Abstract
Background
Research on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission within households and other close settings using serological testing is scarce.
Methods
We invited coronavirus disease 2019 (COVID-19) cases diagnosed between February 27 and April 1, 2020, in Canton of Vaud, Switzerland, to participate, along with household members and other close contacts. Anti-SARS-CoV-2 immunoglobulin G antibodies were measured using a Luminex immunoassay. We estimated factors associated with serological status using generalized estimating equations.
Results
Overall, 219 cases, 302 household members, and 69 other close contacts participated between May 4 and June 27, 2020. More than half of household members (57.2%; 95% CI, 49.7%–64.3%) had developed a serologic response to SARS-CoV-2, while 19.0% (95% CI, 10.0%–33.2%) of other close contacts were seropositive. After adjusting for individual and household characteristics, infection risk was higher in household members aged ≥65 years than in younger adults (adjusted odds ratio [aOR], 3.63; 95% CI, 1.05–12.60) and in those not strictly adhering to simple hygiene rules like hand washing (aOR, 1.80; 95% CI, 1.02–3.17). The risk was lower when more than 5 people outside home were met during semiconfinement, compared with none (aOR, 0.35; 95% CI, 0.16–0.74). Individual risk of household members to be seropositive was lower in large households (22% less per each additional person).
Conclusions
During semiconfinement, household members of a COVID-19 case were at very high risk of getting infected, 3 times more than close contacts outside home. This highlights the need to provide clear messages on protective measures applicable at home. For elderly couples, who were especially at risk, providing external support for daily basic activities is essential.
Keywords: COVID-19, household, SARS-CoV-2, serology, transmission
The understanding of transmission patterns is especially critical to guide interventions aiming at limiting the occurrence of new cases of coronavirus disease 2019 (COVID-19). In this respect, transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in promiscuous settings such as households is of particular interest and is at the core of the early investigation protocols provided by the World Health Organization (WHO Unity Studies) to address the many unknowns related to the COVID-19 pandemic [1, 2].
Studies dealing with the transmission of SARS-CoV-2 within households have found secondary attack rates (SARs) ranging from 3.9% to 44.6%, reflecting heterogeneous settings and study designs [3]. The evidence regarding transmission to close contacts outside the household tends to show lower SARs (from 0.7% to 5.1%), but attack rates above 50% have been reported in certain circumstances [4–8]. Most studies conducted so far are based on the identification of active disease through nucleic acid amplification tests (NAATs), whose sensitivity can be hampered by various factors [9].
The availability of serological assays allows the identification of past infection and thus provides key input into our understanding of the epidemiology of SARS-CoV-2. Nevertheless, studies on SARS-CoV-2 transmission in close settings using serological testing remain scarce. So far, most of them found SARs close to 35% within households [10–13]. However, none of them includes a thorough investigation of factors associated with seropositivity. Regarding close contacts outside the household, research shows SARs ranging from 0% to 13.7%, but study designs and settings are disparate [12, 14–16]. Furthermore, the amount of available serological assays is quickly growing, often with limited external validation of their accuracy, and concerns are emerging regarding their accuracy in the setting of seroepidemiological studies because of the lower median level of antibodies in participants compared with clinical studies [17].
This work was part of SerocoViD, a community-based seroepidemiological study of SARS-CoV-2 infection conducted in Canton of Vaud, Switzerland, embedded within a nationwide program, Corona Immunitas [18]. Taking advantage of prior development and validation of a highly sensitive serological assay carried out locally [19], the objective was to determine the prevalence of anti-SARS-CoV-2 immunoglobulin G (IgG) antibodies among household members and other close contacts of COVID-19 cases and to identify factors associated with seropositivity in these highly exposed people.
METHODS
Study Design and Participants
SerocoViD is a cross-sectional community-based seroepidemiological study of SARS-CoV-2 infection conducted in Canton of Vaud (French-speaking region of Switzerland, 806 088 inhabitants on December 31, 2019). The study was launched at the end of April 2020, coinciding with the easing of semiconfinement measures taken in Switzerland in mid-March.
From February 27 (first confirmed case in Canton of Vaud) to March 4, 2020, all COVID-19 cases underwent contact tracing by local authorities. At that time, a close contact was any individual who had been within 2 m of an infected person for at least 15 minutes, starting 24 hours before illness onset. Given the exponential growth of the number of cases, contact tracing was stopped from week 2 of the epidemic. For the same reason, from March 9, 2020, diagnostic testing was limited to health care personal, hospitalized people, and individuals at increased risk for severe illness in the entire country.
We sampled confirmed COVID-19 cases from the cantonal registry (total n ≈ 3700). With the exception of 3 people (1 deceased, 2 who returned home abroad), all confirmed cases from week 1 were invited to participate in the study (n = 13), along with their close contacts identified by contact tracing (n = 117). Additionally, all cases aged between 6 months and 19 years (n = 66) and a random sample of noninstitutionalized cases aged ≥20 years (n = 368) who were tested positive during weeks 2–5 (from March 5 to April 1, 2020) were invited to take part in the study. In order to extend the age range of confirmed cases for whom a contact tracing procedure had been performed, the study team conducted complementary tracing procedures for 3 adolescent cases, thus identifying 20 additional close contacts outside the household.
Overall, this resulted in the solicitation of 447 confirmed cases (hereafter called index cases) and 137 close contacts not belonging to the households of the index cases. Moreover, index case participants were asked to invite all their household members aged ≥6 months to take part in the study. Because of testing restrictions, index cases were not necessarily the first infected in their household, but those fulfilling testing criteria. All index cases were diagnosed using NAAT.
Patient Consent Statement
The Cantonal Ethics Committee of Vaud, Switzerland, approved the protocol (ID 2020-00887), and written consent was obtained from participants.
Procedures
Index cases and their close contacts, identified by contact tracing, were invited by letters. Participants completed registration for the study and answered the study questionnaire (available in French and English) via an online platform. The questionnaire covered the following topics: sociodemographic information, medical history, history of symptoms compatible with COVID-19 and use of health services, living conditions and household characteristics, contacts with other people in private and professional settings, and compliance with measures aimed at controlling the epidemic. The full questionnaire is available in the Supplementary Data.
Study visits took place in 4 centers distributed over the cantonal territory between May 4 and June 27, 2020. A venous blood sample was collected to proceed with serological testing. We offered a home visit by a mobile study team to people at increased risk for severe illness from COVID-19. All participants (or their legal representative) provided written informed consent.
Detection of Anti-SARS-CoV-2 Antibodies
We measured anti-SARS-CoV-2 IgG antibodies targeting the spike (S) protein in its native trimeric form using a Luminex immunoassay. This test was developed by the Lausanne University Hospital, Switzerland, in collaboration with the École Polytechnique Fédérale de Lausanne (EPFL), and compared with 5 commercially available immunoassays detecting IgG against the N protein and the monomeric moieties of the S1 protein [19]. The in-house Luminex S protein trimer IgG assay was 99.2% specific in sera from people infected with prepandemic coronaviruses or from patients with autoimmune diseases, and it proved to be more sensitive (96.7%) than commercial tests in hospitalized patients with moderate to severe disease 16 to 33 days postsymptoms. The threshold for a positive result was defined at an antibody Multiplex Fluorescent Immunoassay (MFI) ratio of ≥6.
Statistical Analysis
We calculated the proportion of index cases with a positive serology test result and computed a Clopper-Pearson 95% CI. Significant clustering of infections within households has been reported in previous research [20]. In order to account for correlation between close contacts of the same index case, we used generalized estimating equations (GEEs) with an exchangeable correlation structure to estimate the seroprevalence and corresponding 95% CI among contacts. Odds ratios (ORs) were computed to measure the strength of the association between each independent variable and the serology test result. We used GEEs to account for correlation between contacts of the same index case and calculated ORs with their 95% CIs and P values using a logit link function. Finally, a multivariable regression model using GEE was fitted to measure the adjusted association of individual and household characteristics with serology test results among household members. Considering the potential influence of past diagnostic testing for SARS-CoV-2 on the reporting of symptoms, we proceeded to a sensitivity analysis among contacts not reporting previous nasal or throat swabbing. We performed statistical analysis using Stata/IC, version 16.1. There was no imputation of missing values.
RESULTS
Two-hundred nineteen index cases (49.0%), aged 2 to 90 years (mean [SD], 48.7 [19.3] years), participated in the study, of whom 55.7% considered themselves women. They reported 421 household members, of whom 302 (71.7%), aged 1 to 87 years (mean [SD], 37.0 [21.3] years), took part in the study. Sixty-nine (50.4%) close contacts outside the household, aged 9 to 85 years (mean [SD], 47.8 [17.0] years), participated.
Prevalence of Seropositivity in the Different Groups
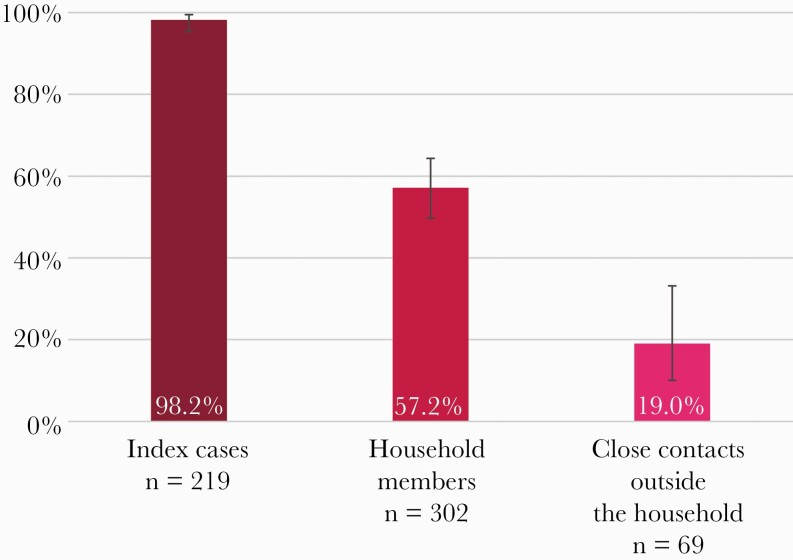
Most index cases (215/219, 98.2%) had a positive serological test result (95% CI, 95.4%–99.5%) (Figure 1). The crude proportion of positives was 53.0% in household members (160/302) and 17.4% among close contacts outside the household (12/69). When taking into account correlation, the seroprevalence was 57.2% in household members (95% CI, 49.7%–64.3%) and 19.0% in close contacts outside the household (95% CI, 10.0%–33.2%).
Figure 1.
Percentage of participants with a positive serology test result, by type of participant. Index cases: crude proportion, calculation of 95% confidence interval using the Clopper-Pearson method. For household members and close contacts outside the household, proportion and corresponding 95% confidence interval were estimated using GEEs (exchangeable correlation structure). Abbreviation: GEEs, generalized estimating equations.
Unadjusted Association of Individual and Household Characteristics With Seropositivity (Bivariable Analysis)
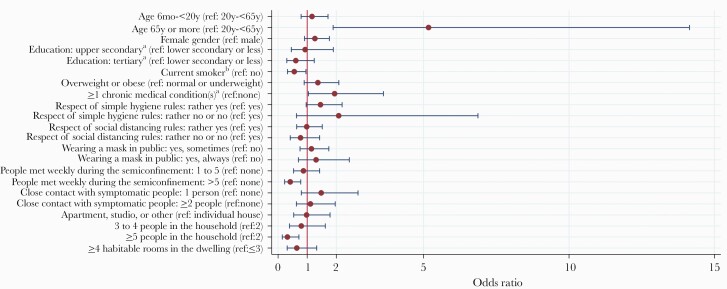
A higher proportion of household members aged 65 to 75 (85.7%) and ≥75 (83.3%) were seropositive (Table 1). No association between serological test result and gender or level of education was found (Figure 2). Household members currently smoking had lower odds of infection than nonsmokers in bivariable analysis (unadjusted OR, 0.56; 95% CI, 0.32–0.96). In close contacts outside the household, seroprevalence was 30.3% and 5.7% in overweight/obese and normal/underweight participants, respectively, but no association was found in household members. Close contacts not strictly adhering to simple hygiene rules tended to have higher odds of infection (Table 2, Figure 2). We found no association between serology and compliance with social distancing rules. Positive test results were less frequent in household members who had met more than 5 people per week during the semiconfinement compared with none (unadjusted OR, 0.42; 95% CI, 0.22–0.78), but there was no association with the number of close encounters with symptomatic individuals. In bivariable analysis, seroprevalence significantly decreased with increasing household size. We found that 66.1% of participants living with 1 other person only (the index COVID-19 case) had a positive test result, contrasting with participants living with ≥5 people, who showed a 26.0% risk of being seropositive (unadjusted OR, 0.19; 95% CI, 0.06–0.62). There was an inverse relationship between household size and mean age of participants in the household (Supplementary Table 1).
Table 1.
Serology Test Result According to General Characteristics and Medical History, Stratified by Type of Participant (Unadjusted Results)
| Index Cases (n = 219) | Household Members (n = 302) | Close Contacts Outside the Household (n = 69) | |||||
|---|---|---|---|---|---|---|---|
| No. (%) Seropositive | No. (%) Seropositive | Unadjusted OR [95% CI] | P Value | No. (%) Seropositive | Unadjusted OR [95% CI] | P Value | |
| All participants | 215/219 (98.2) | 160/302 (53.0) | 12/69 (17.4) | ||||
| Age | .119 | .928 | |||||
| 6 mo–<5 y | 1/2 (50.0) | 5/11 (45.5) | 0.92 [0.37–2.29] | No participant | ·· | ||
| 5 y–<10 y | 1/1 (100.0) | 12/22 (54.6) | 1.16 [0.55–2.44] | 0/1 (0.0) | ·· | ||
| 10 y–<15 y | 2/2 (100.0) | 15/32 (46.9) | 0.93 [0.49–1.75] | 0/3 (0.0) | ·· | ||
| 15 y–<20 y | 20/21 (95.2) | 9/19 (47.4) | 1.17 [0.56–2.45] | 0/2 (0.0) | ·· | ||
| 20 y–<40 y | 42/43 (97.7) | 37/76 (48.7) | Reference | 3/15 (20.0) | Reference | ||
| 40 y–<65 y | 103/104 (99.0) | 60/116 (51.7) | 0.83 [0.52–1.30] | 7/38 (18.4) | 0.92 [0.22–3.87] | ||
| 65 y–<75 y | 31/31 (100.0) | 12/14 (85.7) | 3.98 [1.03–15.44] | 2/9 (22.2) | 1.32 [0.16–11.00] | ||
| ≥75 y | 15/15 (100.0) | 10/12 (83.3) | 5.25 [1.16–23.72] | 0/1 (0.0) | ·· | ||
| Gender | .164 | .124 | |||||
| Male | 94/96 (97.9) | 74/146 (50.7) | Reference | 8/30 (26.7) | Reference | ||
| Female | 120/122 (98.4) | 86/156 (55.1) | 1.27 [0.91–1.76] | 4/39 (10.3) | 0.36 [0.10–1.32] | ||
| Other | 1/1 (100.0) | No participant | ·· | No participant | ·· | ||
| Current smokera | .034 | .825 | |||||
| No | 191/194 (98.5) | 142/256 (55.5) | Reference | 10/59 (17.0) | Reference | ||
| Yes | 23/24 (95.8) | 18/46 (39.1) | 0.56 [0.32–0.96] | 2/9 (22.2) | 1.21 [0.22–6.53] | ||
| Weight status | .146 | .011 | |||||
| Normal or underweight | 104/106 (98.1) | 92/184 (50.0) | Reference | 2/35 (5.7) | Reference | ||
| Overweight or obese | 110/112 (98.2) | 66/111 (59.5) | 1.37 [0.90–2.09] | 10/33 (30.3) | 6.74 [1.54–29.50] | ||
| Adult participants only | |||||||
| Education | .196 | .423 | |||||
| Lower secondary or less | 21/21 (100.0) | 21/33 (63.6) | Reference | 3/10 (30.0) | Reference | ||
| Upper secondary | 58/59 (98.3) | 46/82 (56.1) | 0.93 [0.46–1.90] | 5/23 (21.7) | 0.64 [0.13–3.21] | ||
| Tertiary | 108/109 (99.1) | 51/100 (51.0) | 0.61 [0.30–1.24] | 4/30 (13.3) | 0.33 [0.06–1.83] | ||
| Chronic medical conditionsb | .037 | .148 | |||||
| None | 131/133 (98.5) | 86/169 (50.9) | Reference | 6/44 (13.6) | Reference | ||
| ≥1 | 58/58 (100.0) | 32/47 (68.1) | 1.94 [1.04–3.62] | 6/19 (31.6) | 2.48 [0.73–8.48] | ||
| Hypertension | .110 | .034 | |||||
| No | 152/154 (98.7) | 95/182 (52.2) | Reference | 7/50 (14.0) | Reference | ||
| Yes | 36/36 (100.0) | 21/31 (67.7) | 1.81 [0.87–3.74] | 5/11 (45.5) | 4.48 [1.12–18.01] | ||
| Diabetes | .489 | .082 | |||||
| No | 169/171 (98.8) | 111/206 (53.9) | Reference | 10/57 (17.5) | Reference | ||
| Yes | 15/15 (100.0) | 4/6 (66.7) | 1.75 [0.36–8.48] | 2/3 (66.7) | 8.59 [0.76–96.92] | ||
| Cardiovascular disease | .239 | ·· | |||||
| No | 170/172 (98.8) | 103/196 (52.6) | Reference | 12/58 (20.7) | ·· | ||
| Yes | 12/12 (100.0) | 9/12 (75.0) | 2.03 [0.62–6.62] | 0/2 (0.0) | ·· | ||
| Kidney disease | |||||||
| No | 181/183 (98.9) | 115/212 (54.3) | ·· | 12/60 (20.0) | ·· | ||
| Yes | 3/3 (100.0) | No participant | ·· | 0/1 (0.0) | ·· | ||
| Chronic respiratory disease | .196 | ·· | |||||
| No | 177/179 (98.9) | 106/202 (52.5) | Reference | 12/59 (20.3) | ·· | ||
| Yes | 7/7 (100.0) | 4/5 (80.0) | 3.79 [0.50–28.52] | 0/2 (0.0) | ·· | ||
| Immunodeficiency | .596 | ·· | |||||
| No | 174/176 (98.9) | 110/202 (54.5) | Reference | 12/58 (20.7) | ·· | ||
| Yes | 12/12 (100.0) | 4/9 (44.4) | 0.74 [0.25–2.23] | 0/3 (0.0) | ·· | ||
| Cancer | |||||||
| No | 177/179 (98.9) | 113/209 (54.1) | ·· | 12/58 (20.7) | ·· | ||
| Yes | 4/4 (100.0) | No participant | ·· | 0/2 (0.0) | ·· | ||
| Other chronic condition | .325 | ·· | |||||
| No | 159/160 (99.4) | 97/184 (52.7) | Reference | 12/55 (21.8) | ·· | ||
| Yes | 24/25 (96.0) | 18/28 (64.3) | 1.49 [0.68–3.27] | 0/7 (0.0) | ·· | ||
Calculation of odds ratio and P value: correlation between close contacts of the same index case taken into account using GEE (exchangeable correlation structure, logit link function).
Abbreviations: GEE, generalized estimating equation; OR, odds ratio.
aChildren aged <12 considered nonsmokers.
bAmong all following conditions, except “other chronic condition.”
Figure 2.
Unadjusted association between characteristics of household members of index cases and seropositivity (bivariable analysis). For calculation of odds ratio, the correlation between household members of the same index case was taken into account using GEEs (exchangeable correlation structure, logit link function). Error bars represent the limits of the 95% confidence interval for the odds ratio. aAdult participants only. bChildren aged <12 considered nonsmokers. Abbreviation: GEEs, generalized estimating equations.
Table 2.
Serology Test Result According to Adherence to Measures Aimed at Decreasing Transmission, Contacts With Other People, and Living Conditions, Stratified by Type of Participant (Unadjusted Results)
| Household Members (n = 302) | Close Contacts Outside the Household (n = 69) | |||||
|---|---|---|---|---|---|---|
| No. (%) Seropositive | Unadjusted OR [95% CI] | P Value | No. (%) Seropositive | Unadjusted OR [95% CI] | P Value | |
| Respect of measures and contacts with other people | ||||||
| Respect of simple hygiene rules (washing hands regularly, sneezing into the elbow, etc.) | .123 | .272 | ||||
| Yes | 114/215 (53.0) | Reference | 8/56 (14.3) | Reference | ||
| Rather yes | 40/74 (54.1) | 1.46 [0.97–2.20] | 4/13 (30.8) | 2.22 [0.54–9.21] | ||
| Rather no or no | 4/9 (44.4) | 2.08 [0.63–6.87] | No participant | ·· | ||
| Respect of social distancing rules (physical distancing, avoid shaking hands or kissing, etc.) | .868 | .114 | ||||
| Yes | 87/157 (55.4) | Reference | 7/53 (13.2) | Reference | ||
| Rather yes | 56/105 (53.3) | 0.99 [0.64–1.52] | 5/15 (33.3) | 2.94 [0.77–11.23] | ||
| Rather no or no | 15/36 (41.7) | 0.77 [0.42–1.43] | 0/1 (0.0) | ·· | ||
| Wearing a mask in public | .657 | .093 | ||||
| No | 79/163 (48.5) | Reference | 5/38 (13.2) | Reference | ||
| Yes, sometimes | 54/98 (55.1) | 1.15 [0.75–1.74] | 2/17 (11.8) | 1.06 [0.21–5.35] | ||
| Yes, always | 27/40 (67.5) | 1.31 [0.70–2.45] | 5/13 (38.5) | 4.36 [1.06–17.83] | ||
| Weekly No. of people who met outside home during the semiconfinement | .009 | .761 | ||||
| 0 | 65/100 (65.0) | Reference | 2/17 (11.8) | Reference | ||
| 1–5 | 73/140 (52.1) | 0.87 [0.53–1.43] | 6/34 (17.7) | 1.26 [0.25–6.35] | ||
| >5 | 21/61 (34.4) | 0.42 [0.22–0.78] | 4/17 (23.5) | 1.86 [0.32–10.75] | ||
| Close contact with people outside the home having symptoms suggestive of COVID-19, No. of people | .456 | .099 | ||||
| 0 | 127/233 (54.5) | Reference | 2/14 (14.3) | Reference | ||
| 1 | 17/34 (50.0) | 1.48 [0.80–2.75] | 5/42 (11.9) | 0.88 [0.15–5.01] | ||
| ≥2 | 16/35 (45.7) | 1.11 [0.63–1.96] | 5/12 (41.7) | 4.36 [0.68–27.99] | ||
| Living conditions and household characteristicsa | ||||||
| Housing typeb | .946 | |||||
| Individual house | 83/158 (52.5) | Reference | ||||
| Apartment, studio, or other | 77/144 (53.5) | 0.98 [0.54–1.79] | ||||
| No. of people in the householdb | .046 | |||||
| 2 | 39/59 (66.1) | Reference | ||||
| 3 | 31/51 (60.8) | 0.82 [0.35–1.93] | ||||
| 4 | 44/74 (59.5) | 0.78 [0.34–1.77] | ||||
| 5 | 33/68 (48.5) | 0.43 [0.17–1.06] | ||||
| ≥6 | 13/50 (26.0) | 0.19 [0.06–0.62] | ||||
| No. of habitable rooms (besides kitchen) in the dwellingc | .441 | |||||
| ≤3 | 35/54 (64.8) | Reference | ||||
| 4–6 | 96/183 (52.5) | 0.67 [0.32–1.40] | ||||
| ≥7 | 29/65 (44.6) | 0.55 [0.21–1.47] | ||||
All participants, including children and teens. Calculation of odds ratio and P value: correlation between close contacts of the same index case taken into account using GEE (exchangeable correlation structure, logit link function).
Abbreviations: COVID-19, coronavirus disease 2019; GEE, generalized estimating equation; OR, odds ratio.
aNot relevant for close contacts outside the household.
bAnswer of the index case taken for all household members.
cAnswer of the index case taken for all household members, except 2 households where information from the index case was missing (mean of answers reported by other household members taken instead).
Adjusted Association of Individual and Household Characteristics With Seropositivity (Multivariable Analysis)
We finally estimated the adjusted association of individual and household characteristics with serology test results among household members (Table 3). The odds of infection were almost 4 times higher in household members aged ≥65 than in the younger age group (adjusted OR, 3.63; 95% CI, 1.05–12.60). The association of current smoking with negative serology observed in bivariable analysis faded in the multivariable model (adjusted OR, 0.73; 95% CI, 0.38–1.39). Although overweight/obesity tended to be associated with higher odds of infection, this association was not statistically significant at the .05 level. In comparison with bivariable analysis, we observed a strengthening of the relationship between the absence of strict adherence to simple hygiene rules and positive serology testing (adjusted OR, 1.80; 95% CI, 1.02–3.17). However, there was no indication of a link with adherence to social distancing rules or mask wearing. The association of a greater number of social contacts during the semiconfinement with lower odds of infection was confirmed in multivariable analysis (adjusted OR, 0.35; 95% CI, 0.16–0.74). On the other hand, close encounters with symptomatic individuals tended to be associated with positive serology, but this tendency was not statistically significant at the .05 level. Household characteristics did not show a significant association with serological test result. Adding characteristics of the index case to the model (age, gender) yielded comparable estimates (results not shown).
Table 3.
Adjusted Association of Individual and Household Characteristics With Serology Test Result Among Household Members
| Adjusted OR [95% CI] | P Value | |
|---|---|---|
| Characteristics of household member | ||
| Age (ref: 20 y–<65 y) | ||
| 6 mo–<20 y | 0.92 [0.54–1.59] | .775 |
| ≥65 y | 3.63 [1.05–12.60] | .042 |
| Gender (ref: male) | ||
| Female | 1.37 [0.90–2.08] | .137 |
| Current smokera (ref: no) | ||
| Yes | 0.73 [0.38–1.39] | .339 |
| Weight status (ref: normal or underweight) | ||
| Overweight or obese | 1.48 [0.90–2.43] | .125 |
| Respect of simple hygiene rules (washing hands regularly, sneezing into the elbow, etc.) (ref: yes) | ||
| Rather yes, rather no, or no | 1.80 [1.02–3.17] | .041 |
| Respect of social distancing rules (physical distancing, avoid shaking hands or kissing, etc.) (ref: yes) | ||
| Rather yes, rather no, or no | 1.06 [0.62–1.82] | .831 |
| Wearing a mask in public (ref: no) | ||
| Yes, sometimes | 1.02 [0.61–1.72] | .926 |
| Yes, always | 0.94 [0.43–2.09] | .885 |
| Weekly No. of people met outside home during semiconfinement (ref: 0) | ||
| 1–5 | 0.70 [0.40–1.21] | .201 |
| >5 | 0.35 [0.16–0.74] | .006 |
| Close contact with people outside home having symptoms suggestive of COVID-19, No. of people (ref: none) | ||
| 1 | 1.29 [0.62–2.67] | .495 |
| ≥2 | 1.72 [0.86–3.45] | .125 |
| Characteristics of household | ||
| Highest education level among adult household members (ref: lower secondary or less) | ||
| Upper secondary | 1.08 [0.21–5.54] | .922 |
| Tertiary | 1.64 [0.34–7.95] | .541 |
| Housing typeb (ref: individual house) | ||
| Apartment, studio, or other | 0.87 [0.39–1.95] | .738 |
| No. of people in the householdb | ||
| 1-person increase | 0.78 [0.56–1.08] | .135 |
| No. of habitable rooms (besides kitchen) in the dwellingc | ||
| 1-room increase | 0.98 [0.76–1.25] | .843 |
| Mean age of participating household membersd | ||
| 1-y increase | 1.00 [0.97–1.04] | .793 |
Multivariable regression model; 291/302 household members included in model. Within-household correlation taken into account using GEE (exchangeable correlation structure, logit link function). The variable “chronic medical conditions,” which was not available for children and teens, was not included in the model.
Abbreviations: COVID-19, coronavirus disease 2019; GEE, generalized estimating equation; OR, odds ratio.
aChildren aged <12 considered nonsmokers.
bAnswer of the index case taken for all household members.
cAnswer of the index case taken for all household members, except 2 households where information from the index case was missing (mean of answers reported by other household members taken instead).
dGiven the association between household size and mean age of participating household members, this variable was included in the model.
Prevalence and Clinical Presentation of Flu-Like Episodes and Use of Health Services
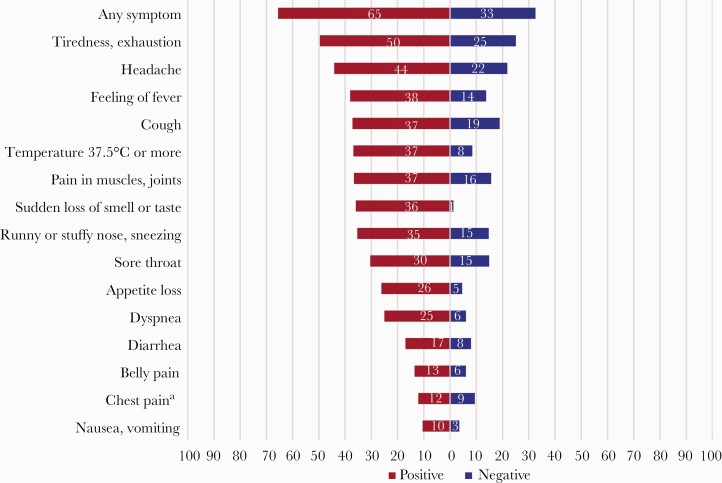
The occurrence of ≥1 flu-like episode since the end of February 2020 was strongly associated with positive serological testing, both in household members (OR, 3.55; 95% CI, 2.37–5.32) (Table 4) and close contacts outside the household (OR, 8.64; 95% CI, 1.77–42.12). The proportion of asymptomatic seropositive individuals (ie, not reporting any flu-like episode) was 21.4% in household members and 16.7% in close contacts outside the household. With the exception of chest pain, all reported symptoms were associated with a positive serology. This was particularly evident in household members mentioning new-onset anosmia or ageusia, of whom 92.8% were seropositive (OR, 6.24; 95% CI, 3.46–11.24). When limiting the analysis to participants not reporting previous nasal or throat swabbing, the strength of the association between symptoms and serology generally increased (Supplementary Table 2). Half the seropositive household members not mentioning prior PCR testing reported tiredness (49.6%) (Figure 3), followed by headache (44.1%), cough (37.1%), fever (36.8%), aching muscles or joints (36.6%), and anosmia or ageusia (35.9%). Gastrointestinal symptoms were infrequent. Half of the seropositive household members (46.3%) reported contact with a medical provider, and 6.3% were hospitalized. Figures were comparable among seropositive close contacts outside the household (41.7% and 8.3%, respectively). However, the hospitalization rate was higher in index cases (14.7%).
Table 4.
Serology Test Result According to Symptoms and Use of Health Services Since the End of February 2020, Stratified by Type of Participant
| Index Cases (n = 219) | Household Members (n = 302) | Close Contacts Outside the Household (n = 69) | |||||
|---|---|---|---|---|---|---|---|
| No. (%) Seropositive | No. (%) Seropositive | Odds Ratio [95% CI] | P Value | No. (%) Seropositive | Odds Ratio [95% CI] | P Value | |
| Symptoms | |||||||
| Flu-like episodes | .000 | .008 | |||||
| 0 | 25/26 (96.2) | 34/117 (29.1) | Reference | 2/39 (5.1) | Reference | ||
| ≥1 | 190/193 (98.5) | 125/184 (67.9) | 3.55 [2.37–5.32] | 10/29 (34.5) | 8.64 [1.77–42.12] | ||
| Cough | .001 | .003 | |||||
| No | 58/58 (100.0) | 84/192 (43.8) | Reference | 5/53 (9.4) | Reference | ||
| Yes | 157/161 (97.5) | 76/110 (69.1) | 2.07 [1.36–3.15] | 7/16 (43.8) | 7.76 [2.02–29.84] | ||
| Runny or stuffy nose, sneezing | .001 | .008 | |||||
| No | 105/107 (98.1) | 93/208 (44.7) | Reference | 5/52 (9.6) | Reference | ||
| Yes | 110/112 (98.2) | 67/94 (71.3) | 2.18 [1.38–3.44] | 7/17 (41.2) | 6.33 [1.64–24.46] | ||
| Sore throat | .049 | .729 | |||||
| No | 137/140 (97.9) | 109/224 (48.7) | Reference | 8/52 (15.4) | Reference | ||
| Yes | 78/79 (98.7) | 51/78 (65.4) | 1.53 [1.00–2.33] | 4/17 (23.5) | 1.27 [0.33–4.84] | ||
| Dyspnea | .000 | .203 | |||||
| No | 123/125 (98.4) | 108/235 (46.0) | Reference | 9/61 (14.8) | Reference | ||
| Yes | 92/94 (97.9) | 52/67 (77.6) | 2.86 [1.74–4.70] | 3/8 (37.5) | 2.82 [0.57–13.97] | ||
| Feeling of fever | .000 | .901 | |||||
| No | 93/95 (97.9) | 87/206 (42.2) | Reference | 9/54 (16.7) | Reference | ||
| Yes | 122/124 (98.4) | 73/96 (76.0) | 2.74 [1.71–4.40] | 3/15 (20.0) | 1.09 [0.27–4.44] | ||
| Temperature ≥37.5°C (measured) | .000 | .007 | |||||
| No | 80/82 (97.6) | 82/209 (39.2) | Reference | 8/63 (12.7) | Reference | ||
| Yes | 135/137 (98.5) | 78/93 (83.9) | 4.64 [2.82–7.65] | 4/6 (66.7) | 12.61 [1.98–80.32] | ||
| Headache | .000 | .001 | |||||
| No | 79/81 (97.5) | 76/180 (42.2) | Reference | 4/52 (7.7) | Reference | ||
| Yes | 136/138 (98.6) | 84/122 (68.9) | 2.14 [1.43–3.19] | 8/17 (47.1) | 10.29 [2.55–41.62] | ||
| Pain in muscles, joints | .000 | .940 | |||||
| No | 86/89 (96.6) | 88/203 (43.4) | Reference | 10/58 (17.2) | Reference | ||
| Yes | 129/130 (99.2) | 72/99 (72.7) | 2.44 [1.61–3.70] | 2/11 (18.2) | 0.94 [0.18–4.84] | ||
| Chest pain | .444 | .018 | |||||
| No | 150/152 (98.7) | 131/258 (50.8) | Reference | 7/58 (12.1) | Reference | ||
| Yes | 65/67 (97.0) | 29/44 (65.9) | 1.23 [0.72–2.09] | 5/11 (45.5) | 5.56 [1.34–23.07] | ||
| Tiredness, exhaustion | .000 | .001 | |||||
| No | 31/33 (93.9) | 62/162 (38.3) | Reference | 3/49 (6.1) | Reference | ||
| Yes | 184/186 (98.9) | 98/140 (70.0) | 2.66 [1.79–3.95] | 9/20 (45.0) | 11.03 [2.64–45.97] | ||
| Appetite loss | .000 | .003 | |||||
| No | 89/93 (95.7) | 104/237 (43.9) | Reference | 7/61 (11.5) | Reference | ||
| Yes | 126/126 (100.0) | 56/65 (86.2) | 4.52 [2.50–8.17] | 5/8 (62.5) | 11.30 [2.28–55.89] | ||
| Nausea, vomiting | .004 | .181 | |||||
| No | 178/182 (97.8) | 138/275 (50.2) | Reference | 11/67 (16.4) | Reference | ||
| Yes | 37/37 (100.0) | 22/27 (81.5) | 2.93 [1.40–6.13] | 1/2 (50.0) | 6.51 [0.42–101.56] | ||
| Diarrhea | .001 | .475 | |||||
| No | 154/157 (98.1) | 123/250 (49.2) | Reference | 10/62 (16.1) | Reference | ||
| Yes | 61/62 (98.4) | 37/52 (71.2) | 2.37 [1.41–3.99] | 2/7 (28.6) | 1.90 [0.33–11.00] | ||
| Belly pain | .002 | .530 | |||||
| No | 175/178 (98.3) | 134/267 (50.2) | Reference | 10/62 (16.1) | Reference | ||
| Yes | 40/41 (97.6) | 26/35 (74.3) | 2.76 [1.44–5.28] | 2/7 (28.6) | 1.75 [0.31–10.01] | ||
| Sudden loss of smell or taste | .000 | .001 | |||||
| No | 74/77 (96.1) | 96/233 (41.2) | Reference | 6/62 (9.7) | Reference | ||
| Yes | 141/142 (99.3) | 64/69 (92.8) | 6.24 [3.46–11.24] | 6/7 (85.7) | 65.25 [5.47–779.10] | ||
| Use of health services | |||||||
| Contact with a medical provider | .000 | .027 | |||||
| No | 28/28 (100.0) | 86/209 (41.2) | Reference | 7/57 (12.3) | Reference | ||
| Yes | 185/189 (97.9) | 74/93 (79.6) | 3.61 [2.19–5.95] | 5/12 (41.7) | 4.62 [1.20–17.86] | ||
| Nasal or throat swabbing to detect SARS-CoV-2 | .000 | .002 | |||||
| No | (a) | 110/240 (45.8) | Reference | 7/63 (11.1) | Reference | ||
| Yes | (a) | 50/62 (80.7) | 2.74 [1.61–4.65] | 5/6 (83.3) | 38.52 [3.95–375.53] | ||
| Test result for SARS-CoV-2b | |||||||
| Negative or unknown | (a) | 5/16 (31.3) | ·· | 4/5 (80.0) | ·· | ||
| Positive | (a) | 45/46 (97.8) | ·· | 1/1 (100.0) | ·· | ||
| Hospitalization | .028 | ·· | |||||
| No | 181/185 (97.8) | 150/291 (51.6) | Reference | 11/68 (16.2) | ·· | ||
| Yes | 32/32 (100.0) | 10/11 (90.9) | 4.96 [1.19–20.62] | 1/1 (100.0) | ·· | ||
| Admission to the ICU | ·· | ·· | |||||
| No | 203/207 (98.1) | 158/300 (52.7) | ·· | 12/69 (17.4) | ·· | ||
| Yes | 10/10 (100.0) | 2/2 (100.0) | ·· | No participant | ·· | ||
| Intubation | ·· | ·· | |||||
| No | 210/214 (98.1) | 160/302 (53.0) | ·· | 12/69 (17.4) | ·· | ||
| Yes | 3/3 (100.0) | No participant | ·· | No participant | ·· | ||
All participants, including children and teens. Calculation of odds ratio and P value: correlation between close contacts of the same index case taken into account using GEEs (exchangeable correlation structure, logit link function).
Abbreviations: GEEs, generalized estimating equations; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aIndex cases all tested positive.
bNucleic acid amplification test.
Figure 3.
Percentage of household members reporting specific symptoms, according to serology test result (household members not reporting prior nasal or throat swabbing to detect severe acute respiratory syndrome coronavirus 2). The correlation between household members of the same index case was taken into account using GEEs (exchangeable correlation structure). aDifference not statistically significant at the .05 level. Abbreviation: GEEs, generalized estimating equations.
DISCUSSION
More than 1 in 2 participants living with a confirmed COVID-19 case developed a serologic response to SARS-CoV-2, while 1 in 5 close contacts outside the household was seropositive. Our findings confirm that households represent high-risk transmission settings [4, 5, 8, 12, 21–23]. The SAR we observed is substantially higher than those reported in previous seroepidemiological studies, including a large nationwide survey conducted in Spain (37.4%) and a retrospective cohort study conducted in Singapore (11%, estimation based on Bayesian modeling) [11–13, 23]. One study disclosed an SAR of 80% in household members of essential workers, but the estimation was based on 30 participants only [10]. Besides serological testing characteristics, differences could be due to variable average household sizes (2.2 members in Switzerland vs 2.6 in Spain) [24], unequal adoption of protective behaviors within households [25], or different levels of confinement. Regarding close contacts outside the household, previous seroepidemiological studies have provided SAR estimations ranging from 0% to 13.7% [12, 14–16, 23]. The heterogeneity of results could reflect different study designs and settings and varying adherence to public health protective recommendations [25]. The strong difference observed between the prevalence in household members and in close contacts outside the home is probably due the fact that contacts at home are closer and last longer than those that occur outside, due to the difficulty of applying social distancing in limited spaces and with family members. Moreover, simple hygiene rules may be more neglected at home, maybe due to a feeling of security.
We found that older household members were at particularly high risk, corroborating the findings of previous research on transmission using NAAT [8, 26, 27]. This association was not found for close contacts outside the home. This suggests that elderly couples are even less able to apply protective measures at home, due to their high level of mutual dependency. There was no difference in infection susceptibility according to gender, which is in line with other works [5, 23, 26]. The impact of smoking on the risk of SARS-CoV-2 infection is a controversial issue [28]. Although household members currently smoking were less frequently positive, this association vanished in multivariable analysis, suggesting that it may be confounded by other factors. The importance of hygiene measures to avoid transmission within the household is confirmed by our observations [29]. Mask wearing in public and respect of social distancing rules, which is particularly difficult when living under the same roof, were not associated with infection risk in households. In contrast, the association of a greater number of social contacts with a lower probability of infection seems surprising in the first place. In fact, our study took place during a period of semiconfinement, during which most people stayed at home, except those who had to go out to work in essential sectors. Our findings thus show that the individual risk of being infected is higher when staying at home than when working outside, with the aim of confinement (or quarantine) being to break the transmission chain. We have thus to accept that this works well but at the price of a higher risk for household members of COVID-19 cases to be infected. Like previous studies, we found an inverse relationship between household size and the proportion of seropositive household members [26, 27]. This seems counterintuitive, as prevalence of infectious diseases is well known to be associated with crowded housing. However, having many in a household decreases mutual dependency and thus decreases close contacts. This association was weakened by inclusion of the mean age of household members in the multivariable model, suggesting that the apparent protective effect of a high number of household members could reflect the fact that large families are, on average, younger. However, disentangling the respective contributions of household size and age distribution of household members remains difficult.
Regarding the clinical presentation of COVID-19, the proportion of asymptomatic seropositive individuals was close to the findings of Pollán and colleagues in Spain (28.5%) [12]. Even if not specific, a large number of symptoms were still associated with SARS-CoV-2 infection, especially new-onset smell and/or taste disturbance, confirming the clinical utility of this symptom in suspicion of COVID-19 [30]. Interestingly, the prevalence of flu-like symptoms was high also in seronegative people, maybe because the first epidemic wave occurred just after the winter, when other respiratory infections were still quite prevalent.
Limitations need to be acknowledged. The Swiss testing policy during the first epidemic wave, which limited diagnostic testing mainly to individuals at increased risk for severe illness, made the sample of index cases not representative of all cases that occurred in the community during this period. Index cases were thus not necessarily the first infected in their household, but those fulfilling testing criteria. However, this would be especially problematic if the purpose were to identify factors associated with infectivity of the index case, which we deliberately avoided.
Incidence of new COVID-19 cases remains high worldwide, and prevention of transmission is, for now, the only way to tackle the pandemic. If concerns regarding the transmission of SARS-CoV-2 in shops, restaurants, and public gatherings are justified, our findings emphasize that the risk of being infected is much higher at home. However, this remains overlooked in collective awareness and public health discourse, precisely because quarantine and confinement are methods used to break the transmission chain. Early testing of the first case in a household is important to support immediate self-isolation within the house. Our results suggest in particular that it is essential for noninstitutionalized elderly couples to receive strong external support for daily basic needs during the infectious period of the index case. Further research is needed to determine the efficacy and acceptability of specific measures aimed at limiting SARS-CoV-2 transmission within households and at motivating early testing and self-isolation.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to warmly thank all study participants for their involvement. This study was made possible by the strong involvement of all the SerocoViD operational team (Julia Baldwin, Giovanna Bonsembiante-Poidomani, Ophélie Hoffmann, Emilie Jendly, Athiththan Kanthasami, Daria Mapelli, Virginie Schlüter, Kevin Schutzbach, Auriane Soris, and Lucie Wuillemin).
Financial support. This work was supported by the operating budget of the Center for Primary Care and Public Health (Unisanté), University of Lausanne, Switzerland, and by contributions of local health authorities (Department of Health and Social Action, Canton of Vaud) and the following Swiss nonprofit institutions: Leenaards Foundation, Fondation pour l’Université de Lausanne. SerocoViD is part of the national Corona Immunitas program coordinated by the Swiss School of Public Health Plus (SSPH+).
Potential conflicts of interest. The authors declare no competing interests. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. J.D. did the statistical analyses and drafted the first version of the manuscript. J.D., M.B., S.G.N., and V.D.A. designed the article, accessed the data, and contributed to the interpretation of data. M.B., S.G.N., and V.D.A. conceived and conducted the study and contributed to drafting sections of the manuscript. J.D., A.B., O.D., S.E., V.F., J.T., C.Z., M.E., A.S.D., S.V., M.B., S.G.N., and V.D.A. participated in the planning of the study and collection of data. G.G. and G.P. were involved in development and validation of the serological test. J.P. and V.R. provided support for planning and performing statistical analyses. E.M. contributed to study design. All authors commented on drafts and read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing. All data and materials used in this work are accessible to researchers upon reasonable request for data sharing to the corresponding author.
References
- 1. Pitzer VE, Cohen T. Household studies provide key insights on the transmission of, and susceptibility to, SARS-CoV-2. Lancet Infect Dis 2020; 20:1103–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Unity studies: early investigation protocols. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations. Accessed 30 June 2020.
- 3. Madewell ZJ, Yang Y, Longini IM, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis of secondary attack rate. JAMA Netw Open 2020; 3:e2031756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis 2020; 26:2465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Y, Song S, Kao Q, et al. Risk of SARS-CoV-2 infection among contacts of individuals with COVID-19 in Hangzhou, China. Public Health 2020; 185:57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at a choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:606–10. [DOI] [PubMed] [Google Scholar]
- 7. Böhmer MM, Buchholz U, Corman VM, et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis 2020; 20:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng H-Y, Jian S-W, Liu D-P, et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med 2020; 180:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caruana G, Croxatto A, Coste AT, et al. Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results. Clin Microbiol Infect 2020; 26:1178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDade TW, McNally EM, Zelikovich AS, et al. High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay. PLoS One 2020; 15:e0237833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silveira MF, Barros AJD, Horta BL, et al. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med 2020; 26:1196–9. [DOI] [PubMed] [Google Scholar]
- 12. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cox RJ, Brokstad KA, Krammer F, Langeland N; Bergen COVID-19 Research Group . Seroconversion in household members of COVID-19 outpatients. Lancet Infect Dis 2021; 21:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown NE, Bryant-Genevier J, Bandy U, et al. Antibody responses after classroom exposure to teacher with coronavirus disease, March 2020. Emerg Infect Dis 2020; 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang H-J, Su Y-Y, Xu S-L, et al. Asymptomatic and symptomatic SARS-CoV-2 infections in close contacts of COVID-19 patients: a seroepidemiological study. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chu VT, Freeman-Ponder B, Lindquist S, et al. Investigation and serologic follow-up of contacts of an early confirmed case-patient with COVID-19, Washington, USA. Emerg Infect Dis 2020; 26:1671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deeks JJ, Dinnes J, Takwoingi Y, et al. ; Cochrane COVID-19 Diagnostic Test Accuracy Group . Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 2020; 6:CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. West EA, Anker D, Amati R, et al. Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int J Public Health 2020; 65:1529–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fenwick C, Croxatto A, Coste AT, et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol 2021; 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 2020; 396:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020; 383:1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng OT, Marimuthu K, Koh V, et al. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis 2021; 21:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. United Nations, Department of Economic and Social Affairs, Population Division. Database on household size and composition 2019. Available at: https://population.un.org/Household/index.html. Accessed 27 August 2020.
- 25. Clark C, Davila A, Regis M, Kraus S. Predictors of COVID-19 voluntary compliance behaviors: an international investigation. Glob Transit 2020; 2:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jing Q-L, Liu M-J, Zhang Z-B, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis 2020; 20:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenberg ES, Dufort EM, Blog DS, et al. COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York state—March 2020. Clin Infect Dis 2020; 71:1953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Usman MS, Siddiqi TJ, Khan MS, et al. Is there a smoker’s paradox in COVID-19? BMJ Evid-Based Med. In press. [DOI] [PubMed] [Google Scholar]
- 29. Wu J, Huang Y, Tu C, et al. Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis 2020; 71:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makaronidis J, Mok J, Balogun N, et al. Seroprevalence of SARS-CoV-2 antibodies in people with an acute loss in their sense of smell and/or taste in a community-based population in London, UK: an observational cohort study. PLoS Med 2020; 17:e1003358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.