Abstract
Background
Acute kidney injury (AKI) is a common and important complication of coronavirus disease 2019 (COVID-19). Further characterization is required to reduce both short- and long-term adverse outcomes.
Methods
We examined registry data including adults with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection admitted to five London Hospitals from 1 January to 14 May 2020. Prior end-stage kidney disease was excluded. Early AKI was defined by Kidney Disease: Improving Global Outcomes creatinine criteria within 7 days of admission. Independent associations of AKI and survival were examined in multivariable analysis. Results are given as odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals.
Results
Among 1855 admissions, 455 patients (24.5%) developed early AKI: 200 (44.0%) Stage 1, 90 (19.8%) Stage 2 and 165 (36.3%) Stage 3 (74 receiving renal replacement therapy). The strongest risk factor for AKI was high C-reactive protein [OR 3.35 (2.53–4.47), P < 0.001]. Death within 30 days occurred in 242 (53.2%) with AKI compared with 255 (18.2%) without. In multivariable analysis, increasing severity of AKI was incrementally associated with higher mortality: Stage 3 [HR 3.93 (3.04–5.08), P < 0.001]. In 333 patients with AKI surviving to Day 7, 134 (40.2%) recovered, 47 (14.1%) recovered then relapsed and 152 (45.6%) had persistent AKI at Day 7; an additional 105 (8.2%) patients developed AKI after Day 7. Persistent AKI was strongly associated with adjusted mortality at 90 days [OR 7.57 (4.50–12.89), P < 0.001].
Conclusions
AKI affected one in four hospital in-patients with COVID-19 and significantly increased mortality. Timing and recovery of COVID-19 AKI is a key determinant of outcome.
Keywords: AKI, CKD, epidemiology, ethnicity, survival analysis
GRAPHICAL ABSTRACT
GRAPHICAL ABSTRACT.
INTRODUCTION
Development of acute kidney injury (AKI) appears common in coronavirus disease 2019 (COVID-19), however, geographical differences in incidence and severity have been described [1–6]. In a meta-analysis of 20 cohorts worldwide, overall prevalence during admission was 17%, ranging from 0.5% to 80.3% [7]. This variation likely reflects differences in sample sizes and cohort characteristics including in monitoring and detection of AKI [8]. Few studies have reported timing and severity of AKI, or temporal relationships to interventions including renal replacement therapy (RRT) [9]. Short follow-up periods have also limited analysis of longer-term survival and kidney recovery. Importantly, more severe AKI has been strongly and consistently associated with short-term risk of death [10, 11], making AKI a potentially pivotal complication in the deteriorating COVID-19 patient. Better understanding of risk factors involved may enable early recognition and use of preventative and therapeutic measures to limit AKI development or progression, reducing overall morbidity and mortality.
The mechanisms involved in kidney damage in COVID-19 are likely to be multifactorial. These potentially include direct viral effects on the kidney [12, 13], systemic immune dysregulation and hypercoagulability [11, 14], as well as traditional risk-factors for primary tubular injury including hypovolaemia, sepsis-related haemodynamic instability and consequences of intensive care unit (ICU) therapies [8, 15–17]. Patient risk factors for COVID-19 AKI are common to those of AKI in other settings, including older age and comorbid diseases [1, 18]. Importantly, while racial and socioeconomic background have been identified as potential risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and adverse outcomes in COVID-19 disease, the influence of these factors on the incidence of COVID-19-associated AKI is unclear despite pre-existing evidence for racial and socioeconomic disparities leading to higher AKI incidence and poorer outcomes outside of COVID-19 [19]. Accordingly, we aimed to define the incidence and severity of AKI during hospital admission, examine trajectories of AKI progression and kidney recovery following hospital discharge, and identify risk factors for AKI and adverse outcomes including the influence of ethnicity and measures of socioeconomic status.
MATERIALS AND METHODS
Study cohort
We included all patients aged 16 years or over admitted to one of five acute hospitals within Barts Health National Health Service (NHS) Trust between 1 January and 13 May 2020 with SARS-CoV-2 infection confirmed on PCR [20]. The first emergency admission encompassing the first positive SARS-CoV-2 test or within 2 weeks of positive outpatient testing was defined as the index admission. For this analysis, patients with no available creatinine data and patients with prior history of end-stage kidney disease (ESKD) were excluded. Follow-up data were available up to 1 December 2020.
Definitions of key variables
AKI was defined and severity staged using the Kidney Disease: Improving Global Outcomes (KDIGO) creatinine criteria, namely an acute ≥0.3 mg/dL rise in serum creatinine within a 48-h window or a ≥1.5-fold increase in creatinine above pre-morbid baseline value [21]. Baseline creatinine was set using the median result from all available blood tests in the 7–365 days before admission. If no prior results were available, baseline creatinine was imputed based on an estimated glomerular filtration rate (eGFR) of 75 mL/min/1.72 m2 or admission creatinine, whichever was lower. Early AKI was defined as AKI criteria being met within the first 7 days of hospital admission. In patients who survived to Day 7, early AKI was further subdivided based on trajectory during admission: recovered (absence of AKI criteria by Day 7 and no further AKI); relapsed (absence of AKI criteria by Day 7, but subsequent re-occurrence of AKI later in admission); and persistent (persistence of AKI criteria at Day 7 or requirement for RRT). Late AKI was defined as AKI occurring only after Day 7. Ethnicity was defined using the NHS ethnic category codes. Relative measures of socioeconomic deprivation were assessed using the English Indices of Deprivation 2020 by matching patient postcode to national index of multiple deprivation (IMD) divided into quintiles within our study population [22]. Baseline comorbid diseases and Hospital Frailty Risk Score (HFRS) were identified by mapping to International Classification of Diseases, Tenth Revision (ICD-10) coding [23, 24]. Pre-existing chronic kidney disease (CKD) defined as baseline eGFR ˂60 mL/min/1.72 m2 was calculated using the last creatinine value available from results earlier than 7 days before hospitalization using the CKD Epidemiology Collaboration formula [25]. ESKD was defined by ICD-10 coding [26]. Rockwood Clinical Frailty Scoring was assessed by the admitting medical team and recorded in the electronic medical records [27]. The median C-reactive protein (CRP) value for the cohort was used as a cut-off for high and low groups. Full definitions of AKI categories and baseline variables are detailed in the Online Supplementary Material.
Outcome measures
The primary outcome was survival to 30 days from time of index COVID-19 hospital admission. We assessed the composite outcome of death, new dialysis and worsened renal function constituting the major adverse kidney event (MAKE) outcome at 90 days (MAKE90) as a secondary outcome [28]. In 90-day survivors, worsened renal function was defined as eGFR <70% of baseline using the last serum creatinine value before 90 days. Other secondary endpoints were 90-day survival, ICU admission, ICU length of stay, duration of organ support on ICU, need for mechanical ventilation, ICU and hospital length of stay, and discharge destination if discharged alive from hospital.
Statistical analyses
A prospective statistical analysis plan was developed [29]. Baseline characteristics are presented as median and interquartile range (IQR) or number and percentage, as appropriate. We compared proportions using Pearson’s chi-square test or Fisher’s exact test and continuous variables using two-sample t-test or Wilcoxon rank-sum or signed rank (for paired data) tests, as appropriate. Multivariable logistic regression was carried out to assess risk factors for development of early AKI. Cox proportional hazards models were used to assess survival comparing AKI versus no AKI adjusted for age and sex. A further multivariable Cox model was developed to assess the effect of pre-defined risk factors described associated with adverse outcomes in COVID-19: ethnicity, IMD quintile, smoking status, body mass index (BMI), diabetes, hypertension (HTN) and CKD. We assessed the effects of an interaction between ethnicity and early AKI on 30-day survival. Logistic regression modelling for the association between early AKI and ICU admission, as well as treatment using mechanical ventilation and RRT were carried out. Survival at 90 days comparing trajectory of AKI was assessed using logistic regression as relapsed and late AKI are not baseline hazards. Effect measures are presented as hazard ratios (HRs) or odds ratios (ORs) with 95% confidence intervals (CIs). All analyses were performed using R version 4.0.2 (R Development Core Team 2020).
Sensitivity analyses
Subgroup analyses were carried out including only patients with known baseline creatinine.
RESULTS
STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines were followed (Supplementary data, Table S1). A total of 1996 patients were included in our cohort, 28 without creatinine results available during their admission and 113 with pre-existing ESKD were excluded from analyses (Supplementary data, Figure S1). A total of 455 out of 1855 patients (24.5%) developed AKI during the first 7 days of admission. Of these, 200 (44.0%) had Stage 1, 90 (19.8%) had Stage 2 and 165 (36.3%) had Stage 3 AKI.
Early AKI
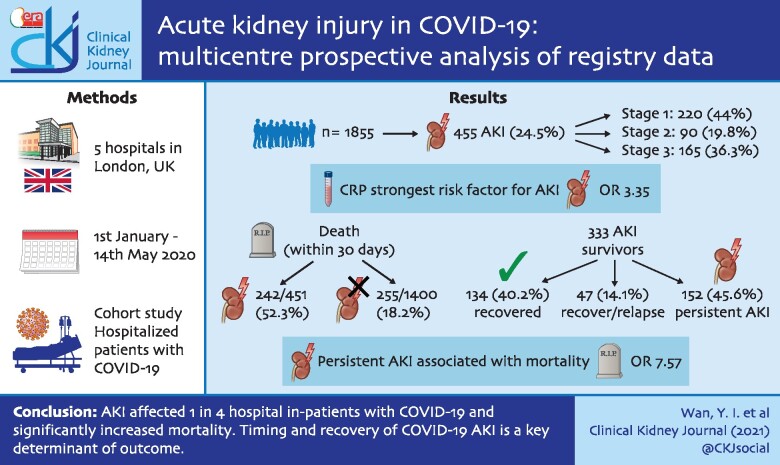
Baseline patient characteristics are presented in Table 1. Patients with early AKI were older (median age 71 versus 62 years), and greater proportions were male, more deprived and had comorbid disease. Baseline creatinine values were available for 1179/1855 patients, the remainder were imputed. Excluding imputed values, baseline kidney function was worse in the early AKI group [median eGFR 65.4 (42.3–82.9) versus 74.3 (56.9–91.6) mL/min/1.72 m2, P < 0.001]. A significantly greater proportion of patients with Black ethnicity developed early AKI, which tended to be more severe (Table 1). Patients with early AKI had higher CRP during admission [median 249.0 (137.0–347.3) versus 123.0 (62.0–213.8) mg/L, P < 0.001]. In a multivariable analysis (Figure 1), the risk of early AKI was significantly higher in older patients [OR 1.13 (1.02–1.25), P = 0.02] and those with peak CRP >145 [OR 3.35 (2.53–4.47) mg/L, P < 0.001]. The comorbidities most strongly associated with early AKI were diabetes [OR 1.50 (1.12–2.02), P = 0.007] and congestive heart failure [CHF; OR 1.47 (1.01–2.13), P = 0.04].
Table 1.
Baseline characteristic table by stages of early AKI compared with no early AKI
| All | No early AKI | Early AKI |
Early AKI by stage |
|||||
|---|---|---|---|---|---|---|---|---|
| All | P-value | Stage 1 | Stage 2 | Stage 3 | P-value | |||
| N | 1855 | 1400 | 455 | 200 | 90 | 165 | ||
| Age (years) Median (IQR) | 65 (51–79) | 62 (48–77) | 71 (60–82) | <0.001 | 74 (62–84) | 74 (60–85) | 66 (58–77) | <0.001 |
| Male (%) | 1122 (60.5) | 820 (58.6) | 302 (66.4) | 0.004 | 131 (65.5) | 56 (62.2) | 115 (69.7) | 0.02 |
| Ethnic group | 0.001 | <0.001 | ||||||
| Asian or Asian British | 491 (26.5) | 395 (28.2) | 96 (21.1) | – | 43 (21.5) | 13 (14.4) | 40 (24.2) | – |
| Black or Black British | 299 (16.1) | 203 (14.5) | 96 (21.1) | – | 34 (17.0) | 15 (16.7) | 47 (28.5) | – |
| Mixed and Other | 147 (7.9) | 118 (8.4) | 29 (6.4) | – | 15 (7.5) | 5 (5.6) | 9 (5.5) | – |
| Unknown | 253 (13.6) | 189 (13.5) | 64 (14.1) | – | 26 (13.0) | 14 (15.6) | 24 (14.5) | – |
| White | 665 (35.8) | 495 (35.4) | 170 (37.4) | – | 82 (41.0) | 43 (47.8) | 45 (27.3) | – |
| IMD quintile (n = 1840) | 0.20 | 0.70 | ||||||
| 1 (most deprived) | 368 (20.0) | 271 (19.5) | 97 (21.6) | – | 37 (18.6) | 20 (22.7) | 40 (24.5) | – |
| 2 | 366 (19.9) | 265 (19.1) | 101 (22.4) | – | 44 (22.1) | 19 (21.6) | 38 (23.3) | – |
| 3 | 368 (20.0) | 292 (21.0) | 76 (16.9) | – | 38 (19.1) | 15 (17.0) | 23 (14.1) | – |
| 4 | 366 (19.9) | 282 (20.3) | 84 (18.7) | – | 39 (19.6) | 16 (18.2) | 29 (17.8) | – |
| 5 (least deprived) | 372 (20.2) | 280 (20.1) | 92 (20.4) | – | 41 (20.6) | 18 (20.5) | 33 (20.2) | – |
| Smoking (n = 1572) | 153 (9.7) | 122 (10.3) | 31 (7.9) | 0.19 | 16 (9.3) | 7 (9.1) | 8 (5.6) | 0.34 |
| Comorbidities [n = 1572] | ||||||||
| Moderate to severe CKD | 248 (15.8) | 132 (11.2) | 116 (29.6) | <0.001 | 46 (26.7) | 23 (29.9) | 47 (32.9) | <0.001 |
| Obesity | 366 (23.3) | 273 (23.1) | 93 (23.7) | 0.87 | 37 (21.5) | 26 (33.8) | 30 (21.0) | 0.14 |
| Ischaemic heart disease | 288 (18.3) | 202 (17.1) | 86 (21.9) | 0.04 | 36 (20.9) | 15 (19.5) | 35 (24.5) | 0.13 |
| Myocardial infarction | 150 (9.5) | 103 (8.7) | 47 (12.0) | 0.07 | 19 (11.0) | 11 (14.3) | 17 (11.9) | 0.23 |
| CHF | 209 (13.3) | 133 (11.3) | 76 (19.4) | <0.001 | 32 (18.6) | 14 (18.2) | 30 (21.0) | 0.001 |
| Diabetes | 585 (37.2) | 394 (33.4) | 191 (48.7) | <0.001 | 79 (45.9) | 40 (51.9) | 72 (50.3) | <0.001 |
| HTN | 1120 (71.2) | 810 (68.6) | 310 (79.1) | <0.001 | 135 (78.5) | 67 (87.0) | 108 (75.5) | <0.001 |
| Peripheral vascular disease | 110 (7.0) | 74 (6.3) | 36 (9.2) | 0.07 | 18 (10.5) | 8 (10.4) | 10 (7.0) | 0.14 |
| CEVD | 271 (17.2) | 179 (15.2) | 92 (23.5) | <0.001 | 44 (25.6) | 20 (26.0) | 28 (19.6) | 0.001 |
| COPD | 365 (23.2) | 281 (23.8) | 84 (21.4) | 0.37 | 42 (24.4) | 15 (19.5) | 27 (18.9) | 0.48 |
| Liver disease | 131 (7.1) | 99 (7.1) | 32 (7.0) | 1 | 15 (7.5) | 10 (11.1) | 7 (4.2) | 0.23 |
| Dementia | 163 (10.4) | 111 (9.4) | 52 (13.3) | 0.04 | 27 (15.7) | 13 (16.9) | 12 (8.4) | 0.01 |
| Cancer | 136 (8.7) | 100 (8.5) | 36 (9.2) | 0.74 | 22 (12.8) | 9 (11.7) | 5 (3.5) | 0.02 |
| Charlson comorbidity index (n = 1572) | <0.001 | <0.001 | ||||||
| 0 | 469 (29.8) | 389 (33.0) | 80 (20.4) | – | 38 (22.1) | 8 (10.4) | 34 (23.8) | – |
| 1–2 | 603 (38.4) | 470 (39.8) | 133 (33.9) | – | 57 (33.1) | 25 (32.5) | 51 (35.7) | – |
| 3–4 | 276 (17.6) | 179 (15.2) | 97 (24.7) | – | 36 (20.9) | 29 (37.7) | 32 (22.4) | – |
| ≥5 | 224 (14.2) | 142 (12.0) | 82 (20.9) | – | 41 (23.8) | 15 (19.5) | 26 (18.2) | – |
| Rockwood frailty score (n = 782) | 0.04 | 0.10 | ||||||
| 1–2 (very fit, well) | 92 (11.8) | 73 (12.9) | 19 (8.8) | – | 11 (10.3) | 0 (0.0) | 8 (11.8) | – |
| 3–4 (managing well, vulnerable) | 283 (36.2) | 207 (36.6) | 76 (35.0) | – | 37 (34.6) | 13 (31.0) | 26 (38.2) | – |
| 5–6 (mildly to severely frail) | 338 (43.2) | 244 (43.2) | 94 (43.3) | – | 45 (42.1) | 24 (57.1) | 25 (36.8) | – |
| 8–9 (very severely frail, terminally ill) | 69 (8.8) | 41 (7.3) | 28 (12.9) | – | 14 (13.1) | 5 (11.9) | 9 (13.2) | – |
| HFRS (n = 1572) | <0.001 | <0.001 | ||||||
| <5 (low risk) | 724 (46.1) | 623 (52.8) | 101 (25.8) | – | 47 (27.3) | 13 (16.9) | 41 (28.7) | – |
| 5–15 (intermediate risk) | 435 (27.7) | 284 (24.1) | 151 (38.5) | – | 57 (33.1) | 31 (40.3) | 63 (44.1) | – |
| ≥15 (high risk) | 413 (26.3) | 273 (23.1) | 140 (35.7) | – | 68 (39.5) | 33 (42.9) | 39 (27.3) | – |
| eGFR (mL/min/1.72 m2) | – | |||||||
| Baseline median (IQR) (n = 1179) | 72.0 (53.8–89.9) | 74.3 (56.9–91.6) | 65.4 (42.3–82.9) | <0.001 | 64.6 (42.6–83.0) | 68.3 (55.4–83.6) | 61.4 (36.5–82.5) | <0.001 |
| <60 mL/min/1.72 m2 (n = 1179) | 394 (33.4) | 258 (29.5) | 136 (44.9) | <0.001 | 63 (44.4) | 20 (37.0) | 53 (49.5) | <0.001 |
| Last median (IQR) | 78.5 (51.4–100.0) | 87.1 (65.7–103.7) | 39.3 (20.7–67.5) | <0.001 | 54.3 (36.7–76.7) | 36.9 (26.7–63.6) | 19.5 (11.0–40.6) | <0.001 |
| <60 mL/min/1.72 m2 | 578 (31.2) | 267 (19.1) | 311 (68.4) | <0.001 | 109 (54.5) | 65 (72.2) | 137 (83.0) | <0.001 |
| During admission | ||||||||
| Peak CRP mg/L median (IQR) (n = 1646) | 145.0 (72.0–254.0) | 123.0 (62.0–213.8) | 249.0 (137.0–347.3) | <0.001 | 215.0 (102.0–326.0) | 189.0 (106.0–279.0) | 328.0 (243.0–439.0) | <0.001 |
| Peak CRP mg/L > 145 (n = 1646) | 822 (49.9) | 536 (42.7) | 286 (73.0) | <0.001 | 115 (65.3) | 49 (62.0) | 122 (89.1) | <0.001 |
| ICU | ||||||||
| ICU admission | 338 (18.2) | 172 (12.3) | 166 (36.5) | <0.001 | 48 (24.0) | 21 (23.3) | 97 (58.8) | <0.001 |
| Invasive mechanical ventilation | 267 (79.0) | 114 (66.3) | 153 (92.2) | <0.001 | 41 (85.4) | 19 (90.5) | 93 (95.9) | <0.001 |
| RRT | 83 (24.6) | 9 (5.2) | 74 (44.6) | <0.001 | 0 (0.0) | 0 (0.0) | 74 (76.3) | <0.001 |
| ICU length of stay median (IQR) | 8.5 (4.0–15.0) | 7.9 (4.0–13.0) | 10.0 (4.5–16.4) | 0.03 | 9.5 (4.8–16.0) | 6.0 (3.0–15.0) | 10.0 (5.9–18.0) | 0.09 |
| Total number of organ systems | <0.001 | <0.001 | ||||||
| 0 | 3 (0.9) | 2 (1.2) | 1 (0.6) | – | 0 (0.0) | 0 (0.0) | 1 (1.0) | – |
| 1 | 13 (3.8) | 7 (4.1) | 6 (3.6) | – | 5 (10.4) | 1 (4.8) | 0 (0.0) | – |
| 2 | 239 (70.7) | 152 (88.4) | 87 (52.4) | – | 42 (87.5) | 20 (95.2) | 25 (25.8) | – |
| 3 | 83 (24.6) | 11 (6.4) | 72 (43.4) | – | 1 (2.1) | 0 (0.0) | 71 (73.2) | – |
| Hospital length of stay median (IQR) | 7.0 (4.0–13.0) | 6.0 (3.0–11.0) | 10.0 (5.0–17.0) | <0.001 | 10.0 (5.0–17.0) | 8.0 (4.0–14.0) | 10.5 (6.0–19.0) | <0.001 |
| Outcomes | ||||||||
| Died | 574 (30.9) | 309 (22.1) | 265 (58.2) | <0.001 | 99 (49.5) | 56 (62.2) | 110 (66.7) | <0.001 |
| Days to death Median (IQR) | 9.0 (4.0–18.0) | 10.0 (4.0–20.0) | 8.0 (4.0–15.0) | 0.05 | 9.0 (4.0–15.0) | 6.5 (4.0–16.3) | 8.5 (5.0–14.0) | 0.20 |
| Died within 30 days | 497 (26.8) | 255 (18.2) | 242 (53.2) | <0.001 | 87 (43.5) | 51 (56.7) | 104 (63.0) | <0.001 |
| Died within 90 days | 549 (29.6) | 291 (20.8) | 258 (56.7) | <0.001 | 95 (47.5) | 53 (58.9) | 110 (66.7) | <0.001 |
| MAKE90 | 610 (32.9) | 305 (21.8) | 305 (67.0) | <0.001 | 104 (52.0) | 55 (61.1) | 146 (88.5) | <0.001 |
| Survivors who required RRT | 30 (4.9) | 0 (0.0) | 30 (9.8) | <0.001 | 0 (0.0) | 0 (0.0) | 30 (20.5) | <0.001 |
| Final eGFR <70% of baseline | 37 (6.1) | 14 (4.6) | 23 (7.5) | 0.18 | 9 (8.7) | 2 (3.6) | 12 (8.2) | 0.25 |
| Discharge destination (n = 1343) | <0.001 | <0.001 | ||||||
| Care home or equivalent | 56 (4.2) | 42 (3.7) | 14 (6.9) | – | 6 (5.6) | 7 (16.7) | 1 (1.9) | – |
| Health-related institution | 83 (6.2) | 60 (5.3) | 23 (11.3) | – | 12 (11.2) | 5 (11.9) | 6 (11.1) | – |
| Usual place of residence | 1175 (87.5) | 1016 (89.1) | 159 (78.3) | – | 86 (80.4) | 26 (61.9) | 47 (87.0) | – |
| Hospice or equivalent | 4 (0.3) | 2 (0.2) | 2 (1.0) | – | 1 (0.9) | 1 (2.4) | 0 (0.0) | – |
| Temporary place of residence | 25 (1.9) | 20 (1.8) | 5 (2.5) | – | 2 (1.9) | 3 (7.1) | 0 (0.0) | – |
Cohort baseline characteristics grouped by early AKI status, n (%) unless otherwise stated. Total n = 1855 unless otherwise stated. P-values based on chi-square (for categorical) or Kruskal–Wallis test (for continuous) comparing early AKI all or by stage to no early AKI. CEVD, cerebral vascular disease; COPD, chronic obstructive pulmonary disease.
FIGURE 1:
Forest plots comparing baseline risk factors for development of early AKI. IMD (1, most deprived, as reference), obesity defined as BMI ≥30 kg/m2, White ethnicity as reference, CKD defined as baseline eGFR <60 mL/min/1.72 m2. CEVD, cerebral vascular disease. Age modelled per 10-year increment. Effect sizes are shown as OR with 95% CIs. This model includes 1397 patients who had complete data on all covariates with 334 AKI events.
Survival
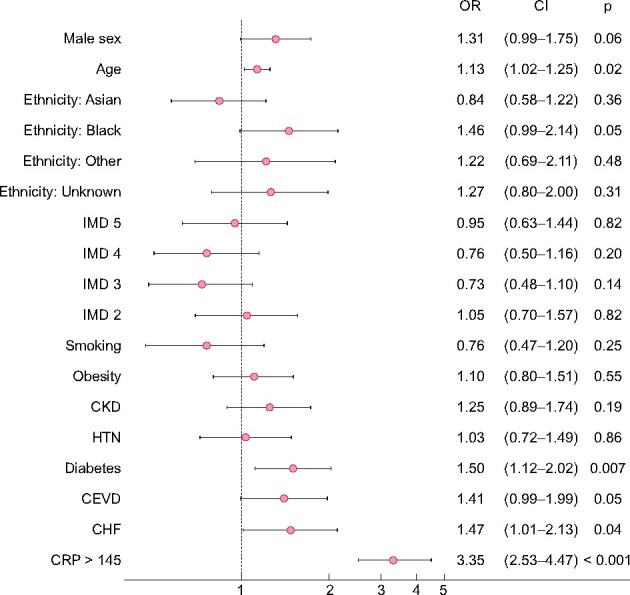
A total of 497 (26.8%) patients died within 30 days of admission, 242 (53.2%) who developed early AKI compared with 255 (18.2%) who did not (Supplementary data, Table S2). Patients with early AKI had a significantly higher age- and sex-adjusted risk of death than those who did not (Supplementary data, Figure S3). Risk increased with increasing severity of AKI: Stage 1 [HR 1.88 (1.47–2.40), P < 0.001], Stage 2 [HR 2.79 (2.06–3.77), P < 0.001] and Stage 3 [HR 4.25 (3.37–5.35), P < 0.001] (Supplementary data, Figure S3). After inclusion of pre-specified confounders including ethnicity, IMD, smoking history, BMI ≥30 kg/m2, diabetes, HTN and CKD in a multivariable survival analysis, the association between early AKI and death persisted: Stage 1 [HR 1.92 (1.48–2.49), P < 0.001], Stage 2 [HR 2.75 (1.97–3.85), P < 0.001] and Stage 3 [HR 3.93 (3.04–5.08), P < 0.001] (Figures 2 and 3).
FIGURE 2:
Forest plots comparing 30-day survival by stage of early AKI compared with no early AKI, results from multivariable analysis. IMD (1, most deprived, as reference), obesity defined as BMI ≥30 kg/m2, White ethnicity as reference, CKD, defined as baseline eGFR <60 mL/min/1.72 m2. Age modelled per 10-year increment. Effect sizes shown as HRs with 95% CIs. This model includes 1561 patients who had complete data on all covariates with 442 deaths by Day 30.
FIGURE 3:
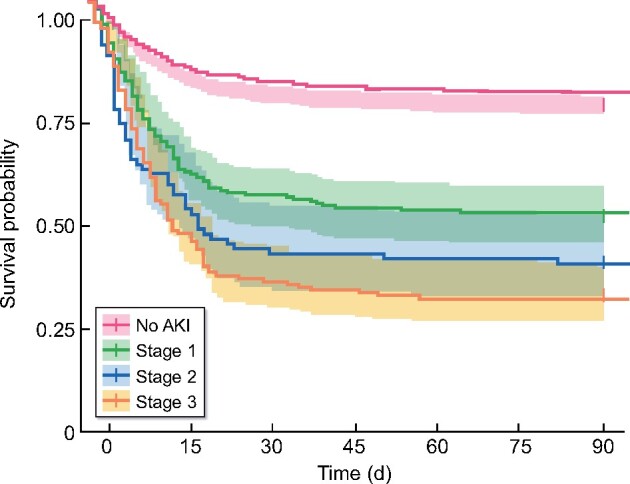
Kaplan–Meier plot showing survival to 90 days by stage of early AKI.
Trajectories of early AKI and late AKI
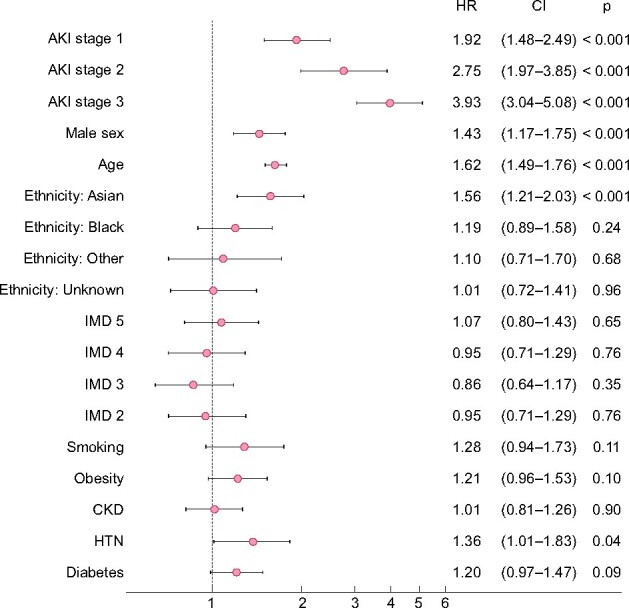
Of 333 patients who developed early AKI and were still alive at Day 7 after admission, 134 (40.2%) recovered, 47 (14.1%) relapsed and 152 (45.6%) had persistent AKI at Day 7. A total of 105 (8.2%) patients who did not have early AKI and still alive at Day 7 developed late AKI after this timepoint. Baseline characteristics are presented in Supplementary data, Table S3. Patients with persistent or relapsed trajectories were younger (median age 65 years persistent, 72 years relapsed and 74 years recovered). Compared with other ethnic groups, a greater proportion of Asian patients with early AKI had persistent or relapsed AKI than recovered (Supplementary data, Figure S6). Patients with persistent or relapsed AKI had higher CRP values (median 330.0 mg/L persistent, 199.0 mg/L relapsed and 179.0 mg/L recovered). Baseline risk factors for early AKI were also more frequent in patients with late compared with no AKI. In multivariable logistic regression, survival to 90 days was worst for persistent AKI [OR 7.57 (4.50–12.89), P < 0.001] (Supplementary data, Figure S7).
Secondary outcomes
There was no clear evidence for any interaction between ethnicity and early AKI on 30-day survival (P = 0.60). Patients with early AKI had higher serum creatinine values throughout admission, with an increasing trend with increasing severity of AKI (Supplementary data, Figure S2A, B, E and F). Compared with baseline, creatinine values on discharge were significantly higher in patients with Stage 3 AKI (median 2.0 versus 1.1, P < 0.001), whereas for patients without AKI they were significantly lower (median 0.8 versus 0.9, P < 0.001) (Supplementary data, Figure S2A and C). Where available (n = 721) post-discharge creatinine closest to Day 90 remained significantly lower than baseline (0.9 versus 1.0), whereas values in patients who experienced early AKI did not significantly differ from baseline (Supplementary data, Figure S2B and D). Patients with increasing severity of early AKI had lower age- and sex-adjusted survival and worse MAKE90 outcomes (Supplementary data, Figures S4 and S5). MAKE90 outcomes were principally driven by mortality and need for RRT in Stage 3 AKI (Table 1). Of 30 patients who required RRT and survived hospital admission, 5 remained dialysis-dependent at discharge. Admission to ICU occurred in 338/1853 (18.2%) and need for ICU admission, and mechanical ventilation on ICU were all significantly associated with incidence and severity of early AKI (Supplementary data, Figures S8 and S9). Of 338 patients admitted to ICU, 166 (49.1%) had early AKI with a further 36 (10.6%) developing late AKI after Day 7. In the ICU, 92.2% of patients with early AKI received invasive mechanical ventilation compared with 66.3% without (Table 1). Patients with early AKI had longer hospital and ICU lengths of stay and lower proportions discharged to their usual place of residence (Table 1).
Sensitivity analyses
Analyses were repeated in 1179 patients with measured baseline creatinine values (Supplementary data, Table S4 and Figures S10–S12). Overall associations of AKI were similar. However, baseline CKD became a significant association of development of early AKI [OR 1.64 (1.13–2.38), P = 0.009]. Associations with survival were similar, although in these analyses, the risk of death associated with AKI Stage 2 was comparatively greater.
DISCUSSION
In our cohort of the first wave hospitalized patients, one in four COVID-19 patients developed early AKI and a further 8% developed late AKI. Over a third of early AKI cases were of Stage 3 severity using KDIGO grading. Importantly, adverse outcomes were strongly associated with increased severity and persistence of AKI. The majority of early AKI was persistent, meaning 45.6% of early AKI patients surviving to Day 7 fulfilled criteria for AKI by the continued presence of a serum creatinine ≥1.5-fold baseline or need for RRT [21]. Importantly, persistent AKI was most strongly associated with ongoing risk of death. In addition, after Day 7, relapsed AKI after initial recovery and late AKI were both associated with worse outcomes compared with recovered early AKI.
Rates of early COVID-19-associated AKI in this study are similar to previous studies in Europe and the USA [8, 15, 30]. As in many previous studies, early AKI was associated with poorer short-term survival [7, 30–32]; however, severity and duration of AKI are clearly interlinked with aspects of the underlying COVID-19 disease process. This supports a comparative study of COVID-19 and non-COVID-19 hospitalized patients, which demonstrated a higher incidence of AKI and a disproportionate burden of severe AKI in COVID-19 patients [33].
Baseline risk factors for early AKI such as older age, male sex and diabetes in this study support those of previous studies for both COVID-19 and non-COVID-19 AKI [1, 11, 15, 34]. However, we found that different demographic and comorbidity profiles were associated with higher risk of COVID-19 AKI. In particular, male sex and diabetes were associated with greater adjusted risk, suggesting that AKI could be a complication partially mediating risk of death in these subgroups previously identified as being at higher risk of death in COVID-19. We also demonstrate that patients of Black ethnicity were at higher risk of developing AKI and more severe AKI as shown in other cohorts; this may be linked to a higher prevalence of CKD in Black populations, as this association disappeared in favour of CKD when only patients with known baseline creatinine were considered [9]. Similarly, more persistent or relapsing AKI trajectories were also seen in Asian patients who developed early AKI, reflecting a less benign course of AKI in this group. Furthermore, the prevalence of diabetes and CKD were significantly greater at younger ages in Asian and Black patients, respectively, potentially increasing risk of AKI in these populations. These observations suggest that patients of Black and Asian backgrounds could in part be at greater risk of death in COVID-19 due to greater prevalence of measured and unmeasured risk factors for development and progression of AKI.
Our finding that high CRP values were associated with development, greater severity and worse trajectories of AKI support hypotheses underlying hyperinflammatory and cytokine release syndrome-mediated damage to kidneys in COVID-19 [9, 14]. Some of this dysregulated immune response may be mediated through lung–kidney cross-talk and associations with other markers of systemic disease such as ferritin and d-dimer, and measures of severity of pulmonary disease could help elucidate pathophysiological mechanisms involved [35]. Of particular importance would be if these processes were modifiable through management strategies such as lung-protective ventilation [15].
As the impact of COVID-19 continues with surges of cases, limited hospital resources including dialysis availability need to be taken into consideration. Identifying and classifying AKI by timing and trajectory may help in tailoring patient monitoring, initiation of AKI prevention and treatment strategies, and timely consideration of patients who will benefit from RRT [30, 31].
Little is known currently about long-term outcomes and kidney recovery after COVID-19 AKI, with only a small number of studies reporting post-hospital outcomes [36]. Reported recovery of kidney function and dependence on RRT following hospital admission has been variable, with recovery at 41–65% and RRT dependence at 8–33% [37–39]. While the majority of patients in our study who received RRT and survived recovered independent kidney function, and the number of patients with worsened kidney function at follow-up was small, assessment of kidney function in COVID-19 survivors is likely to be complex. In individual patients, there was considerable variability in serum creatinine at follow-up, with some, both with and without early AKI, developing new significantly elevated creatinine compared with baseline. Conversely, many other patients, in all AKI categories, had creatinine levels substantially lower than baseline both at hospital discharge and afterwards. This suggests that both worsened renal function and substantial loss of muscle mass (and creatinine generation) may be occurring in COVID-19 survivors, potentially confounding assessment of kidney function by serum creatinine in COVID-19 survivors. Longer-term follow-up is required to better understand recovery mechanisms for COVID-19 AKI, which may differ from other forms of AKI [30]. This may require methods for assessing renal function that are less confounded by the effects of prolonged major illness, such as cystatin-c [40].
Strengths and limitations
This study comprises a comprehensive analysis of the epidemiology and timing of COVID-19-associated AKI in a large and ethnically diverse urban population. The detailed dataset allowed inclusion of multiple demographic and baseline risk factors into multivariable analyses. However, more data on differences in clinical management, medication use including pre-existing renin–angiotensin system blockade, ventilation and haemodynamic parameters could help to identify specific AKI causes. The follow-up period was longer compared with the majority of previous reports, however important long-term outcomes such as development and progression of CKD and ESKD over the following years are not yet available. Definitions of AKI were based only on biochemistry results and there were no data on urine output or other markers of kidney health including proteinuria. Furthermore, imputation of baseline creatinine values was required for 675 (36.4%) patients lacking pre-admission results; however, this was a much smaller proportion compared with many other AKI studies and we carried out a sensitivity analysis excluding those with unknown baseline. Finally, while AKI is associated with adverse outcomes and we hypothesize that it could be an important pathway in mediating risk of death and severity of illness in COVID-19, the direction of causality in these associations cannot be proven in an observational study.
CONCLUSIONS
Characterization of COVID-19 AKI by timing and trajectory could help risk stratification and target monitoring, prevention and treatment strategies. This study highlights the continued need for vigilance and monitoring of AKI throughout all stages of COVID-19, especially in subgroups with higher risk of death in the disease. Finally, longer-term follow-up and better understanding of kidney recovery after COVID-19 is warranted as outcomes are currently unclear.
DATA AVAILABILITY STATEMENT
The statistical analysis plan can be accessed online. The authors will be happy to consider additional analyses of the anonymized dataset on request. The need for stringent measures to prevent re-identification of individuals within a discrete geographical location and limited time-period, however, preclude sharing of patient-level dataset in a GDPR compliant form.
ETHICAL APPROVAL
Study approval was obtained from the Health Research Authority and protocols reviewed by the Research Ethics committee. No patient-level consent was required for this study as it involved the aggregated analysis of an anonymized dataset collated by members of the direct care team.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
AUTHORS’ CONTRIBUTIONS
Y.I.W. and J.R.P. carried out study concept, design and manuscript writing, and also ethics application and approvals. Y.I.W., Z.B. and J.R.P wrote the study protocol and analysis plan. J.R.P. is responsible for data extraction. Y.I.W. and Z.B. carried out data analysis. All authors were involved in critical review of findings and review of the final submission.
CONFLICT OF INTEREST STATEMENT
All authors have completed the ICMJE uniform disclosure form and have no relevant financial or non-financial interests associated with the submitted work to disclose. The results presented in this article have not been published previously in whole or part, except in abstract form.
Supplementary Material
Contributor Information
Yize I Wan, William Harvey Research Institute, Queen Mary University of London, London, UK; Adult Critical Care Unit, Royal London Hospital, Barts Health NHS Trust, London, UK.
Zuzanna Bien, William Harvey Research Institute, Queen Mary University of London, London, UK; Adult Critical Care Unit, Royal London Hospital, Barts Health NHS Trust, London, UK.
Vanessa J Apea, Blizard Institute, Queen Mary University of London, London, UK; Department of Infection and Immunity, Royal London Hospital, Barts Health NHS Trust, London, UK.
Chloe M Orkin, Blizard Institute, Queen Mary University of London, London, UK; Department of Infection and Immunity, Royal London Hospital, Barts Health NHS Trust, London, UK.
Rageshri Dhairyawan, Blizard Institute, Queen Mary University of London, London, UK; Department of Infection and Immunity, Royal London Hospital, Barts Health NHS Trust, London, UK.
Christopher J Kirwan, William Harvey Research Institute, Queen Mary University of London, London, UK; Adult Critical Care Unit, Royal London Hospital, Barts Health NHS Trust, London, UK.
Rupert M Pearse, William Harvey Research Institute, Queen Mary University of London, London, UK; Adult Critical Care Unit, Royal London Hospital, Barts Health NHS Trust, London, UK.
Zudin A Puthucheary, William Harvey Research Institute, Queen Mary University of London, London, UK; Adult Critical Care Unit, Royal London Hospital, Barts Health NHS Trust, London, UK.
John R Prowle, William Harvey Research Institute, Queen Mary University of London, London, UK; Adult Critical Care Unit, Royal London Hospital, Barts Health NHS Trust, London, UK.
REFERENCES
- 1. Cui X, Yu X, Wu Xet al. Acute kidney injury in patients with the coronavirus disease 2019: a multicenter study. Kidney Blood Press Res 2020; 45: 612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasselli G, Zangrillo A, Zanella Aet al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323: 1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan Met al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu Cet al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu Jet al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farouk SS, Fiaccadori E, Cravedi Pet al. COVID-19 and the kidney: what we think we know so far and what we don’t. J Nephrol 2020; 33: 1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robbins-Juarez SY, Qian L, King KLet al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep 2020; 5: 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gabarre P, Dumas G, Dupont Tet al. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med 2020; 46: 1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsch JS, Ng JH, Ross DWet al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98: 209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du Ret al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng Y, Luo R, Wang Ket al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su H, Yang M, Wan Cet al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larsen CP, Bourne TD, Wilson JDet al. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep 2020; 5: 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Xiao M, Zhang Set al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med 2020; 382: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ronco C, Reis T, Husain-Syed F.. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med 2020; 8: 738–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanley B, Naresh KN, Roufosse Cet al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe 2020; 1: e245–e253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santoriello D, Khairallah P, Bomback ASet al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 2020; 31: 2158–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhatraju PK, Ghassemieh BJ, Nichols Met al. Covid-19 in critically ill patients in the seattle region—case series. N Engl J Med 2020; 382: 2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grams ME, Matsushita K, Sang Yet al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol 2014; 25: 1834–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Apea VJ, Wan YI, Dhairyawan Ret al. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. BMJ Open 2021; 11: e042140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120: c179–184 [DOI] [PubMed] [Google Scholar]
- 22. Office for National Statistics (ONS). English Indices of Deprivation 2019. 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019/ (30 December 2020, date last accessed)
- 23. Gilbert T, Neuburger J, Kraindler Jet al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018; 391: 1775–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quan H, Sundararajan V, Halfon Pet al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139 [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crellin E, Mansfield KE, Leyrat Cet al. Clinical Code List—ICD-10—End-Stage Renal Disease. London: London School of Hygiene & Tropical Medicine, 2017 [Google Scholar]
- 27. Rockwood K, Song X, MacKnight Cet al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Billings FT, Shaw AD.. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 2014; 127: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wan YI, Bien Z, Prowle JR.. Acute Kidney Injury in COVID-19: Secondary Analysis of Prospective Data from the EthICAL Study (Statistical Analysis Plan). https://qmro.qmul.ac.uk/xmlui/handle/123456789/69987 (accessioned date: 26 January 2021)
- 30. Nadim MK, Forni LG, Mehta RLet al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) workgroup. Nat Rev Nephrol 2020; 16: 747–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. NICE: COVID-19 Rapid Guideline: Acute Kidney Injury in Hospital NICE Guideline [NG175]. https://www.nice.org.uk/guidance/ng175/chapter/5-Detecting-and-investigating-AKI-in-patients-with-suspected-or-confirmed-COVID-19 (2 October 2020, date last accessed) [PubMed]
- 32. Qian JY, Wang B, Liu BC.. Acute kidney injury in the 2019 novel coronavirus disease. Kidney Dis (Basel) 2020; 323: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fisher M, Neugarten J, Bellin Eet al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol 2020; 31: 2145–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ronco C, Bellomo R, Kellum JA.. Acute kidney injury. Lancet 2019; 394: 1949–1964 [DOI] [PubMed] [Google Scholar]
- 35. Joannidis M, Forni LG, Klein SJet al. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 workgroup. Intensive Care Med 2020; 46: 654–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pei G, Zhang Z, Peng Jet al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 2020; 31: 1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stevens JS, King KL, Robbins-Juarez SYet al. High rate of renal recovery in survivors of COVID-19 associated acute renal failure requiring renal replacement therapy. PLoS ONE 2020; 15: e0244131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan L, Chaudhary K, Saha Aet al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol 2021; 32: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta S, Coca SG, Chan Let al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol 2021; 32: 161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ravn B, Prowle JR, Mårtensson Jet al. Superiority of serum cystatin C over creatinine in prediction of long-term prognosis at discharge from ICU. Crit Care Med 2017; 45: e932–e940 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The statistical analysis plan can be accessed online. The authors will be happy to consider additional analyses of the anonymized dataset on request. The need for stringent measures to prevent re-identification of individuals within a discrete geographical location and limited time-period, however, preclude sharing of patient-level dataset in a GDPR compliant form.