV-2-mediated immunosuppression. Although Coccidioides infection has been described during the management of COVID-19 before,16 it is recognized that our patient may have had two unrelated primary infectious processes occurring simultaneously. This is improbable: coccidioidomycosis is not a contagious disease between humans, and Coccidioides is not endemic in Guam, with his only potential location for infection having been in California before deployment. Additionally, although disseminated coccidioidomycosis occurs in a minority of infected patients, such rapid dissemination occurring months following primary infection is extremely rare in otherwise-immunocompetent patients.17 We hypothesize that patients infected with SARS-CoV-2 may experience diminished cell-mediated signaling as a consequence of functional exhaustion and CD8+ T-cell senescence with consequential impairment in cellular response to Coccidioides infection. Further investigation should be pursued in patients with coccidioidomycosis and newly diagnosed COVID-19 with attention to monitoring CD57 levels among CD8+ T-cells. Doing so could characterize a unique clinical relevance of cellular senescence in COVID-19 immunology. In the ongoing COVID-19 pandemic, this may have significant ramifications for not only persons exposed to Coccidioides, but potentially as well as with other infectious diseases dependent on CD8+ T-cell function for host protection.
ACKNOWLEDGMENTS
We would like to thank Dr. Julia H. Cheringal and Dr. Robert L. Fenequito of U.S. Naval Hospital.
Contributor Information
LT Daniel S Krauth, Division of Infectious Diseases, Naval Medical Center, San Diego, CA 92134, USA.
LCDR Christina M Jamros, Division of Infectious Diseases, Naval Medical Center, San Diego, CA 92134, USA.
LCDR Shayna C Rivard, Department of Internal Medicine, US Naval Hospital, Tutuhan, GU 96910, USA; Department of Dermatology, US Naval Hospital, Tutuhan, GU 96910, USA.
CDR Niels H Olson, Department of Pathology, US Naval Hospital, Tutuhan, GU 96910, USA.
CAPT Ryan C Maves, Division of Infectious Diseases, Naval Medical Center, San Diego, CA 92134, USA.
FUNDING
No funding was utilized for this work.
CONFLICT OF INTEREST STATEMENT
Drs. Krauth, Jamros, Rivard, Olson, and Maves have no relevant financial conflicts of interest to report.
REFERENCES
- 1. Brown J, Benedict K, Park BJ, Thompson GR: Coccidioidomycosis: epidemiology. Clin Epidemiol 2013; 5(1): 185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mease L: Pulmonary and extrapulmonary cocciodioidomycosis, active component, U.S. Armed Forces, 1999–2011. MSMR 2012; 19(12): 2–5. [PubMed] [Google Scholar]
- 3. Fierer J, Waters C, Walls L: Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against coccidioides immitis infection in mice. J Infect Dis 2006; 193(9): 1323–31. [DOI] [PubMed] [Google Scholar]
- 4. Duplessis CA, Tilley D, Bavaro M, Hale B, Holland SM: Two cases illustrating successful adjunctive interferon-γ immunotherapy in refractory disseminated coccidioidomycosis. J Infect 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM: Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-γ receptor 1 deficiency. Clin Infect Dis 2009; 49(6): 12–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nanjappa SG, Heninger E, Wüthrich M, Sullivan T, Klein B: Protective antifungal memory CD8 + T cells are maintained in the absence of CD4 + T cell help and cognate antigen in mice. J Clin Invest 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamilloux Y, Henry T, Belot A, et al. : Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 2020; 19(7): 102567.doi: 10.1016/j.autrev.2020.102567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann M, Kleine-Weber H, Schroeder S, et al. : SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181(2): 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingraham NE, Lotfi-Emran S, Thielen BK, et al. : Immunomodulation in COVID-19. Lancet Respir Med 2020; 8(6): 544–6.doi: 10.1016/S2213-2600(20)30226-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang J, Jiang M, Chen X, Montaner LJ: Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 2020; 108(1): 17–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Biasi S, Meschiari M, Gibellini L, et al. : Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun 2020; 11(1): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Payne DC, Smith-Jeffcoat SE, Nowak G, et al. : SARS-CoV-2 infections and serologic responses from a sample of U.S. Navy Service Members — USS Theodore Roosevelt, April 2020. MMWR Morb Mortal Wkly Rep 2020; 69(23): 714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang F, Nie J, Wang H, et al. : Characteristics of peripheral lymphocyte subset alteration in COVID-19 Pneumonia. J Infect Dis 2020; 221(11): 1762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grifoni A, Weiskopf D, Ramirez SI, et al. : Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181(7): 1489–1501.e15.doi: 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng M, Gao Y, Wang G, et al. : Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020; 17(5): 533–5.doi: 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang C, Senining R, Kim J, Goyal R: An acute pulmonary coccidioidomycosis coinfection in a patient presenting with multifocal pneumonia with COVID-19. J Investig Med High Impact Case Rep 2020; 8: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adam R, Elliott S, Taljanovic M: The spectrum and presentation of disseminated coccidioidomycosis. Am J Med 2009; 122(8): 770–7. [DOI] [PubMed] [Google Scholar]


