Abstract
Introduction
The SARS-CoV-2/COVID-19 may produce neurological manifestations, including its occurrence in children, and newborns, which has been little reported so far in newborns with COVID-19.
Case
We present a case in Colombia, of community-acquired neonatal infection of SARS-CoV-2, with suggestive symptoms, such as fever, and showing neurological findings, such as drowsiness, poor suction and mild hypotonia for a short time.
Discussion
The clinical manifestations of SARS-COV-2 in neonates are beginning to be described in detail. We report a case of SARS-COV-2-associated neurological compromise in a newborn, with features of drowsiness, poor suction and hypotonia.
Keywords: SARS-CoV-2, COVID-19, children, congenital, neonatal
INTRODUCTION
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), primarily manifests as a severe respiratory infection, affecting other organs and is currently considered systemic, especially in adults. Nevertheless, may affect children, including newborns, compromising 2–5% of reported cases in some countries [1].
Although 70% of COVID-19 cases in newborns are due to horizontal transmission by the infected mother or people in close contact, congenital infection has been reported. Most of them are asymptomatic, and sometimes the neonate is the index case at households. The main symptom is fever, associated with other findings, such as rhinorrhea, cough, apnea and hypoxemia, generally less severe than the observed in adults. Besides that, other symptoms have been reported, such as gastrointestinal, neurological and skin lesions, among others, including neurological findings [2, 3].
Some case series highlight the need for long-term follow-up in this scenario, and specific guidelines for the long-term follow-up of newborns to mothers with COVID-19 in pregnancy are needed, as these were not developed [4]. In a systematic review of neonatal COVID-19, neurological manifestations were found in 18% [5].
We present a case in Colombia, of community-acquired neonatal infection of SARS-CoV-2, with suggestive symptoms, and presenting neurological findings, which has been little reported in newborns COVID-19.
CASE REPORT
Male newborn of 39 weeks, product of a 40-year-old mother, with a history of one pregnancy, born by C-section due to fetal macrosomia without complications. His prenatal care was without complications. Apgar scores were 8/9 (1st/5th minute), weight 4000 g (classified at the percentile >P95, according to the WHO), length 52 cm (P90) and head circumference 36 cm (P 90). He was discharged after 24 h post-partum, at home with exclusive breastfeeding and ambulatory follow-up. A 21 days old male neonate start with episodes of hyperthermia (38.5°C) without other symptoms. Members of the family, three persons and the newborn, were asymptomatic at the time. For this reason, the neonate was assessed in the local hospital, and a physical examination on admission revealed fever and a well-appearing baby. Thus, community-acquired neonatal sepsis was suspected, and complete blood count reported white blood cells 3750/µl, lymphocytes 40%, neutrophils 53%, monocytes 7%, hemoglobin 11.2 g/dl, hematocrit 31%, platelets 191 000/µl, C-reactive protein (CRP) 10.5 mg/dl, normal urinalysis and coproscopic with leucocytes 10–15 per field. Ampicillin and gentamicin were started and was referred to our institution.
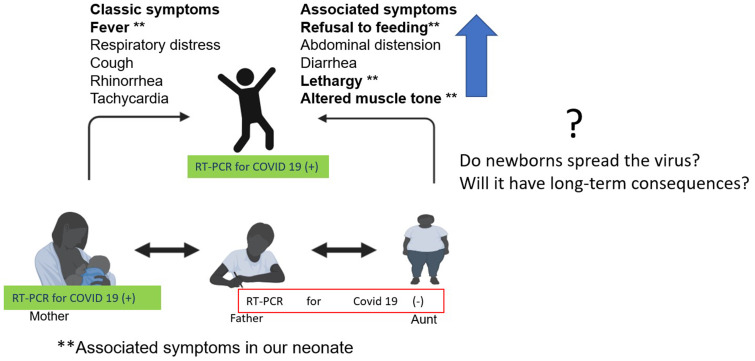
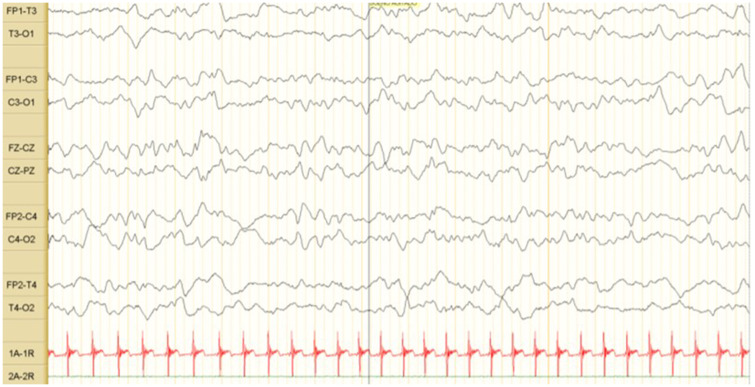
On examination, he looked well, febrile (38.5°C), heart rate of 178 beats/min, blood pressure of 85/50 mmHg, respiratory rate of 45 per minute and SPO2 of 95% in room air. Other symptoms such as rash, pleuropulmonary or abdominal alterations were rule out. We made complete blood count (white blood cells 4580/µl, lymphocytes 43%, neutrophils 29.9%, monocytes 22%, hemoglobin 12.4 g/dl, hematocrit 36% and platelets 191 000/µl) and CRP <6 mg/dl. Blood and urine culture samples were taken. Samples for RT-PCR for SARS-CoV-2 by a nasopharyngeal exudate/aspirate were taken. Antibiotic therapy was continued and acetaminophen initiated. In the following 24 h, changes in the state of consciousness were observed, showing temporary neurological symptoms, such as drowsiness, poor suction and hypotonia. A lumbar puncture was performed. However, it was traumatic; for this reason, no cerebrospinal fluid (CSF) study was performed. Antibiotic therapy was changed to cefepime and gentamicin. The neurological changes lasted 24 h. The result of the SARS-CoV-2 PCR test was positive. The diagnosis of community-acquired late-onset neonatal sepsis due to SARS-CoV-2 was made. The baby remained afebrile for 48 h, with negative cultures and antibiotics were stopped. Given his good condition, he was discharged. The RT-PCR SARS-CoV-2 was made in the close family group, and the mother was positive (Fig. 1); however, she was asymptomatic. Brain ultrasound and electroencephalogram (Fig. 2) were performed (outpatient clinic); both were normal. The follow-up was done by their national social security system and to date (3 months) with adequate growth and development.
Fig. 1.
COVID-19 in newborns, symptoms and transmission.
Fig. 2.
EEG of the newborn. Tracing where the sleep–wake cycle differs. No epileptiform activity.
DISCUSSION
Early during the SARS-CoV-2/COVID-19 pandemic, it was noted that this emerging infection also affects children and neonates considerably [4–6]. But only recently, the clinical manifestations of SARS-CoV-2 in neonates are beginning to be described in detail. Our patient showed neurological alterations such as drowsiness, poor suction and mild hypotonia for a short time. Previous reports have found lethargy and refusal feed, without other clinical manifestations [3]. The SARS-CoV-2 infection during the neonatal period may be asymptomatic or symptomatic, as in our case. Very few reported cases of SARS-CoV-2 described neurological compromise in newborns, with clinical variability that include paroxysmal episodes, upward gaze deviation, stiffening, irritability, hypertonia, apnea and seizures [7]. Even the seizures can be the only manifestation in children, sometimes without electroencephalographical changes [8]. There are reports of encephalitis, seizure, headache, irritability and meningismus in older children, in addition to changes in CSF such as increased proteins and pleocytosis with neutrophilia [9]. Unfortunately, our tap was traumatic.
SARS-CoV-2 RNA was detected in the CSF in adult patients with meningoencephalitis, but the cases report in children show negative CSF for SARS-CoV-2 [10]. About 36% of adult patients have neurological manifestations, increasing to 84% in critical patients [7, 11]. The possibility that SARS-CoV-2 could invade the brain has been raising. Furthermore, growing evidence shows that neurotropism is a central feature of coronaviruses, such as SARS-CoV-2. It remains unclear if neurologic manifestations are due to viral damage, fever or immunological phenomena [10, 12]. The clinical manifestations of SARS-CoV-2 infection in neonates are still under study, but neurological manifestations are beginning to emerge. With the ongoing pandemic, signs of encephalopathy, encephalitis and meningismus should be considered by physicians to suspect SARS-CoV-2 infection [6]. This report highlights the importance of rare but important neurological manifestations of SARS-CoV-2 in neonates.
Although our case was a community-acquired, is still unclear if congenital transmission occurred. Some studies demonstrate the transplacental transmission of SARS-CoV-2 in some cases, including some with neurological compromise. Clinical and imaging alterations can be found, with hypertonia or hypotonia, as in our case, and even the detection of SARS-CoV-2 antigens at the placental immunohistochemistry [6]. Ideally, congenital infection is considered proven if the virus is detected in the amniotic fluid collected before the membranes rupture or in blood drawn early in life; otherwise, the rest of cases would be classified as possible or unlikely [13].
Most cases are asymptomatic, maybe because newborns may have maternal antibodies with IgG [4, 14]. While SARS-CoV-2 has been found in breast milk, it is unclear if this route is associated with transmission and infection [13, 15, 16]. In our case, we did not assess breastmilk, therefore we cannot exclude it. It is also essential the follow-up of all the newborns with COVID-19 because other coronaviruses have shown neurological impairment, and long-term consequences are unknown. Then, early surveillance of SARS-CoV-2 should begin with maternal screening [17–19]. Finally, long-term follow-up of children with COVID-19 should be considered as they may present future alterations that deserve study and intervention [17, 20].
REFERENCES
- 1. Zimmermann P, Curtis N.. Coronavirus infections in children including CoCOVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 2020;39:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trevisanuto D, Cavallin F, Cavicchiolo ME, et al. Coronavirus infection in neonates: a systematic review. Arch Dis Child Fetal Neonatal Ed 2020. doi: 10.1136/archdischild-2020-319837. [DOI] [PubMed] [Google Scholar]
- 3. Nayak M, Panda S, Ballav Pradhan J, et al. Coronavirus disease 2019 in neonates - what is known and what needs to be known. Cureus 2020;12:e10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buonsenso D, Costa S, Sanguinetti M, et al. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol 2020;37:869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raschetti R, Vivanti AJ, Vauloup-Fellous C, et al. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun 2020;11:5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020;11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stafstrom CE, Jantzie LL.. COVID-19: neurological considerations in neonates and children. Children (Basel) 2020;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mark EG, Golden WC, Maureen M, et al. Community-onset SARS-CoV-2 infection in young infants: a systematic review. J Pediatr 2020;228:31133–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arango Ferreira C, Correa-Roda M.. Acute meningoencephalitis as initial presentation of SARS-CoV-2 infection in pediatrics. Pediatr Infect Dis J 2020;39:e386–7. [DOI] [PubMed] [Google Scholar]
- 10. Lorenz N, Treptow A, Schmidt S, et al. Neonatal early-onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J 2020;39:e212. [DOI] [PubMed] [Google Scholar]
- 11. Orozco-Hernández J, Marin-Medina D, Sánchez-Duque J.. Manifestaciones neurológicas de la infección por SARS-CoV-2. Medicina de Familia SEMERGEN 2020;46:106–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Bai W, Hashikawa T.. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol 2020;92:552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa S, Posteraro B, Marchetti S, et al. Excretion of SARS-CoV-2 in human breast milk. Clin Microbiol Infect 2020;26:1430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flannery DD, Gouma S, Dhudasia MB, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr 2021;e210038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel SK, Pathak M, Rana J, et al. Possibility of SARS-CoV-2 transmission from the breast milk of COVID-19 affected women patients to their infants: worries and strategies to counter it. Infez Med 2020;28:291–4. [PubMed] [Google Scholar]
- 16. McGuire MK, Seppo A, Goga A, et al. Best practices for human milk collection for COVID-19 research. Breastfeed Med 2021;16:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zambrano LI, Fuentes-Barahona IC, Bejarano-Torres DA, et al. Pregnant woman with COVID-19 in Central America. Travel Med Infect Dis 2020;36:101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dhama K, Patel SK, Pathak M, et al. An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med Infect Dis 2020;37:101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panda PK, Sharawat IK, Panda P, et al. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J Trop Pediatr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willi S, Lüthold R, Hunt A, et al. COVID-19 sequelae in adults aged less than 50 years: a systematic review. Travel Med Infect Dis 2021;40:101995. [DOI] [PMC free article] [PubMed] [Google Scholar]