Abstract
We sought to assess the proportion of elicited close contacts diagnosed with coronavirus disease 2019 at the start of and before exiting quarantine in San Francisco. From June 8 to August 31, 6946 contacts were identified: 3008 (46.3%) were tested, 940 (13.5%) tested positive, and 90% tested positive in the first 9 days of quarantine.
Keywords: contact tracing, COVID-19, epidemiology, quarantine, testing
Contact tracing, in combination with the quarantining and testing of contacts, is considered a key element of any coronavirus disease 2019 (COVID-19) response [1–3]. Mounting evidence that up to half of persons with COVID-19 are entirely asymptomatic, including contacts identified in contact tracing efforts [4–6], has highlighted the importance of ensuring that contact tracing efforts successfully link all elicited close contacts to testing during quarantine. Leveraging data from the San Francisco Department of Public Health (SFDPH) case investigation and contact tracing program [7], we aimed to investigate the proportion of elicited close contacts who developed COVID-19 during their 14-day postexposure quarantine period and the proportion of those who were asymptomatic. In addition, we sought to determine when during quarantine most secondary cases were likely to have been identified, as to better understand if the duration of quarantine could be abbreviated for a subset of contacts, contingent of negative “exit” test.
METHODS
Data from case investigations for all individuals with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcriptase polymerase chain reaction (RT-PCR) test result in San Francisco from June 8 to August 31, 2020, were extracted from the public health database. Cases were defined as any individual with a laboratory-confirmed diagnosis of COVID-19. Contacts were identified by cases and defined in accordance with the current Centers for Disease Control and Prevention (CDC) criteria [8]. Close contacts were then defined as household contacts (HCs) if the case reported that the contact lived in the same dweling unit and sharing common spaces or nonhousehold contacts (NHCs) if the case reported that the contact was exposed in a nonhousehold setting, such as workplaces, classrooms, transportation vehicles, etc. [9].
All contacts, reached by the SFDPH contact tracing team, were instructed to quarantine at home or in an isolation and quarantine hotel for 14 days following the date of last exposure to the index case, in accordance with CDC guidance at that time. Contacts were also referred for SARS-CoV-2 RT-PCR testing during quarantine regardless of symptoms. All those who tested negative at the start of quarantine were then referred for a second test toward the end of quarantine.
Patient Constent
This work was conducted as part of SFDPH’s COVID-19 surveillance; institutional review board approval and informed consent from cases and contacts were not required.
Main Outcomes and Measures
To assess the impact of the SFDPH contact tracing program, we sought to determine (1) the proportion of contacts who were tested during their quarantine for SARS-CoV-2; (2) of those who tested negative or had an indeterminate result at the first test, those who got a second test; and (3) of all those who got tested, the overall proportion that tested positive. Sociodemographic and clinical characteristics of contacts at the initial contact tracing interview, including contact type (HCs vs NHCs), were summarized using descriptive statistics; proportions testing positive were compared using chi-square testing, with significance set at P = .001. A deterministic match based on personal identifiers was performed between contact and testing databases to (1) exclude contacts who were known to have tested positive for COVID-19 before the date of last exposure with the case; (2) deduplicate previously named household contacts; and (3) ascertain testing results. Survival curves for time from last exposure to positive test were plotted using the Kaplan-Meier method and compared with a log-rank test to determine the probability of testing positive. All analyses were conducted with R Statistical Software, version 4.0.2.
RESULTS
Outcomes of Case Investigation and Contact Tracing
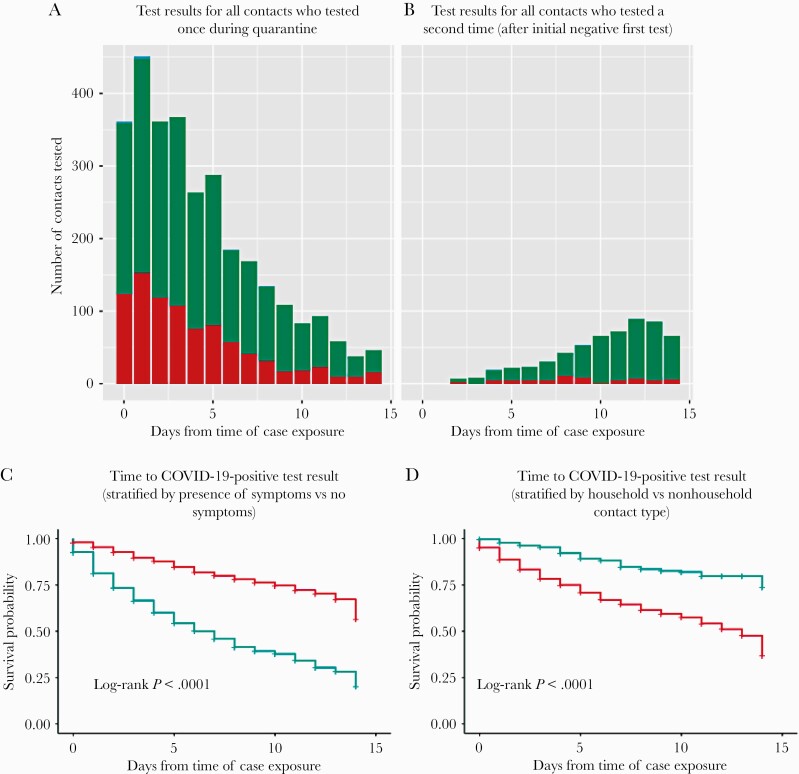
Between June 8 and August 31, 2020, a total of 2506 laboratory-confirmed cases reported to SFDPH identified at least 1 contact. From interviews with those cases, 6946 close contacts not previously positive for COVID-19 (in the 90 days pre-exposure) were elicited, 3008 (43.3%) of whom were tested during their postexposure window and 880 (29.3%) tested positive at their first test. Among 2128 contacts who initially tested negative, the majority (n = 1586, 72.6%) did not get retested. However, 582 (27.3%) completed a second test, and 60 (10.3%) additional positive secondary cases were identified (Figure 1A; Supplementary Tables 1–3). Notably, 90% (880/940) of all those testing positive tested positive by day 9 of quarantine.
Figure 1.
A, Histogram of test results for those tested at least once during quarantine (red indicating negative result, green indicating positive result). B, Histogram of test results for those tested a second time during quarantine, after an initial negative first test (red indicating negative result, green indicating positive result). C, Kaplan-Meier curves showing time to positive COVID test result stratified by presence of symptoms (red line representing results for contacts with no symptoms, green indicating results for contact with symptoms). D, Kaplan-Meier curves showing time to positive COVID test result, stratified by contact type (red line representing household contacts, green representing nonhousehold contacts). Abbreviation: COVID, coronavirus disease.
Symptom data were collected from 4990 contacts (71.8% of all contacts elicited) during the initial interview, and, of the 1237 (24.8%) contacts endorsing symptoms, 791 (63.9%) were tested and 412 (52.1%) tested positive. Among 379 symptomatic contacts who initially tested negative or indeterminate, 132 (34.8%) received a second test and 23 (17.4%) subsequently tested positive (Figure 1B; Supplementary Table 4, Supplementary Figure 1). Among 3753 asymptomatic contacts, 1964 (52.3%) were tested, of whom 1601 (81.5%) had a negative or indeterminate result and 363 (18.5%) tested positive. Among 1601 asymptomatic contacts with an initial negative or indeterminate test result, 423 (26.4%) received a second test, among whom 31 (7.3%) tested positive upon repeat testing. Of the 31 secondary cases who were asymptomatic at the initial interview, 11 were symptomatic at or after the second test. Taken together, asymptomatic contacts accounted for 47.5% (394/829) of all secondary cases. Among contacts for whom symptom data were available, those with symptoms were more likely to get tested compared with those who did not have symptoms (63.9% [791/1237] vs 52.3% [1964/3753]; P < .001). Moreover, the probability of testing positive earlier in quarantine was greater among contacts with symptoms compared with those without (P < .001) (Figure 1C).
Of 6616 contacts for whom contact type data were collected (95.2% of all contacts elicited), 5345 (80.8%) were HCs, among whom 2381 (44.5%) were initially tested and 786 (33.0%) tested positive (Supplementary Table 5). Among 1595 HCs who initially tested negative or indeterminate, 448 (28.1%) received a second test and 53 (11.8%) subsequently tested positive at a later point during quarantine. Among 1271 (19.2%) NHCs, 495 (38.9%) were tested and 68 (13.7%) tested positive. Among 427 NHCs who initially tested negative, 109 (25.5%) received a second test and 2 (1.8%) subsequently tested positive, 1 of whom had symptoms at the initial interview. Of note, 36 (51.4%) of the secondary cases identified from nonhousehold exposures reported symptoms. Among all contacts for whom contact type data were available, HCs were more likely to get tested compared with NHCs (44.5% [2381/5345] vs 25.5% [109/1271]; P < .001).
As the Kaplan-Meier curve (Figure 1D) illustrates, the probability of testing positive earlier in quarantine was greater among HCs compared with NHCs (P < .0001; with 84.3% [59/70] of secondary cases among NHCs testing positive before day 7).
CONCLUSIONS
To our knowledge, this is the first report assessing the utility of a 2-test strategy to ascertain the proportion of contacts who developed COVID-19 during the 14-day quarantine period after exposure to an index case. We believe that these findings have at least 3 critical programmatic implications for contact tracing programs.
First, we highlight how NHCs, especially those without symptoms, may be less likely to test positive after day 7 of quarantine. These data highlight how a longer quarantine may be unnecessary for NHCs, especially in the absence of symptoms and contingent on a negative exit test after day 7, assuming that the exit test is performed with a highly sensitive RT-PCR assay. These findings are consistent with research elsewhere demonstrating that the secondary attack rate among NHCs is substantially lower when compared with HCs [9, 10]. Given significant social and economic costs, not to mention the public health challenges of ensuring adherence to a 14-day quarantine recommendation, our findings highlight the need for rigorous research to determine if more efficient but equally effective quarantine and testing strategies may be appropriate for NHCs. Moreover, our findings validate the current CDC guidance that a 10-day duration of quarantine for all asymptomatic close contacts is an acceptable alternative to 14 days [11].
Second, our findings underscore the importance of testing all contacts, regardless of symptoms, as 47.5% (394/829) of those testing positive endorsed no symptoms at initial interview and would have been missed if testing had been restricted to only those with symptoms. This finding builds on substantial recent research on the importance of testing asymptomatic contacts [4, 12–14].
Third, this report highlights the possible utility of repeat testing for contacts both at the start of and then before exiting quarantine. Although only 27.3% (582/2128) of all eligible contacts got a second test during quarantine, 10.3% (60/582) of those were positive. Recognizing that resource constraints and logistical challenges make repeat testing challenging, we assert that a prioritized approach to repeat testing may be helpful. First testing contacts with symptoms at the time of the start of quarantine or at the contact tracing interview, or at the onset of symptoms if symptoms develop later; and then testing asymptomatic contacts before exiting quarantine. Such an approach is supported by modeling analyses demonstrating that testing on exit from quarantine can reduce 14-day quarantine by 50% [15] and also by contact tracing programmatic data from Vermont, where testing contacts at day 7 was found to be useful in determining who could exit quarantine early [16].
Limitations
Our analysis has several limitations. We note that the proportion of contacts who were tested is low, a reflection of the fact that even despite SFDPH guidance recommending 2 tests, many contacts were reluctant or lacked adequate resources to get tested. We speculate that more contacts may have been tested during quarantine than captured in this analysis, including a proportion that was tested before being notified by the contact tracing team; unfortunately, the process of reconciling the contact tracing database and the COVID-19 test results database was imperfect. Rapid SARS-CoV-2 antigen detection assays were not being widely used in San Francisco at the time of this study, but have since been shown to increase speed and uptake of testing among close contacts [17]. While we note that false-negative RT-PCR results have been reported [18] and could have led to an underestimate of the secondary attack rate among tested contacts, we speculate that this was unlikely to have impacted our findings. Finally, we only collected data on the presence of typical symptoms, as listed by the CDC [8], and not on less typical symptoms; as such, we may have under-reported those who had atypical presentations.
CONCLUSIONS
This analysis provides robust evidence for tracing and testing all contacts of COVID-19 cases during quarantine. Moreover, these data support the policy of testing all elicited close contacts, regardless of symptoms, as critical to mitigating COVID-19 transmission. More research is necessary to determine if a shorter quarantine period for close contacts, in particular NHCs, contingent on robust exit testing, can minimize economic impacts as well as COVID-related public health risks.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are grateful to Cinthia Blat and George Rutherford for reviewing drafts of this manuscript.
Financial support. None.
Potential conflicts of interest. None of the authors listed report any conflicts of interest: M.J.A.R., P.P., H.B., E.A., H.S., J.C., M.S.W., D.S. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A 2004; 101:6146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keeling MJ, Hollingsworth TD, Read JM. Efficacy of contact tracing for the containment of the 2019 novel coronavirus (COVID-19). J Epidemiol Community Health 2020; 74:861–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klinkenberg D, Fraser C, Heesterbeek H. The effectiveness of contact tracing in emerging epidemics. PLoS One 2006; 1:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med 2020; 173:362–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hung IF, Cheng VC, Li X, et al. SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: a case series. Lancet Infect Dis 2020; 20:1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ooi EE, Low JG. Asymptomatic SARS-CoV-2 infection. Lancet Infect Dis 2020; 20:996–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sachdev DD, Brosnan HK, Reid MJA, et al. Outcomes of contact tracing in San Francisco, California—test and trace during shelter-in-place. JAMA Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Overview of testing for SARS-CoV-2 (COVID-19). 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed 30 August 2020.
- 9. Jing QL, Liu MJ, Zhang ZB, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis 2021; 21:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Options to reduce quarantine for contacts of persons with SARS-CoV-2 infection using symptom monitoring and diagnostic testing. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-options-to-reduce-quarantine.html. Accessed 20 March 2021. [PubMed]
- 12. Lavezzo E, Franchin E, Ciavarella C, et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020; 584:425–429. [DOI] [PubMed] [Google Scholar]
- 13. Moriarty LF, Plucinski MM, Marston BJ, et al. Public health responses to COVID-19 outbreaks on cruise ships - worldwide, February-March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang R, Gui X, Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw Open 2020; 3:e2010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells CR, Townsend JP, Pandey A, et al. Optimal COVID-19 quarantine and testing strategies. Nat Commun 2021; 12:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones A, Fialkowski V, Prinzing L, et al. Assessment of day-7 postexposure testing of asymptomatic contacts of COVID-19 patients to evaluate early release from quarantine - Vermont, May-November 2020. MMWR Morb Mortal Wkly Rep 2021; 70:12–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pilarowski G, Marquez C, Rubio L, et al. Field performance and public health response using the BinaxNOW TM Rapid SARS-CoV-2 antigen detection assay during community-based testing. Clin Infect Dis 2020; ciaa1890. doi: 10.1093/cid/ciaa1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One 2020; 15:e0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.