Learning point for clinicians
Of all COVID-19 vaccination-associated adverse events, 2.69% may be neurological in nature. We report the first case of myelitis from India with ChAdOX1 nCoV-19 vaccine; the patient showed excellent recovery. Having occurred after an administration of >50 million doses, benefits of vaccination seem to outweigh the risk of adverse events.
Adverse event following immunization (AEFI) associated with coronavirus diseaes (COVID-19) vaccines are just beginning to unfold. Neurological complications are potentially disabling AEFI that may range from facial palsy to stroke. Out of 9442 AEFI reported in Centers for Disease Control (CDC)’s Vaccine Adverse Event Reporting System (VAERS) related to Pfizer-BioNTech, Moderna and Johnson & Johnson’s COVID-19 vaccines, 254 (2.69%) were neurological in nature; of these, 9 cases had transverse myelitis.1 We report the first case of myelitis from India associated with a different, viral-vectored, recombinant ChAdOX1 nCoV-19 (Oxford/AstraZeneca, COVISHIELDTM) vaccine.
A 36-year-old Hindu gentleman, with no prior comorbidities, received the first dose of COVISHIELDTM around 3 weeks back. On the eighth post-vaccination day, he presented to the primary referral unit with complaints of abnormal sensations in both lower limbs. With abnormal sensations ascending the trunk, an imaging of the spine was ordered. Magnetic resonance imaging (MRI) of the spine, done on 13th post-vaccination day, showed an ovoid T2-hyperintense lesion in the dorsal aspect of spinal cord at C6 and C7 vertebral levels. A possibility of vaccine-associated demyelination was kept, and the patient was initiated on oral methylprednisolone (16 mg; 12 hourly). The patient took treatment for a week and was referred to tertiary care facility for further evaluation.
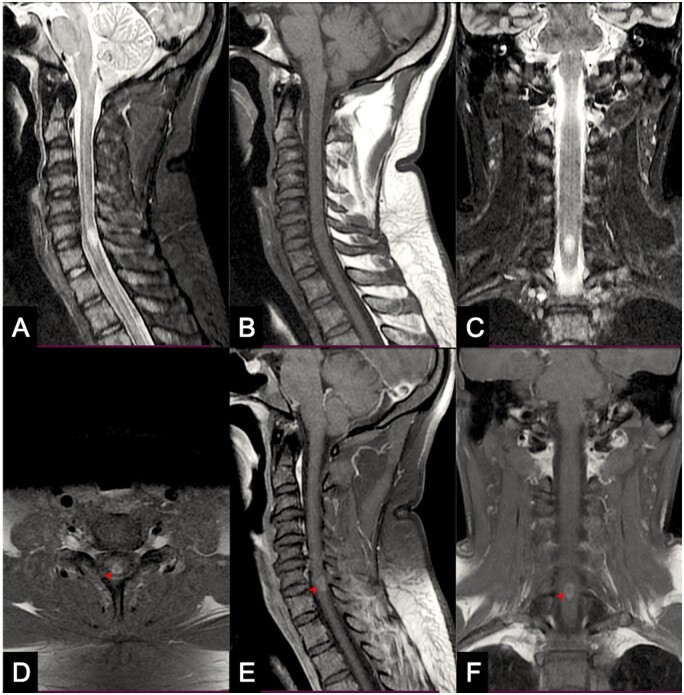
The patient was admitted to our University Hospital on the 24th post-vaccination day. Historically, the patient never experienced fever or had involvement of sphincters. On examination, no motor deficit was found. Deep tendon jerks were exaggerated in the lower limbs with an extensor plantar response on the left side. Amongst sensations, sense of vibration was found to be impaired till manubrium sterni. Neuroelectrophysiology, including visual evoked potentials, was found to be within normal limits. MRI of the spine confirmed the presence of an ovoid T2-hyperintense lesion that showed mild to moderate peripheral enhancement on T1-gadolinium contrast administration. (Figure 1) MRI of the brain was normal. Cerebrospinal fluid examination (CSF) showed an increased protein (54 mg%; normal limit: 15–45 mg%) level; other parameters, including a panel to screen for infection, were normal. Anti-aquaporin 4 and anti-myelin-oligodendrocyte antibody panel was negative; connective tissue disorder and vasculitis profile did not reveal any abnormality.
Figure 1.
MRI of the spine shows a hyperintense lesion on T2-weighted sagittal (A) and coronal (C) scans. The lesion is isointense on T1-weighted (B) scan. Mild to moderate peripheral contrast enhancement (arrowheads) can be seen on axial (D), sagittal (E) and coronal (F) sections.
Based on the Brighton case definition, the patient was labeled with Level-3 myelitis on the certainty algorithm.2 A Level-2 label would have been better in our opinion since a consequential increase in protein level was found in the CSF and pleocytosis is known to normalize earlier than the protein concentration. The patient responded well to intravenous methylprednisolone (1000 mg/day for 5 days) and was discharged after a week of hospital stay.
Recombinant ChAdOX1 nCoV-19 vaccine, during the trial phase, had been associated with two instances of myelitis. Of these, one was found to have multiple sclerosis in the background while the other was referred to as a possibly-related event.3,4 Even with similarly reported occurrences with the current vaccine and those listed in the CDC’s-VAERS, it is extremely important to consider two relevant questions. First, if the strength of association is acceptable (deemed causal) whether myelitis is way above the expected frequency known with other vaccinations and what is usually seen in the general population? Second, is the event serious enough to qualify for recommendations to discontinue the vaccine/s since most of these AEFI can be managed appropriately? To put things in context, a total of 50.84 million doses have been administered in India till 23 March 2021.5 Considering an incidence of 1–4 cases per million per year,6 an event of myelitis occurring after more than 50 million vaccine doses appears fairly acceptable. We feel that benefits of vaccination still seem to outweigh the associated risks.
Conflict of interest. None declared.
References
- 1. Goss AL, Samudralwar RD, Das RR, Nath A.. ANA Investigates: neurological complications of COVID-19 vaccines. Ann Neurol 2021; doi: 10.1002/ana.26065. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sejvar JJ, Kohl KS, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, et al. ; Brighton Collaboration Encephalitis Working Group. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2007; 25:5771–92. [DOI] [PubMed] [Google Scholar]
- 3. Mahase E. Covid-19: oxford researchers halt vaccine trial while adverse reaction is investigated. BMJ 2020; 370:m3525. [DOI] [PubMed] [Google Scholar]
- 4. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Data Explorer. https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&time=40.latest&pickerSort=desc&pickerMetric=total_vaccinations&Metric=Vaccinations&Interval=Cumulative&Relative+to+Population=true&Align+outbreaks=false&country=USA∼GBR∼ISR∼DEU∼ARE∼ARG∼FRA∼IND (23 March 2021, date last accessed).
- 6.Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002; 59:499–505. [DOI] [PubMed] [Google Scholar]