Abstract
Background
Sequencing of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral genome from patient samples is an important epidemiological tool for monitoring and responding to the pandemic, including the emergence of new mutations in specific communities.
Methods
SARS-CoV-2 genomic sequences were generated from positive samples collected, along with epidemiological metadata, at a walk-up, rapid testing site in the Mission District of San Francisco, California during 22 November to 1 December, 2020, and 10–29 January 2021. Secondary household attack rates and mean sample viral load were estimated and compared across observed variants.
Results
A total of 12 124 tests were performed yielding 1099 positives. From these, 928 high-quality genomes were generated. Certain viral lineages bearing spike mutations, defined in part by L452R, S13I, and W152C, comprised 54.4% of the total sequences from January, compared to 15.7% in November. Household contacts exposed to the “California” or “West Coast” variants (B.1.427 and B.1.429) were at higher risk of infection compared to household contacts exposed to lineages lacking these variants (0.36 vs 0.29, risk ratio [RR] = 1.28; 95% confidence interval [CI]: 1.00–1.64). The reproductive number was estimated to be modestly higher than other lineages spreading in California during the second half of 2020. Viral loads were similar among persons infected with West Coast versus non-West Coast strains, as was the proportion of individuals with symptoms (60.9% vs 64.3%).
Conclusions
The increase in prevalence, relative household attack rates, and reproductive number are consistent with a modest transmissibility increase of the West Coast variants.
Summary: We observed a growing prevalence and modestly elevated attack rate for “West Coast” severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants in a community testing setting in San Francisco during January 2021, suggesting its modestly higher transmissibility.
Keywords: SARS-CoV-2, variant, spike mutation, secondary attack rates, household transmission
Genomic surveillance during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic is a critical source of situational intelligence for epidemiological control measures, including outbreak investigations and detection of emergent variants [1]. Countries with robust, unified public health systems and systematic genomic surveillance have been able to rapidly detect SARS-CoV-2 variants with increased transmission characteristics, and mutations that potentially subvert both naturally acquired or vaccination-based immunity (eg, COVID-19 Genomics UK Consortium). Examples include the rapidly spreading B.1.1.7 lineage documented in the United Kingdom and the B.1.351 lineage described from South Africa, or the P.1/P.2 lineages that harbor the spike E484K mutation that is associated with reduced neutralization in laboratory experiments [2–5].
In the United States, genomic surveillance is sparse relative to the number of confirmed cases (27.8 million as of 20 February 2021), with 123 672 genomes deposited in the GISAID database, representing only 0.4% of the total reported cases. Despite the low rates of US genomic surveillance, independent local programs and efforts have contributed to our understanding of variant emergence and spread [6–8]. The appearance of new nonsynonymous mutations highlight the utility of this approach in the United States [9].
Genomic sequencing of SARS-CoV-2 in California has predominantly been conducted by academic researchers and non-profit biomedical research institutions (eg, the Chan Zuckerberg Biohub and the Andersen Lab at the Scripps Research Institute) in conjunction with state and local public health partners. These efforts identified an apparent increase in the prevalence of lineages B.1.427 and B.1.429 (“California” or “West Coast” variant), which share S gene nonsynonymous mutations at sites 13, 152, 452, and 614, during December 2020 to February 2021 when California was experiencing the largest peak of cases observed during the pandemic. Although the cluster of mutations was first observed in a sample from May 2020, these variants rose from representing <1% of the consensus genomes recovered from California samples collected in October 2020 (5/546; 0.91%) to over 50% of those collected during January 2021 (2309/4305; 53.6%; GISAID accessed 20 February 2021).
The majority of sequencing efforts in the United States utilize samples from symptomatic individuals or outbreaks, introducing selection bias making interpretation of trends, such as the rise in lineage prevalence, complex. Furthermore, clinical remnant samples are most often delinked from case information, thus eliminating the possibility of evaluating genotypes with detailed household information, and other metadata useful for investigation of transmission dynamics.
Sequencing cases identified during intensive, longitudinal community-based testing may help address both limitations. Here we describe an investigation of the prevalence of the West Coast variants as well as other variants among persons tested at a community testing site situated in the Mission District of San Francisco, a neighborhood with high coronavirus disease 2019 (COVID-19) incidence, during 2 periods: 22 November to 1 December 2020 and 10–29 January 2021. Using metadata collected at the testing site and supplementary household testing, we estimated secondary household attack rate with respect to viral genotype to evaluate relative transmissibility of identified variants.
METHODS
Study Setting and Population
Over 22 November to 1 December 2020 and 10–29 January 2021, BinaxNOWTM rapid antigen tests were performed at the 24th & Mission BART (public transit) station in the Mission District of San Francisco, a setting of ongoing community transmission, predominantly among Latinx persons [10, 11]. Tests for SARS-CoV-2 were performed free of charge on a walk-up, no-appointment basis, including persons ≥1 year of age and regardless of symptoms, through “Unidos en Salud,” an academic, community (Latino Task Force) and city partnership. Certified lab assistants collected 2 bilateral anterior nasal swabs. The first was tested with BinaxNOWTM, immediately followed by a separate bilateral swab for SARS-CoV-2 genomic sequencing [11, 12]. Results were reported to participants within 2 hours, and all persons in a household (regardless of symptom status) corresponding to a positive BinaxNOW case were offered BinaxNOW testing. All persons testing BinaxNOW positive were offered participation in longitudinal Community Wellness Team support program [13, 14].
SARS-CoV-2 Genomic Sequence Recovery and Consensus Genome Generation
SARS-CoV-2 genomes were recovered using ARTIC Network V3 primers [15] and sequenced on an Illumina NovaSeq platform. Consensus genomes generated from the resulting raw.fastq files using IDseq [16] were used for subsequent analysis. Full details are included in Supplementary materials.
Household Attack Rate Analyses
Households (n = 328) tested in January and meeting the following inclusion criteria were eligible for secondary attack rate analyses: 1) ≥1 adult (aged ≥ 18 years) with a positive BinaxNOW result; 2) ≥1 case in household sequenced; and 3) ≥2 persons tested with BinaxNOW during the study period. Households in which sequences represented both West Coast and non-West Coast variants were excluded (n = 9). The index was defined as the first adult to test positive. Crude household attack rates, stratified by variant classification, were calculated as i) the proportion of positive BinaxNOW results among tested household contacts; and ii) the mean of the household-specific secondary attack rate, with 95% confidence interval (CI) based on cluster-level bootstrap. Generalized estimating equations were used to fit Poisson regressions, with cluster-robust standard errors and an exchangeable working covariance matrix. Because symptoms and disease severity may be affected by strain, these factors were not included in the a priori adjustment set. We evaluated for overdispersion [17] and conducted sensitivity analyses using targeted maximum likelihood estimation (TMLE) combined with Super Learning to relax parametric model assumptions; influence curve-based standard error estimates used household as the unit of independence [18].
Bayesian Phylogenetic Analysis
We compared the growth rates of B.1.427 and B.1.429 Phylogenetic Assignment of Named Global Outbreak (PANGO) lineages against 2 other lineages, B.1.232 and B.1.243, that had been circulating in California during the latter half of 2020. To do this, we built a Bayesian phylogeny for each lineage in BEAST v.1.10.4 and estimated the effective population size over time using the Bayesian SkyGrid model. We fit an exponential model to the median SkyGrid curve and inferred the reproductive numbers based on the exponential growth rates and generation time estimates from literature. Full analysis details are included in Supplementary materials.
Ethics Statement
The UCSF Committee on Human Research determined that the study met criteria for public health surveillance. All participants provided informed consent for dual testing.
RESULTS
Low-Barrier SARS-CoV-2 Testing and Sequencing
From 22 November to 1 December 2020, 3302 rapid direct antigen tests were performed on 3122 unique individuals; sample characteristics from this testing have been previously described [11]. From 10–29 January, using identical methods, 8822 rapid direct antigen tests were performed on 7696 unique individuals, representing 5239 households; household attack rate analyses were restricted to January samples, described here (Supplementary Table 1).
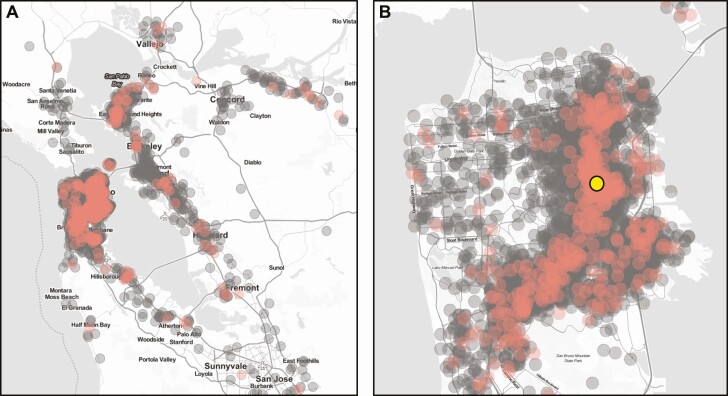
Test subjects originated from addresses in 8 Bay Area counties, indicating a wide catchment area (Figure 1). During this time period, there were 885 (10.0%) samples from 863 unique persons that were BinaxNOW positive for SARS-CoV-2 infection. From this set, a total of 80 samples were sequenced for the S gene only, of which 58 had S gene coverage over 92%. In addition, full SARS-CoV-2 genome sequencing was attempted on a total of 775 samples, of which 737 (95%) samples resulted in a genome coverage over 92% (Supplementary Table 2, sequences deposited in GISAID). These 986 samples, together with an additional 191 SARS-CoV-2 genome sequences generated from the same testing site during the period of 22 November to 1 December 2020 [11, 19] had adequate coverage of the full genome or spike protein for further analysis based on S gene sequence (Supplementary Table 3). Classification as either a West Coast variant or a non-West Coast variant was determined for 846 of all samples sequenced.
Figure 1.
Testing catchment area. The location of the 24th & Mission testing site is denoted by the yellow symbol. Negative tests are in gray, and positive tests are shown in red. Household locations shown have a random offset of up to 750 meters to obfuscate the precise addresses of individuals. The testing catchment area encompasses a substantial number of individuals in the surrounding 8 Bay Area counties (A). The greatest concentration of individuals reside within San Francisco county (B), Map tiles by Stamen Design and data by OpenStreetMap.
Similar to previous observations in San Francisco [20], full length sequences were distributed among the major clades (Supplementary Figure 1) [21]. Notably, mutations at spike position 501 were not observed, and thus no instances of the B.1.1.7 strain or any other strain bearing the N501Y mutation were detected in any sample during this period in January 2021. A single individual was found to have been infected with the P.2 strain, which carries the spike E484K mutation and was described in Brazil from a reinfection case [5]. This mutation has been associated with decreased neutralization in laboratory experiments [2, 4].
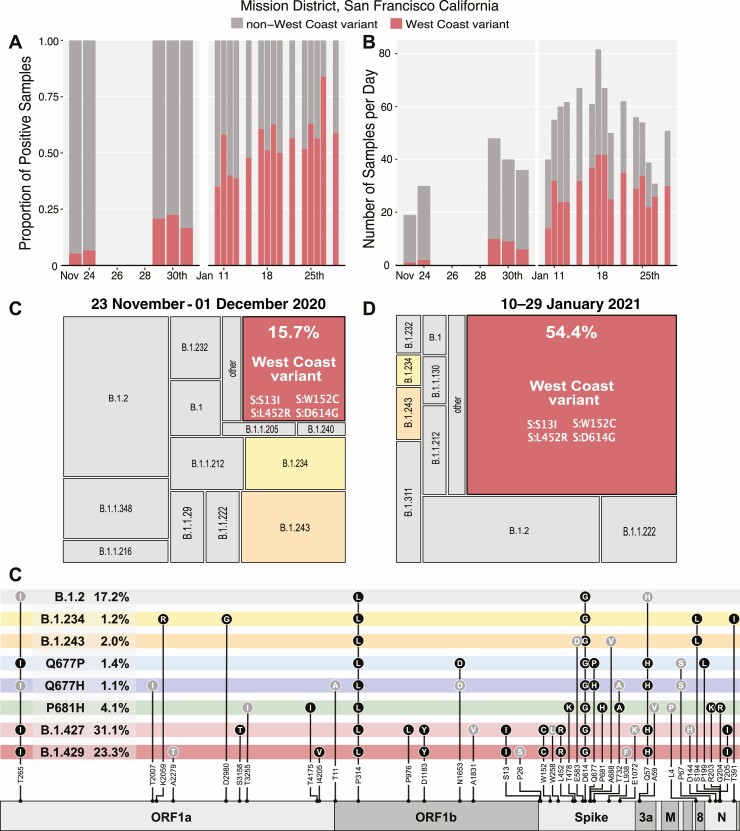
We observed SARS-CoV-2 genome sequences that belonged to PANGO lineages B.1.427 and B.1.429, both of which share a trio of recent mutations in the spike protein (S13I, W152C, and L452R) (Figure 2). These lineages are separated by differing mutations ORF1a and ORF1b, including ORF1b:P976L and ORF1a:I4205V, respectively. Sequencing of 191 viral genomes from 22 November to 1 December 2020 revealed that sequences carrying this trio of mutations represented only 15.7% of the total. A trend of increasing frequency was observed on a daily basis during the January testing period (Figure 2A), and the frequency of these lineages were observed to have increased to 54.4% of the total, representing an increase of more than 3-fold in approximately 1.5 months (Figure 2B, 2C). This increase in frequency is consistent with an expansion of viruses more broadly in California carrying these same mutations [23].
Figure 2.
Variants observed at 24th & Mission. A, Proportion of daily cases belonging to West Coast and non-West Coast variants. B, Total number of samples per day. C, D, Area maps [22] showing the relative proportion of PANGO lineages acquired from full length genomes from the November (N = 191) and January (N = 737) time periods, respectively. E, Genome maps for variants detected in this study. Dominant mutations (filled black circles), and nonsynonymous mutations detected at lower frequency in combination with existing lineages (filled gray circles) are shown in gray. Abbreviation: PANGO, Phylogenetic Assignment of Named Global Outbreak.
Additional nonsynonymous mutations were observed throughout the genome, including 108 unique non-synonymous mutations in the spike gene, several within functionally-significant regions of the protein (Figure 2C, Supplementary Table 3). Twelve unique mutations were observed in the receptor binding domain, most of which have yet to be investigated for functional effects. Additionally, 8 unique mutations were found adjacent to the polybasic furin cleavage site at the S1/S2 junction, which is reported to have a potential role in determination of virulence and host cell tropism [24–27]. Moderately prevalent mutations were observed at spike position 681 (P681H, n = 34 and P681R, n = 1), which is within the furin recognition site, and at spike position 677, where 2 different amino acid substitutions were observed in this cohort (Q677H, n = 22 and Q677P, n = 11). Multiple mutations at both of these sites have been previously observed [9].
Disease Severity
The SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) cycle thresholds (Ct) for nasal swab samples from which whole genomes corresponding to the West Coast variant were recovered were compared to parallel non-West Coast variant samples. Mean Ct values did not differ significantly between persons infected with West Coast (mean Ct 23.56; interquartile range [IQR] 6.4) versus non-West Coast (mean Ct 23.67; IQR 7.8) strains (95% CI: −.77 to .50, P-value = .67) (Supplementary Figure 2, Supplementary Table 2). The proportion of individuals with symptoms was similar among persons infected with West Coast (273/448, 60.9%) versus non-West Coast (250/389, 64.3%) strains. Among 364 sequenced cases with longitudinal follow-up by the Community Wellness Team, 4 (1.1%) were hospitalized (3/183, and 1/181, for West Coast and non-West Coast, respectively).
Household Secondary Attack Rate
A total of 328 households met inclusion criteria for evaluation of secondary attack rate; of these, 9 households had individuals with mixed strains and thus were excluded from analyses. Among the remaining 319 households, characteristics including race/ethnicity, ages of other household members, household size, density, and location were similar, regardless of whether the members were positive for West Coast or non-West Coast variants. (Table 1, Supplementary Table 4).
Table 1.
Characteristics of Households Included in the Household Attack Rate Analysis, Stratified by Strain
| Non-West Coast (N = 156) | West Coast | Total (N = 319) | |||
|---|---|---|---|---|---|
| B.1.427 (N = 90) | B.1.429 (N = 65) | All West Coast (N = 163)a | |||
| Race/Ethnicity (most common in household) | |||||
| Hispanic/Latinx | 143 (91.7%) | 78 (86.7%) | 62 (95.4%) | 146 (89.6%) | 289 (90.6%) |
| Asian | 5 (3.2%) | 5 (5.6%) | 1 (1.5%) | 8 (4.9%) | 13 (4.1%) |
| White/Caucasian | 4 (2.6%) | 3 (3.3%) | 0 (0%) | 3 (1.8%) | 7 (2.2%) |
| Black or African American | 2 (1.3%) | 2 (2.2%) | 2 (3.1%) | 4 (2.5%) | 6 (1.9%) |
| Other | 2 (1.3%) | 2 (2.2%) | 0 (0%) | 2 (1.2%) | 4 (1.3%) |
| Has children | |||||
| Does not have children | 105 (67.3%) | 69 (76.7%) | 35 (53.8%) | 110 (67.5%) | 215 (67.4%) |
| Has children | 51 (32.7%) | 21 (23.3%) | 30 (46.2%) | 53 (32.5%) | 104 (32.6%) |
| Location | |||||
| San Francisco | 118 (75.6%) | 71 (78.9%) | 39 (60.0%) | 115 (70.6%) | 233 (73.0%) |
| Outside San Francisco | 38 (24.4%) | 19 (21.1%) | 26 (40.0%) | 48 (29.4%) | 86 (27.0%) |
| Household size | |||||
| 2 persons | 14 (9.0%) | 12 (13.3%) | 5 (7.7%) | 20 (12.3%) | 34 (10.7%) |
| 3–4 persons | 63 (40.4%) | 33 (36.7%) | 22 (33.8%) | 57 (35.0%) | 120 (37.6%) |
| 5+ persons | 79 (50.6%) | 45 (50.0%) | 38 (58.5%) | 86 (52.8%) | 165 (51.7%) |
| Household densityb | |||||
| Mean (SD) | 1.86 (0.858) | 1.91 (0.881) | 2.27 (1.22) | 2.04 (1.03) | 1.95 (0.955) |
| Median [min, max] | 1.88 [0.250, 7.00] | 1.67 [0.444, 5.00] | 2.00 [0.714, 6.00] | 1.69 [0.444, 6.00] | 1.75 [0.250, 7.00] |
a8 households with S gene only sequence available.
bHousehold density missing for 17 households.
The 319 index cases had a total of 1241 nonindex household members; of these, 867 (69.9%) had a BinaxNOW test result available (452/658 [68.7%] for West Coast variant households; 415/583 [71.2%] of non-West Coast variant households). A total of 35.6% (161/452) of household contacts exposed to the West Coast variant tested BinaxNOW positive (33.2%, 78/235 for B1.427; 40.3%, 79/196 for B.1.429), whereas 29.4% (122/415) of contacts exposed to non-West Coast variant tested positive (Table 2). Secondary cases were identified a median of 1 day after index cases (IQR 0–4).
Table 2.
Secondary Household Attack Rates for West Coast Variants, Combined and Disaggregated by B.1.427 and B.1.429
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Positives Among Tested Contacts (%) | Mean Household Attack Rate (95% CI) | RR (95% CI) | P-value | aRR | P-value | |
| Class | ||||||
| Non-West Coast | 122/415 (29.4%) | 25.6% (20.3–31) | … | … | … | … |
| West Coast | 161/452 (35.6%) | 35.9% (30.1–41.9) | 1.28 (1.00–1.64) | .05 | 1.25 (.98–1.59) | .07 |
| Lineage | ||||||
| B.1.427 | 78/235 (33.2%) | 32.9% (25.4–40.6) | 1.19 (0.89–1.59) | .20 | 1.19 (.90–1.59) | .20 |
| B.1.429 | 79/196 (40.3%) | 40.9% (31.5–50.5) | 1.43 (1.07–1.91) | .02 | 1.36 (1.01–1.83) | .04 |
Relative risks estimated based on Poisson regression using generalized estimating equations and cluster-robust standard errors. Adjustment variables included age group, Latinx/Hispanic race, household size, and household density.
Abbreviations: aRR, adjusted risk ratio; CI, confidence interval; RR, risk ratio.
Based on unadjusted Poisson regression with cluster-robust standard errors, household contacts exposed to the West Coast variant had an estimated 28% higher risk of secondary infection, compared to household contacts exposed to a non-West Coast variant (RR: 1.28, 95% CI: 1.00–1.64, P-value = .05). When exposure to West Coast variants was disaggregated by B.1.427 and B.1.429, corresponding risks of secondary infections relative to exposure to non-West Coast variants were 1.19 (95% CI: .89–1.59, P-value = .20) and 1.43 (95% CI: 1.07–1.91, P-value = .02), respectively. Dispersion ratios were >0.9 in all regression analyses. Estimated relative risks of infection after household exposure to West Coast versus non-West Coast variants were similar after adjustment for household and individual-level characteristics of secondary contacts (adjusted risk ratio [aRR]: 1.25, 95% CI: .98–1.59, P-value: .07 for West Coast vs non-West Coast variants; aRR: 1.19, 95% CI: .90–1.59, P-value = .20 and aRR: 1.36, 95% CI: 1.01–1.83, P-value = .04 for B.1.427 and B.1.429, respectively). Relative attack rates were generally similar when stratified by household characteristics and by the characteristics of secondary contacts (Table 3); secondary attack rates among children aged <12 years were 51.9% (41/79) and 39.7% (31/78) when exposed to West Coast and non-West Coast strains, respectively. Sensitivity analyses in which parametric assumptions were relaxed using TMLE and Super Learning yielded similar estimates (Supplementary Table 5).
Table 3.
Secondary Attack Rate Disaggregated by Covariates
| Non-West Coast Strain | West Coast Strain | |||
|---|---|---|---|---|
| Positives Among Tested Contacts (%) | Mean Household Attack Rate (95% CI) | Positives Among Tested Contacts (%) | Mean Household Attack Rate (95% CI) | |
| Location | ||||
| San Francisco | 88/321 (27.4%) | 22.9% (17.2–28.8) | 113/316 (35.8%) | 37.5% (30.2–44.9) |
| Outside of San Francisco | 34/94 (36.2%) | 34% (22.1–46.2) | 48/136 (35.3%) | 32.1% (22.2–42.1) |
| Age group | ||||
| Age ≤ 12 | 31/78 (39.7%) | … | 41/79 (51.9%) | … |
| Age > 12 | 91/337 (27%) | … | 120/373 (32.2%) | … |
| Race/Ethnicity | ||||
| Latinx/Hispanic | 107/372 (28.8%) | … | 136/379 (35.9%) | … |
| Not Latinx/Hispanic | 15/43 (34.9%) | … | 25/73 (34.2%) | … |
| Household size | ||||
| 2 persons | 1/14 (7.1%) | 7.1% (0–21.4) | 12/20 (60%) | 60% (40–80) |
| 3–4 persons | 30/115 (26.1%) | 26.5% (17.7–35.7) | 35/102 (34.3%) | 33.3% (23.7–43.6) |
| 5+ persons | 91/286 (31.8%) | 28.1% (21.1–35.4) | 114/330 (34.5%) | 32% (25.1–39.2) |
| Household density | ||||
| Bottom half | 43/159 (27%) | 23.8% (15.7–32.2) | 52/176 (29.5%) | 33% (24.2–42.3) |
| Top half | 76/243 (31.3%) | 27.4% (20.1–35) | 101/262 (38.5%) | 35.7% (27.9–43.6) |
Mean household secondary attack rate only reported disaggregated by household level characteristics.
Abbreviation: CI, confidence interval.
Estimation of Reproductive Number
Using Bayesian phylogenetic analysis, we estimated the reproductive number to be 1.27 (95% CI: 1.10–1.46) for B.1.427 and 1.18 (95% CI: 1.05–1.32) for B.1.429 during the second half of 2020. These values were slightly higher than 2 other lineages spreading in California during the same time period: 1.12 (95% CI: 1.10–1.14) for B.1.232, and 1.02 (95% CI: .98–1.05) for B.1.243. As the reproductive numbers are very similar and were calculated from the median SkyGrid estimates, we cannot conclude any statistically significant differences between the lineages.
Discussion
We monitored SARS-CoV-2 viral variants by genomic sequencing and integration of metadata from households at a community based “test-and-respond” program. We found that the West Coast variants (PANGO lineages B.1.427 and B.1.429) increased in prevalence relative to wild type from November to January in the San Francisco Bay Area among persons tested in the same community-based location. These data extend and confirm prior observations from convenience, outbreak, and clinical samples reporting apparent increases in relative prevalence of the West Coast variants [23].
Household secondary attack rates of the West Coast variants were modestly higher than for non-West Coast variants, suggesting the potential for increased transmissibility. The West Coast variants compromise two closely related lineages (B.1.427 and B.1.429) that share identical sets of mutations in the spike protein but differ by additional synonymous and nonsynonymous mutations in other genes. Although the frequency of both lineages increased in this study and in California more widely [23], and the estimated increase in risk of secondary household infection relative to non-West Coast variants was fairly consistent across lineages, the point estimate was somewhat higher for B.1.429. Although moderate compared to increased transmissibility of other previously identified variants, even small increases in transmissibility could contribute to a substantial increase in cases, particularly in the context of reproductive numbers just below 1. Although this finding may be due to chance, future work should continue to monitor individual lineages.
The household attack rate observed here was higher than that reported in a recent global meta-analysis [28], even for the non-West Coast variants. It was similar to, or lower than, attack rates reported in other US settings. Prior US reports, however, were based on substantially smaller sample sizes.
Our findings that the West Coast variants increased in relative prevalence and had higher household secondary attack rates potentially suggest higher transmissibility. However, the West Coast variant has been detected in multiple locations and has been detected since May 2020 in California without relative expansion until the peak associated with the holiday season of November–January. Using Bayesian phylogenetic analysis, the estimated reproductive number for both West Coast lineages was found to be modestly higher than other circulating lineages.
We found no significant differences in viral load (using Ct) between West Coast and non-West Coast variants (Supplementary Figure 2), and recorded hospitalizations (n = 5/388) remained rare, despite the West Coast variant representing 54.4% of positive cases. This highlights the importance of studying walk-up populations, whether they are symptomatic or asymptomatic, as hospitalized populations often are confounded by comorbidities and subject to selection bias.
At the time of this sampling, no instances of B.1.1.7, or independent N501Y mutations were detected in our sample population of 830, despite sporadic observations elsewhere in CA (approximately 3% [69/2423] of genomes reported in California during the January study period; accessed from GISAID 24 February 2021), suggesting that introductions of B.1.1.7 have been rare in this catchment area, despite high SARS-CoV-2 incidence [29]. A single case of the P.2 variant, which carries the E484K mutation [2], was detected in this study. Surprisingly, this case did not have a travel history, highlighting the risk of cryptic transmission.
In addition to the mutations associated with spike L452R in the West Coast variants, we observed, at lower frequencies, other mutations of interest, such as those at spike positions 677 and 681, both of which have been reported previously on their own [9].
This study has several limitations. First, testing was conducted at a walk-up testing site, and thus these are inherently convenience samples; however, this would not be expected to impose a differential selection bias for those with or without any particular variant. Second, clear classification of the index case was not always possible, particularly when multiple adults from a household tested positive on the same date; furthermore, secondary household attack rate calculations do not account for potential external sources of infection other than the index case. However, the relative risk of secondary infection from household exposure to West Coast versus non-West Coast variants was similar among children, a group less likely to have been misclassified as non-index or to be exposed to external infection. Third, household testing coverage was incomplete and, in some cases, consisted of only a single follow-up test; this might contribute to an underestimate (or overestimate) of secondary attack rate, and although we again have no reason to suspect differential ascertainment by strain, this could bias estimates of relative risk.
The occurrence of variants in SARS-CoV-2 was always expected; however, it is often difficult to understand the clinical and epidemiological importance of any given single or set of co-occurring mutations. Although further epidemiological and laboratory experiments will be required to fully understand the community impact and mechanistic underpinnings of each variant, it is clear that enhanced genomic surveillance paired with community engagement, testing, and response capacity is an important tool in the arsenal against this pandemic.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online.
Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to thank the hundreds of academic labs and public health institutions that have been sequencing and publicly depositing genomic sequences, analysis tools, and epidemiological data throughout the pandemic. They thank Bevan Dufty and the BART team, Jeff Tumlin and the San Francisco MUNI, Supervisor Hillary Ronen, Mayor London Breed, Dr Grant Colfax and the Department of Public Health, Salu Ribeiro and Bay Area Phlebotomy and Laboratory services, PrimaryBio COVID testing platform, and our community ambassadors and volunteers. They would like to thank the Chan Zuckerberg Initiative, Greg and Lisa Wendt, Anne-Marie and Wylie Peterson, Gwendolyn Holcombe, Andrew and Dokleida Kawaja, Michael and Hazel Kawaja, Chris and Mitchell Kawaja, Carl Kawaja, the McKinnon Family Foundation, and Kevin and Julia Hartz, for their critical support and input. We also thank Jack Kamm, Peter Kim, Don Ganem, Sandy Schmidt, Cori Bargmann, Norma Neff, and Christopher Hoover for technical assistance and discussion.
Financial support. This work was supported by the UCSF COVID Fund (to J. D., D. H., J. L., and M. L.), the National Institutes of Health (grant numbers UM1AI069496 to J. D. and F31AI150007 to S. S.), the Chan Zuckerberg Biohub (to J. D. and D. H.), the Chan Zuckerberg Initiative (to J. D. and D. H.), and a group of private donors. The BinaxNOW cards were provided by the California Department of Public Health.
Potential conflicts of interest. J. D. is a member of the scientific advisory board of The Public Health Company, Inc., and is a scientific advisor for Allen & Co. J. D. also reports options granted for service on the Scientific Advisory Board of The Public Health Company. D. H. reports non-financial support from Abbott, outside the submitted work. P. A. and D. H. report funding from Chan Zuckerberg Biohub/Initiative. P. A. and A. M. are employed by Chan Zuckerberg Biohub. C. M. reports grant funding from the NIH and gift funds from the Stupski foundation. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Grubaugh ND, Hodcroft EB, Fauver JR, Phelan AL, Cevik M. Public health actions to control new SARS-CoV-2 variants. Cell 2021; 184:1127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Z, VanBlargan LA, Bloyet L-M, et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host & Microbe 2021; 29:477– 488.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voloch CM, Francisco R da S, Almeida LGP de, et al. Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J Virol 2021; 95. Available at: https://jvi.asm.org/content/95/10/e00119-21. Accessed 17 May 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020;9:e61312. [DOI] [PMC free article] [PubMed]
- 5. Ferrareze PAG, Franceschi VB, Mayer A de M, et al. E484K as an innovative phylogenetic event for viral evolution: genomic analysis of the E484K spike mutation in SARS-CoV-2 lineages from Brazil. bioRxiv 2021;2021.01.27.426895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeller M, Gangavarapu K, Anderson C, et al. Emergence of an early SARS-CoV-2 epidemic in the United States. medRxiv 2021;2021.02.05.21251235. [DOI] [PMC free article] [PubMed]
- 7. Fauver JR, Petrone ME, Hodcroft EB, et al. Coast-to-coast spread of SARS-CoV-2 during the early epidemic in the United States. Cell 2020; 181:990–996.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalinich CC, Jensen CG, Neugebauer P, et al. Real-time public health communication of local SARS-CoV-2 genomic epidemiology. PLoS Biol 2020; 18:e3000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodcroft EB, Domman DB, Oguntuyo K, et al. Emergence in late 2020 of multiple lineages of SARS-CoV-2 spike protein variants affecting amino acid position 677. medRxiv 2021;2021.02.12.21251658.
- 10. COVID Testing Pop-up site Established at 24th & Mission to Help Combat Surge in the City’s Most Impacted Community During this Holiday Season. 2020. Available at: https://www.sfdph.org/dph/alerts/files/12.20.20_CCSF_Press_Release.pdf.
- 11. Pilarowski G, Marquez C, Rubio L, et al. Field performance and public health response using the BinaxNOWTM rapid severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Antigen Detection Assay During Community-Based Testing. Clin Infect Dis 2021; 73:e3098–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pilarowski G, Lebel P, Sunshine S, et al. Performance characteristics of a rapid severe acute respiratory syndrome coronavirus 2 antigen detection assay at a public plaza testing site in San Francisco. J Infect Dis 2021; 223:1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubio LA, Peng J, Rojas S, et al. The COVID-19 symptom to isolation cascade in a Latinx community: a call to action. Open Forum Infect Dis 2021; 8:ofab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerkhoff AD, Sachdev D, Mizany S, et al. Evaluation of a novel community-based COVID-19 ‘test-to-care’ model for low-income populations. PLoS One 2020; 15:e0239400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ARTIC Project . hCoV-2019/nCoV-2019 version 3 amplicon set. Available at: https://artic.network/resources/ncov/ncov-amplicon-v3.pdf. Accessed 25 February 2021.
- 16. Kalantar KL, Carvalho T, de Bourcy CFA, et al. IDseq—an open source cloud-based pipeline and analysis service for metagenomic pathogen detection and monitoring. GigaScience 2020; 9. doi: 10.1093/gigascience/giaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gelman A, Hill J.. Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press, 2007. [Google Scholar]
- 18. Laan MJ van der, Rose S.. Targeted learning in data science: causal inference for complex longitudinal studies. London: Springer International Publishing, 2018. Available at: https://www.springer.com/gp/book/9783319653037. Accessed 24 February 2021. [Google Scholar]
- 19. Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob Chall 2017; 1:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chamie G, Marquez C, Crawford E, et al. Community transmission of severe acute respiratory syndrome coronavirus 2 disproportionately affects the Latinx population during Shelter-in-Place in San Francisco. Clin Infect Dis 2021; 73(Suppl. 2):S127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hadfield J, Megill C, Bell SM, et al. Nextstrain: real-time tracking of pathogen evolution. Bioinformatics 2018; 34:4121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laserson U. laserson/squarify. 2021. Available at: https://github.com/laserson/squarify. Accessed 26 February 2021.
- 23. Zhang W, Davis BD, Chen SS, Martinez JMS, Plummer JT, Vail E. Emergence of a novel SARS-CoV-2 strain in Southern California, USA. medRxiv 2021;2021.01.18.21249786.
- 24. Hoffmann M, Kleine-Weber H, Pöhlmann S.A Multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell 2020; 78:779–784.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Örd M, Faustova I, Loog M. The sequence at spike S1/S2 site enables cleavage by furin and phospho-regulation in SARS-CoV2 but not in SARS-CoV1 or MERS-CoV. Sci Rep 2020; 10:16944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson BA, Xie X, Bailey AL, et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature 2021; 591:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jaimes JA, Millet JK, Whittaker GR. Proteolytic cleavage of the SARS-CoV-2 spike protein and the role of the novel S1/S2 site. iScience 2020; 23:101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household Transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open 2020; 3:e2031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Volz E, Mishra S, Chand M, et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021; 593:266–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.