Abstract
Background
Systemic vascular injury occurs in coronavirus disease 2019 (COVID-19) patients; however, the underlying mechanisms remain unknown.
Methods
To clarify the role of inflammatory factors in COVID-19 vascular injury, we used a multiplex immunoassay to profile 65 inflammatory cytokines/chemokines/growth factors in plasma samples from 24 hospitalized (severe/critical) COVID-19 patients, 14 mild/moderate cases, and 13 healthy controls (HCs).
Results
COVID-19 patients had significantly higher plasma levels of 20 analytes than HCs. Surprisingly, only 1 cytokine, macrophage migration inhibitory factor (MIF), was among these altered analytes, while the rest were chemokines/growth factors. Additionally, only matrix metalloproteinase-1 (MMP-1) and vascular endothelial growth factor A (VEGF-A) were significantly elevated in hospitalized COVID-19 patients when compared to mild/moderate cases. We further studied MMP-1 enzymatic activity and multiple endothelial cell (EC) activation markers (soluble forms of CD146, intercellular adhesion molecule 1 [ICAM-1], and vascular cell adhesion molecule 1 [VCAM-1]) and found that they were highly dysregulated in COVID-19 patients.
Conclusions
COVID-19 patients have a unique inflammatory profile, and excessive MMP-1 and hyperactivation of ECs are associated with the severity of COVID-19.
Keywords: COVID-19, inflammation, MMP-1, endothelial cell, vascular injury
SARS-CoV-2 infection induces a chemokine storm. In addition, severe COVID-19 patients have excessive MMP-1 and hyperactive ECs that are associated with the severity of COVID-19. Thus, dysregulated chemokines and MMPs could be explored as therapeutic targets for COVID-19 treatment.
Coronavirus disease 2019 (COVID-19), an infectious disease caused by a novel coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2), has created an unprecedented global health and economic crisis. By the end of 2020, confirmed COVID-19 cases surpassed 84 million globally, resulting in over 1.8 million deaths. COVID-19 patients can experience various clinical manifestations, including asymptomatic, mild, moderate, severe, or critical symptoms. Growing evidence shows that COVID-19 is a vascular illness, not solely a respiratory disease. Histopathological examinations of postmortem tissues of COVID-19 patients have revealed (1) diffuse alveolar damage with perivascular infiltration of inflammatory cells [1], (2) extensive damage to the lining of blood vessels throughout the body [1–3], (3) severe endothelial injury and widespread thrombosis in the lungs, heart, liver, kidney, and small intestine [1–3], (4) viral particles in endothelial cells (ECs) of the glomerular capillary loops [2], and (5) caspase-3–positive apoptotic ECs in the lung and intestine tissues [2]. In addition, a recent study has shown that COVID-19 patients in intensive care units (ICUs) have higher counts of circulating ECs than non-ICU patients [4]. Circulating ECs are stressed cells detached from injured blood vessels, thereby indicating severe vascular injury [5]. Collectively, patients with severe COVID-19 exhibit impaired endothelial and microcirculatory functions across vascular beds of different organs, which may be particularly relevant for vulnerable individuals with preexisting endothelial dysfunctions such as diabetes, hypertension, and cardiovascular diseases, all of which are associated with adverse outcomes in COVID-19 [2, 6, 7].
The pathological mechanisms underlying vascular injury in COVID-19 remain unclear, although cytokine storm syndrome (CSS) and SARS-CoV-2 infection are considered contributors. SARS-CoV-2 is a member of the family Coronaviridae, genus Betacoronavirus, and is closely related to the SARS-CoV that caused the 2003 SARS pandemic [8–10]. CSS plays a critical role in the pathogenesis of SARS-CoV infection and represents a major cause of morbidity in SARS patients [11]. Elevated circulating concentrations of numerous inflammatory factors such as interleukin 6 (IL-6), interferon-γ (IFN-γ), IL-8, and inducible protein-10 (IP-10) have been reported in SARS patients when compared to healthy controls (HCs) [12, 13]. Circulating IL-6 levels are also elevated in COVID-19 patients and are cited as evidence of COVID-19 CSS [14, 15]. However, the levels of IL-6 and other inflammatory cytokines such as IL-8 are significantly less elevated in COVID-19 patients than the values typically reported in patients with CSS caused by conditions such as septic shock [16]. Indeed, the concentrations of IL-6, IL-8, and tumor necrosis factor-α (TNF-α), 3 of the most important inflammatory mediators in human diseases with CSS [17], in COVID-19 patients are similar to those found in ICU patients with cardiac arrest or trauma, conditions that are not notable for cytokine storms [16]. These findings bring into question whether a cytokine storm occurs in COVID-19, and whether IL-6, IL-8, and TNF-α act as key inflammatory mediators for fatal manifestations in patients with severe COVID-19 [17]. Our results echo recent National Institutes of Health (NIH) guidelines that note there are insufficient data to recommend IL-6 inhibitors for the treatment of COVID-19 [18]. In fact, efforts to combat cytokine storm in patients with severe COVID-19 have proven unsuccessful [19].
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as a primary receptor for viral binding and entry [20, 21]. Viral entry is also facilitated by transmembrane protease serine 2 (TMPRSS2) and neuropilin-1 (NRP1) [20, 22]. ACE2, TMPRSS2, and NRP1 are highly abundant on the lung alveolar type II epithelial (AT2) cells, rendering AT2 cells highly susceptible to productive SARS-CoV-2 infection [22–25]. ACE2, TMPRSS2, and NRP1 are also expressed on the surface of ECs [22, 23], albeit at lower levels than in AT2 cells [22], suggesting that these molecules may facilitate SARS-CoV-2 infection of ECs. Indeed, SARS-CoV-2 particles were observed in ECs in kidney tissues from COVID-19 patients [8]; however, the underlying mechanisms are unclear.
To explore the profile and key players of the cytokine storm in COVID-19, we used a multiplex immunoassay to simultaneously measure 65 inflammatory cytokines/chemokines/growth factors in plasma samples from hospitalized (severe/critical) COVID-19 patients, mild/moderate cases, and HCs. Surprisingly, matrix metalloproteinase-1 (MMP-1) and vascular endothelial growth factor A (VEGF-A), not conventional inflammatory cytokines such as IL-6, were 2 of the most unambiguously elevated inflammatory factors in hospitalized COVID-19 patients when compared to mild/moderate cases or HCs. Spearman correlation analysis revealed that the plasma levels of MMP-1 and VEGF-A in hospitalized COVID-19 patients were positively correlated. Given that excessive MMP-1 plays a central role in tissue destruction in a wide variety of vascular diseases and that elevated VEGF-A, an EC activation marker, increases vascular permeability [26], we further studied MMP-1 enzymatic activity and other EC activation markers, including soluble forms of CD146, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) in our cohort of COVID-19 patients and HCs. We found that excessive MMP-1 and hyperactivation of ECs occurred in COVID-19 patients and were correlated with the severity of COVID-19.
METHODS
Study Subjects and Ethical Considerations
This study was performed with the approval of the Institutional Review Boards at Indiana University School of Medicine. Blood samples were drawn after each participant provided a written informed consent form.
The study subjects included 24 hospitalized COVID-19 patients, 14 mild/moderate cases, and 13 HCs. Hospitalized COVID-19 patients included SARS-CoV-2–infected individuals who developed severe illness (dyspnea, hypoxia, >50% lung involvement on imaging, or required oxygen support [27]) or critical disease with complications such as respiratory failure, thrombosis, and/or multiorgan failure [27]. Some of these hospitalized patients were admitted to ICUs. Patients with mild/moderate COVID-19 were characterized with mild respiratory symptoms (nasal congestion, runny nose, and a sore throat) or mild pneumonia [27]. All COVID-19 patients were treated in Indiana University Health hospitals in Indianapolis, Indiana, during May to December 2020. Plasma samples from HCs were selected from our banked blood samples that were collected before the COVID-19 pandemic as described in our previous reports [28, 29]. Demographics of HCs were matched with COVID-19 patients so that there were no significant differences between age or sex of HCs and COVID-19 subjects. The demographic and clinical characteristics of COVID-19 patients and HC demographics are summarized in Table 1.
Table 1.
Demographics and Clinical Characteristics of COVID-19 Patients vs HCs
| Characteristic | HC (n = 13) | Mild (n = 14) | Hosp (n = 24) |
|---|---|---|---|
| Age, y, median (IQR) | 56.6 (42.1–65.5) | 59.0 (43.0–63.0) | 64.0 (48.5 -73.0) |
| Sex, male, No. (%) | 6 (46.3) | 6 (42.9) | 10 (41.7) |
| Race, No. (%) | |||
| African American | 5 (38.5) | 7 (50.0) | 10 (41.7) |
| Caucasian American | 7 (53.8) | 6 (42.9) | 13 (54.2) |
| Other | 1 (7.7) | 1 (7.1) | 1 (4.1) |
| D-Dimer, ng/mL, median (IQR) | <250a | 282 (259–290) | 527 (244–814)*** |
| CRP, mg/L, median (IQR) | <10a | 126 (89–268)* | 830 (642–1273)*** |
| Ferritin, ng/mL, median (IQR) | <300a | 350 (145–939) | 311 (88–1078) |
| WBC, No./μL, median (IQR) | 4000–11 000a | 5450 (4200–8100) | 8000 (5800–12 150) |
| Neutrophils, ANC/μL, median (IQR) | 1500–8000a | 3600 (2550–4800) | 6300 (4400–10 300) |
| Lymphocytes, ALC/μL, median (IQR) | 1000–4800a | 1100 (650–1850) | 610 (522–660)# |
***P < .001 (increased), #P < .05 (decreased), comparison between mild/moderate and hospitalized COVID-19 patients.
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; HC, healthy control; Hosp, hospitalized COVID-19 patient; IQR, interquartile range; Mild, patients with mild/moderate COVID-19; WBC, white blood cell count.
aValues of D-dimer, CRP, ferritin, WBC, ANC, and ALC in HCs are the reference values or range for healthy population.
Multiplex and ELISA Immunoassays
Peripheral blood was collected in heparin-coated BD Vacutainer Blood Collection tubes (BD Biosciences). Blood samples were centrifuged within 12 hours of collection at 700g for 20 minutes at room temperature without brake. The top layer (plasma) was harvested and stored at −80°C until use. Plasma concentrations of 65 human cytokines/chemokines/growth factors were simultaneously measured using a magnetic bead-based multiplex kit (EPX650-10065–901; Invitrogen) according to the manufacturer’s instructions. The beads were read on a BioPlex 200 system (Bio-Rad). The standards at 4-fold serial dilutions were run on each plate in duplicate and used to calculate the concentrations of cytokines/chemokines/growth factors using the Bio-Plex Manager Software (Bio-Rad).
Plasma levels of soluble CD146 (sCD146), soluble ICAM-1 (sICAM-1), soluble VCAM-1 (sVCAM-1), and intestinal fatty-acid binding protein (I-FABP) were quantified using the human CD146, ICAM-1, VCAM-1, and I-FABP DuoSet enzyme-linked immunosorbent assay (ELISA) kits (all from R&D Systems), respectively, according to manufacturer’s instructions. ELISA results were recorded using a microplate reader system (Bio-Tek).
Quantitative Determination of Human Active MMP-1 in Plasma Samples
Enzymatic activity of plasma MMP-1 was determined using the Human Active MMP-1 Fluorokine E kit (F1M00; R&D Systems) as per manufacturer’s instructions. Briefly, diluted plasma samples and MMP-1 standards were added to the wells that were precoated with a monoclonal antibody specific for human MMP-1. After washing, amino-phenyl mercuric acetate, an activation reagent of MMP-1, was added to the standards, but not the plasma samples. After washing, a fluorogenic substrate linked to a quencher molecule was added and any active enzyme present would cleave the peptide linker between the fluorophore and the quencher molecule, generating a fluorescent signal that is proportional to the amount of enzyme activity in an individual sample. Thus, plasma levels of active MMP-1 were quantitatively detected using a Synergy H1 Hybrid Multi-Mode Reader (BioTek), where fluorescence emission was recorded in relative light fluorescence units (RLU).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6.0. Data were expressed as mean ± standard error of the mean. Differences between 2 groups were calculated using the Mann-Whitney test. Kruskal-Wallis test with Dunn corrections was used for comparisons among 3 groups. χ 2 test was used for comparison between groups for categorical variables. The linear relationship between 2 variables was analyzed using the Spearman correlation test. P < .05 was considered statistically significant.
RESULTS
Characteristics of Study Subjects
The study subjects included 24 hospitalized patients with severe/critical COVID-19, 14 patients with mild/moderate COVID-19, and 13 HCs. Severe/critical COVID-19 patients were hospitalized in Indiana University Health hospitals in Indianapolis, Indiana and mild/moderate COVID-19 patients visited Indiana University Health hospitals between May and December 2020. HCs were recruited before the COVID-19 pandemic as described in our previous report [29]. The demographics and clinical characteristics of these subjects are summarized in Table 1. There were no differences in age, sex, or race distributions between these 3 groups. Hospitalized COVID-19 patients had lower absolute lymphocyte count and higher levels of D-dimer, C-reactive protein, and ferritin than patients with mild/moderate COVID-19 (Table 1). There were no differences in white blood cell or neutrophil counts between the hospitalized patients and patients with mild/moderate COVID-19 (Table 1).
Profiles of Inflammatory Cytokines/Chemokines/Growth Factors in Hospitalized COVID-19 Patients, Mild/Moderate Cases, and HCs
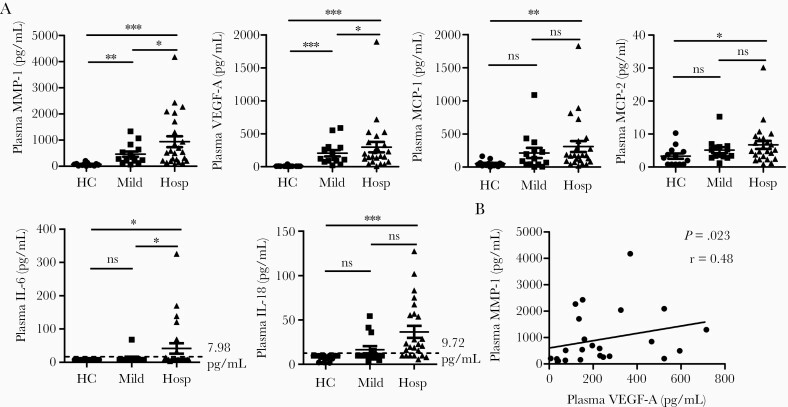
The pathogenesis of severe or critical COVID-19 is complex and has been suggested to include a cytokine storm that sustains an aberrant systemic immune response [30]. To elucidate the profile and key pathogenic inflammatory mediators of the cytokine storm in COVID-19 patients, we used a multiplex immunoassay to simultaneously detect plasma levels of 65 cytokines/chemokines/growth factors in hospitalized (severe/critical) patients, mild/moderate cases, and HCs. In comparison to HCs, COVID-19 patients had significantly higher plasma levels of 20 of these analytes (Table 2). Among these altered analytes, macrophage migration inhibitory factor (MIF) was the only inflammatory cytokine, all others were chemokines (BLC/CXCL13, ENA-78/CXCL5, eotaxin-3/CCL26, fractalkine/CX3CL1, IP-10/CXCL10, I-TAC/CXCL11, MIG/CXCL9, MIP-1α/CCL3, MIP-1β/CCL4, and SDF-1α/CXCL12), growth factors (HGF, MMP-1, SCF, and VEGF-A), and soluble receptors (APRIL, CD30, IL-2R/CD25, TNF-RII, and TRAIL/CD253) (Table 2). Surprisingly, MMP-1 and VEGF-A were the only 2 elevated inflammatory factors that were significantly higher in hospitalized COVID-19 patients when compared to mild/moderate cases (Table 2 and Figure 1A), while all others did not show differences between hospitalized and mild/moderate COVID-19 patients (Table 2). Spearman correlation analysis revealed that the plasma levels of MMP-1 and VEGF-A in hospitalized COVID-19 patients were positively correlated (Figure 1B), indicating that there are interactions between MMP-1 and VEGF-A in COVID-19. Thus, the highly elevated plasma levels of MMP-1 and VEGF-A were associated with the severity of COVID-19.
Table 2.
Plasma Levels of Cytokines/Chemokines/Growth Factors in COVID-19 Patients and HC
| Analyte | HC (n = 13) | Mild (n = 14) | Hosp (n = 24) |
|---|---|---|---|
| BLC (CXCL13) | 36 (17–73) | 92 (45–183)* | 107 (75–185)*** |
| ENA-78 (CXCL5) | 22.8 (4.6–32.4) | 94.5 (68.0–69.0)*** | 108.1 (66.6–178.2)*** |
| Eotaxin-3 (CCL26) | 2.2 (2.2–2.2) | 6.0 (2.2–20.7)** | 9.6 (6.3 -18.9)*** |
| Fractalkin (CX3CL1) | 2.5 (1.7–2.5) | 3.9 (2.8–5.2)* | 3.6 (2.7–5.6)*** |
| IP-10 (CXCL10) | 6.4 (3.1–9.7) | 29.1 (9.2–69.9)** | 28.8 (16.7–87.4)*** |
| I-TAC (CXCL11) | 11.1 (11.1–35.8) | 165.6 (92.6–287.1)** | 196.8 (114.2–491.2)*** |
| MIG (CXCL9) | 8.6 (8.6–10.3) | 37.8 (27.4–69.1)*** | 26.2 (12.7–53.2)** |
| MIP-1α (CCL3) | 5.3 (3.1–26.3) | 10.0 (3.1–19.2)*** | 9.4 (3.5–19.4)*** |
| MIP-1β (CCL4) | 4.6 (4.6–4.6) | 20.0 (10.0–45.8)*** | 19.6 (8.8–32.7)*** |
| SDF-1α (CXCL12) | 679 (472–989) | 1099 (815–1274)* | 1087 (718–1275)** |
| HGF | 11.9 (6.4–17.9) | 54.8 (28.4–141.1)* | 163.2 (77.1–357.3)*** |
| MMP-1 | 73.8 (30.9–96.1) | 316.8 (189.0–589.5)** | 863.4 (551.4–1880)***,# |
| SCF | 4.6 (3.4–8.6) | 15.4 (12.4–43.6)** | 16.6 (8.3–26.2)** |
| VEGF-A | 6.8 (6.8–8.2) | 124.5 (86.0–293.0)*** | 353.9 (235.1–701.7)****,# |
| APRIL | 745 (602–913) | 1951 (1236–4852)*** | 1883 (1239–2515)*** |
| CD30 | 100 (38–126) | 409 (194–936)*** | 274 (178–703)*** |
| IL-2R (CD25) | 84 (84–1414) | 6474 (2006–9316)** | 6884 (4217–9539)*** |
| TNF-RII | 78.6 (59.1–96.4) | 221.2 (158.7–262.3)*** | 202.2 (150.2–256.1)*** |
| TRAIL (CD253) | 11.0 (11.0–29.5) | 63.3 (41.9–103.3)** | 41.4 (24.5–415.2)* |
| MIF | 37.7 (19.7–48.4) | 90.7 (80.3–125.1)*** | 89.5 (70.7–157.2)*** |
| MCP-1 (CCL2) | 41.1 (21.8–59.5) | 109.4 (40.1–281.5) | 172.2 (84.2–363.4)** |
| MCP-2 (CCL8) | 2.8 (0.8–4.9) | 4.4 (3.1–6.2) | 5.0 (2.6–8.6)* |
| IL-6 | 7.9 (7.9–7.9) | 7.9 (7.9–7.9) | 41.9 (7.9–76.1)* |
| IL-18 | 9.7 (4.2–9.7) | 9.7 (9.1–20.5) | 21.8 (9.7–56.5)*** |
| CD40L (CD154) | 4.8 (4.8–82.0) | 29.5 (17.6–71.2) | 36.6 (19.7–59.7) |
| Eotaxin (CCL11) | 4.2 (21.9–99.0) | 54.8 (17.8–103.0) | 51.2 (31.5–128.7) |
| G-CSF | 9.9 (9.9–33.8) | 16.5 (9.9–36.4) | 28.8 (13.7–38.9) |
| GM-CSF | 15.9 (15.9–54.0) | 15.9 (15.9–15.9) | 15.9 (15.9–15.9) |
| IFN-γ | 13.1 (6.4–13.1) | 11.2 (7.3–15.5) | 18.3 (8.9–16.4) |
| IL-2 | 36.3 (22.0–104.1) | 42.4 (22.0–106.7) | 53.9 (22.0–78.8) |
| IL-10 | 2.2 (2.2–2.9) | 1.8 (0.9–3.2) | 2.3 (1.3–4.4) |
| IL-15 | 9.0 (3.3–27.9) | 3.3 (3.3–3.7) | 3.3 (3.3–19.4) |
| IL-16 | 799 (412–1175) | 1091 (865–1362) | 936 (736–1386) |
| IL-17A | 24.3 (24.3–358.2) | 24.3 (24.3–180.3) | 24.3 (24.3–187.0) |
| IL-20 | 9.1 (7.6–50.2) | 9.1 (6.1–31.7) | 8.8 (2.0–21.2) |
| IL-21 | 9.1 (9.1–9.1) | 9.6 (8.9–33.8) | 13.8 (7.3–27. 2) |
| IL-22 | 17.8 (17.8–17.8) | 17.8 (17.8–34.5) | 17.8 (17.8–23.6) |
| IL-27 | 17.4 (17.4–17.4) | 17.4 (17.4–22.8) | 17.4 (17.4–17.4) |
| MDC (CCL22) | 46.2 (20.1–74.5) | 104.6 (51.9–164.8) | 62.9 (20.0–119.3) |
| MIP-3α | 21.0 (21.0–21.0) | 24.5 (15.2–57.9) | 44.8 (16.8–96.4) |
| TSLP | 4.7 (2.4–11.6) | 4.6 (2.3–13.2) | 6.4 (4.0–8.8) |
| TWEAK | 409 (216–1859) | 935 (469–1867) | 799 (566–1566) |
| BAFF | 3/13 | 3/14 | 7/24 |
| Eotaxin-2 (CCL24) | 0/13 | 2/14 | 4/24 |
| FGF-2 | 0/13 | 0/14 | 1/24 |
| Gro-α (CXCL1) | 0/13 | 0/14 | 1/24 |
| IFN-α | 1/13 | 1/14 | 4/24 |
| IL-1α | 0/13 | 1/14 | 2/24 |
| IL-1β | 3/13 | 3/14 | 6/24 |
| IL-3 | 1/13 | 5/14 | 8/24 |
| IL-4 | 0/13 | 0/14 | 2/24 |
| IL-5 | 1/13 | 1/14 | 2/24 |
| IL-7 | 0/13 | 0/14 | 0/24 |
| IL-8 | 1/13 | 1/14 | 4/24 |
| IL-9 | 0/13 | 1/14 | 1/24 |
| IL-12p70 | 0/13 | 1/14 | 0/24 |
| IL-13 | 0/13 | 1/14 | 1/24 |
| IL-23 | 0/13 | 0/13 | 1/24 |
| IL-31 | 0/13 | 1/14 | 1/24 |
| LIF | 0/13 | 0/14 | 1/24 |
| MCP-3 (CCL7) | 0/13 | 1/14 | 0/24 |
| M-CSF | 0/13 | 0/14 | 0/24 |
| NGF-β | 0/13 | 1/14 | 1/24 |
| TNF-α | 0/13 | 0/14 | 0/24 |
| TNF-β | 0/13 | 0/14 | 0/24 |
Data are median (interquartile range) or number of detected out of total subjects in rows BAFF to TNF-β.
Kruskal-Wallis test with Dunn corrections was used for comparisons among 3 groups of HCs, mild/severe cases, and hospitalized COVID-19 patients. *P < .05, **P < .01, ***P < .001 for comparison between hospitalized COVID-19 and HCs, #P < .05 for comparison between hospitalized COVID-19 patients and mild/moderate cases.
Sixty-five human cytokines/chemokines/growth factors were measured: 33 cytokines (G-CSF, GM-CSF, IFN-α, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-16, IL-17A, IL-18, IL-20, IL-21, IL-22, IL-23, IL-27, IL-31, LIF, M-CSF, MIF, TNF-α, TNF-β, and TSLP), 18 chemokines (CXCL13, CXCL5, CCL11, CCL24, CCL26, CX3CL1, CXCL1, CXCL10, CXCL11, MCP-1, MCP-2, MCP-3, MDC, MIG, MIP-1α, MIP-1β, MIP-3α, and SDF-1α), 6 growth factors/regulators (FGF-2, HGF, MMP-1, NGF-β, SCF, VEGF-A), and 8 soluble receptors (APRIL, BAFF, CD30, CD40L, IL-2R, TNF-RII, TRAIL, and TWEAK).
Abbreviations: COVID-19, coronavirus disease 2019; HC, healthy control; Hosp, hospitalized COVID-19 patients; Mild, patients with mild/moderate COVID-19.
Figure 1.
Plasma levels of inflammatory factors were highly elevated in hospitalized COVID-19 patients. A, Scatter plots demonstrating the plasma levels of MMP-1, VEGF-A, MCP-1, MCP-2, IL-6, and IL-18 in hospitalized COVID-19 patients (n = 24), mild/moderate COVID-19 patients (n = 14), and HCs (n = 13). Kruskal-Wallis test with Dunn correction for pairwise comparisons among hospitalized COVID-19 patients, mild/moderate COVID-9 patients, and HCs. *P < .05; **P < .01; ***P < .001; ns, not significant. Lines represent the mean and the standard error of the mean. B, Spearman correlation analysis between plasma levels of MMP-1 and VEGF-A in hospitalized COVID-19 patients. Abbreviations: COVID-19, coronavirus disease 2019; HC, healthy controls; Hosp, hospitalized COVID-19 patients; IL, interleukin; MCP, monocyte chemoattractant protein; Mild, mild/moderate COVID-19 patients; MMP-1, matrix metalloproteinase-1; r, Spearman correlation coefficient; VEGF-A, vascular endothelial growth factor A.
Monocyte chemoattractant protein-1 (MCP-1), MCP-2, IL-6, and IL-18 were the 4 analytes that were significantly increased in hospitalized patients, but not in mild/moderate cases, when compared to HCs (Table 2 and Figure 1A). However, plasma levels of IL-6 and IL-18 were elevated in some, but not all, patients with severe/critical COVID-19. As shown in Figure 1A, IL-6 was detected in 0 HCs (limit of detection ≥7.98 pg/mL), 1 mild/moderate case (67.8 pg/mL), and 7 hospitalized cases (13.8–325.4 pg/mL). Similarly, IL-18 was detected in 0 HCs (limit of detection ≥9.72 pg/mL), 4 mild/moderate cases (15.3–54.4 pg/mL), and 18 hospitalized patients (15.7–127.3 pg/mL). Notably, the levels of IL-6 are significantly less elevated in patients with critical COVID-19 than the values typically reported in patients with CSS caused by conditions such as septic shock [16].
Eighteen analytes were detected in all 3 groups of research subjects (CD40L, Eotaxin, G-CSF, GM-CSF, IFN-γ, IL-2, IL-10, IL-15, IL-16, IL-17A, IL-20, IL-21, IL-22, IL-27, MDC, MIP-3a, TSLP, and TWEAK), albeit with no difference in the analyte levels (Table 2). Twenty-three analytes, primarily inflammatory cytokines such as IL-8 and TNF-α, were below the limit of detection in the majority of hospitalized cases, mild/moderate cases, and HCs (Table 2).
Enzymatic Activity of MMP-1 and Activation Markers of ECs Increased in the Peripheral Blood in Hospitalized COVID-19 Patients
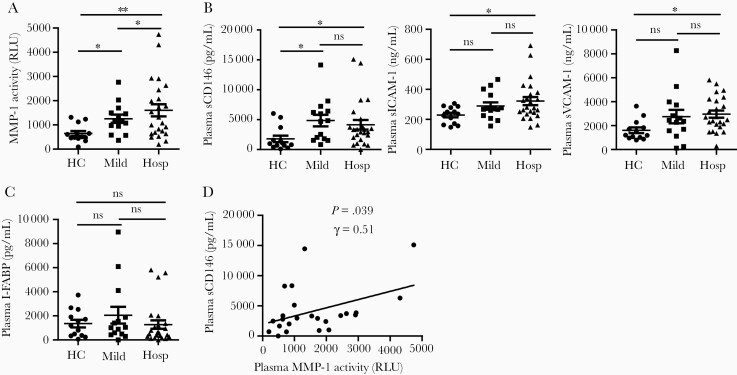
MMP-1 and VEGF-A were 2 of the most unambiguously elevated inflammatory factors in hospitalized COVID-19 patients (Table 2). Their plasma levels were positively correlated (Figure 1), suggesting that there are interactions between MMPs and ECs in COVID-19. We further studied plasma MMP-1 enzymatic activity and the EC activation marker profiles. To date, only one MMP, MMP-9, has been analyzed in one pilot COVID-19 study [31]. This study measured plasma MMP-9 levels using an enzyme immunoassay and showed that hospitalized COVID-19 patients had higher MMP-9 levels than HCs [31]. However, no MMP enzymatic activity has been studied in COVID-19 patients. We found that the enzymatic activity of MMP-1 was significantly increased in hospitalized COVID-19 patients when compared to HCs and those with mild/moderate COVID-19 (Figure 2A). Thus, both the levels and the enzymatic activity of MMP-1 were upregulated in the peripheral blood of COVID-19 patients, particularly in patients with severe/critical COVID-19.
Figure 2.
Enzymatic activity of MMP-1 and activation markers of ECs were elevated in the peripheral blood of hospitalized COVID-19 patients. A–C, Scatter plots demonstrating enzymatic activity of MMP-1, levels of EC activation markers (sCD146, sICAM-1, and sVCAM-1), and levels of intestinal epithelial injury marker (I-FABP), respectively, in the peripheral blood of hospitalized COVID-19 patients (n = 24), mild/moderate COVID-19 patients (n = 14), and HCs (n = 13). Kruskal-Wallis test with Dunn correction for pairwise comparisons among HC, hospitalized COVID-19 patients, and mild/moderate COVID-9 patients. *P < .05; **P < .01; ns, not significant. Lines represent the mean and the standard error of the mean. D, Spearman correlation analysis between plasma levels of MMP-1 and sCD146 in hospitalized COVID-19 patients. Abbreviations: COVID-19, coronavirus disease 2019; EC, endothelial cell; HC, healthy control; Hosp, hospitalized COVID-19 patients; I-FABP, intestinal fatty-acid binding protein; Mild, mild/moderate COVID-19 patients; MMP-1, matrix metalloproteinase-1; r, Spearman correlation coefficient; sCD146, soluble CD146; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1.
We also found that hospitalized COVID-19 patients had significantly higher plasma levels of sCD146, sICAM-1, and sVCAM-1 than HCs. Mild/moderate COVID-19 patients also had higher levels of sCD146, but neither sICAM-1 nor sVCAM-1, than HCs (Figure 2B). There were no differences in the plasma levels of sCD146, sICAM-1, or sVCAM-1 between hospitalized and mild/moderate COVID-19 patients (Figure 2B). As I-FABP is solely expressed in epithelial cells of the mucosal layer of the small intestine and used as a plasma marker of intestinal epithelial injury [32], an I-FABP ELISA assay was also performed to study and compare the effects of COVID-19 on epithelial cells versus ECs. In contrast to EC activation markers, plasma I-FABP levels were not different between hospitalized COVID-19 patients, mild/moderate cases, and HCs (Figure 2C), suggesting that systemic ECs, not small intestine epithelial cells, are significantly affected by COVID-19.
Spearman correlation analysis was performed to identify any associations between plasma levels of active MMP-1 and EC activation markers. There was a positive correlation between plasma active MMP-1 and plasma levels of sCD146 (r = 0.51 and P = .039; Figure 2D). There were no correlations between the plasma levels of active MMP-1 and plasma levels of sICAM-1 or sVCAM-1 in hospitalized COVID-19 patients (data not shown).
Taken together, our data demonstrate that both plasma levels and enzymatic activity of MMP-1 and plasma levels of EC activation markers are highly elevated and positively correlated in COVID-19 and their dysregulations are associated with the severity of COVID-19.
Association of Elevated MMP-1 and EC Activation Markers With Demographics in Hospitalized COVID-19 Patients
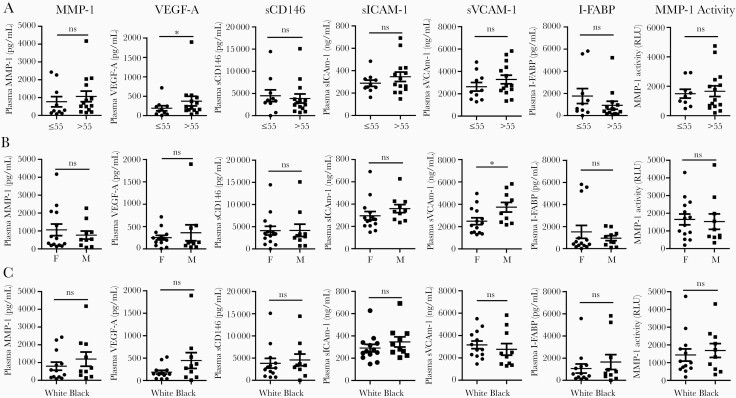
We analyzed the relationship between demographic factors (age, sex, and race) and circulating MMP-1 and EC activation markers in hospitalized COVID-19 patients. Twenty-four hospitalized COVID-19 patients were split into 2 age groups: 10 cases ≤ 55 years old and 14 cases > 55 years old. As shown in Figure 3A, plasma VEGF-A levels were significantly higher in hospitalized COVID-19 patients >55 years old when compared to cases ≤ 55 years old. Plasma levels of MMP-1, sICAM-1, sVCAM-1, and active MMP-1 trended higher in the older group (Figure 3A). We also found that plasma sVCAM-1 levels were significantly higher in men (n = 10) when compared to women (n = 14) (Figure 3B). There were no differences in the plasma levels of MMP-1, active MMP-1, or EC activation markers in African Americans (n = 10) when compared to Caucasian Americans (n = 13) (Figure 3C).
Figure 3.
Relationship of MMP-1 and EC activation with demographics of hospitalized COVID-19 patients. Scatter plots demonstrating levels of MMP-1, levels of EC activation markers (VEGF-A, sCD146, sICAM-1, and sVCAM-1), levels of intestinal epithelial injury marker (I-FABP), and enzymatic activity of MMP-1 in hospitalized COVID-19 patients. Comparison of these factors between hospitalized COVID-19 patients (A) >55 years old (n = 14) and ≤ 55 years old (n = 10), (B) female (n = 14) and male (n = 10), and (C) African Americans (n = 10) and white Americans (n = 13). All comparisons were conducted using Mann-Whitney test. White, Caucasian American; Black, African American. *P < .05; ns, not significant. Lines represent the mean and the standard error of the mean. Abbreviations: COVID-19, coronavirus disease 2019; EC, endothelial cell; I-FABP, intestinal fatty-acid binding protein; MMP-1, matrix metalloproteinase-1; sCD146, soluble CD146; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; VEGF-A, vascular endothelial growth factor A.
DISCUSSION
In the present study, we systematically profiled and compared 65 inflammatory cytokines/chemokines/growth factors in the plasma samples from hospitalized COVID-19 patients, mild/moderate COVID-19 cases, and HCs. COVID-19 patients had significantly higher levels of 20 out of 65 factors analyzed when compared to HCs. These altered factors include 10 chemokines (BLC/CXCL13, ENA-78/CXCL5, eotaxin-3/CCL26, fractalkine/CX3CL1, IP-10/CXCL10, I-TAC/CXCL11, MIG/CXCL9, MIP-1α/CCL3, MIP-1β/CCL4, and SDF-1α/CXCL12), 4 growth factors (HGF, MMP-1, SCF, and VEGF-A), 5 soluble receptors (APRIL, CD30, IL-2R/CD25, TNF-RII, and TRAIL/CD253), and 1 cytokine (MIF). In addition, MCP-1, MCP-2, IL-6, and IL-18 were significantly increased in hospitalized patients when compared to HCs. However, IL-6 and IL-18 were only elevated in some patients with severe/critical COVID-19, and the IL-6 levels were much less elevated than the values typically reported in patients with CSS-associated diseases such as septic shock [16].
The vast majority of altered circulating inflammatory factors in COVID-19 patients were chemokines, growth factors, and soluble receptors, not inflammatory cytokines. Our results indicate that COVID-19 patients have inflammatory profiles that are distinct from the cytokine storms found in other human diseases [30, 33], and also question whether a cytokine storm occurs in COVID-19. The term “cytokine storm” was first coined to describe the excessive release of inflammatory cytokines by immune cells in graft-versus-host disease [30, 33]. Since then, CSS has been reported in a wide range of human diseases including cancer patients undergoing chimeric antigen receptor (CAR) T-cell therapy [34], sepsis [35], and viral infections [13, 36]. Indeed, CSS is a common complication of viral respiratory infections such as infection with SARS-CoV [13, 37, 38], and the Middle East respiratory syndrome coronavirus (MERS-CoV) [39]. In these CSS-linked diseases, IL-6, IFN-γ, IL-1β, and TNF-α are highly elevated and act as hallmarks. In HCs, mean levels of circulating IL-6 have been reported to be <5 pg/mL [40], which can be increased to >10 000 pg/mL in cancer patients on CAR T-cell therapy [41]. The substantial elevation of IL-6 and its correlation with disease severity have resulted in IL-6 inhibitors such as sarilumab, siltuximab, and tocilizumab becoming therapeutic agents that can effectively treat CSS-associated diseases [41]. Circulating IL-6 levels are also elevated in COVID-19 patients and cited as evidence of COVID-19 CSS [14, 15]. However, as previously reported, elevated IL-6 levels in COVID-19 patients are minuscule compared to those found in individuals on CAR T-cell therapy and other CSS-associated diseases [17, 42], suggesting that IL-6 does not act as a key inflammatory mediator for fatal manifestations in patients with severe or critical COVID-19 [17]. Thus, IL-6 inhibitors are not recommended to be used as therapeutic agents to treat patients with critical COVID-19, as noted by NIH guidelines [18]. Our results provide evidence that explains why efforts to combat cytokine storm have proven unsuccessful in severe COVID-19 patients. Our study implies a chemokine storm, not a conventional cytokine storm, occurs in COVID-19 pathogenesis. This chemokine storm may play an important role in the pathogenesis of COVID-19 via recruitment of inflammatory cells to the lungs and other organs. Several clinical trials are ongoing that use antagonists to target specific chemokine receptors (CCR2, CCR5, and CXCR8) involved in recruitment of immune cells in COVID-19 patients [43–45]. The ligands for those receptors include several chemokines, such as MCP-1, MCP-2, MIP-1α, and MIP-1β, which were found to be elevated in the COVID-19 patients in our study.
MMP-1 and VEGF-A were 2 of the most unambiguously elevated inflammatory factors in hospitalized COVID-19 patients when compared to mild/moderate cases or HCs, while all other inflammatory factors did not show differences between hospitalized and mild/moderate COVID-19 patients. The plasma levels of MMP-1 and VEGF-A in hospitalized COVID-19 patients were positively correlated, suggesting interactions between MMP-1 and VEGF-A in COVID-19. MMP-1 is an interstitial collagenase capable of degrading collagen types I, II, and III, and plays a critical role in vascular remodeling and vascular diseases [46]. In addition, MMP-1 acts as a potent agonist for protease-activated receptor-1 (PAR1) [47], a G protein-coupled protease-activated receptor, on the surface of a variety of cell types such as ECs [47]. MMP-1/PAR1 signaling can increase expression of VEGF receptor-2 (VEGFR2) [48], the main receptor for VEGF, on ECs to trigger EC activation. We found that the levels of MMP-1, VEGF-A, and MMP-1 enzymatic activity were significantly elevated in the peripheral blood in hospitalized (severe/critical) COVID-19 patients compared to mild/moderate cases or HCs, suggesting that MMP-1/PAR1/VEGFR2/VEGF-A signaling may be in a hyperactivation state in ECs in COVID-19 patients.
In addition to VEGF-A, other EC activation markers including sCD146, sICAM-1, and sVCAM-1, but not epithelial cell activation marker I-FABP, were highly elevated in COVID-19. These findings indicate that systemic ECs, not epithelial cells, are significantly affected by COVID-19.
Age is a well-known factor that influences the severity and fatality of COVID-19 [49]. The risk for severe illness with COVID-19 increases with age, with older adults at highest risk [49]. Other demographic factors such as sex and race have also been linked with risk for the severity of COVID-19. We found that excessive MMPs and/or circulating markers of EC activation increased with age or were higher in hospitalized male COVID-19 patients, but were not affected by race. However, we realize that our sample size of hospitalized COVID-19 patients, after splitting into 2 demographic groups, is too small to make significant statistical differences.
Additional limitations of our study include the lack of COVID-19–negative hospitalized patients, which might have allowed us to identify a unique profile of inflammatory mediators in hospitalized COVID-19 patients [50]. We were unable to detect IL-8 and TNF-α, 2 factors shown to be elevated by multiplex and ELISA immunoassays in critically ill COVID-19 patients with sepsis [50]. This discrepancy is likely due to differences in COVID-19 patient populations, clinical parameters, and detection limits of the different multiplex immunoassay kits used.
In conclusion, our study demonstrates that SARS-CoV-2 infection induces a chemokine storm. In addition, severe COVID-19 patients have excessive MMP-1 and hyperactive ECs that are associated with the severity of COVID-19. Thus, dysregulated chemokines and MMPs could be explored as therapeutic targets for COVID-19 treatment.
Notes
Disclaimer. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH National Institute on Alcohol Abuse and Alcoholism (grant number UH2AA026218 to Q. Y.); the Bill and Melinda Gates Foundation (grant number OPP1035237 to Q. Y.); the Indiana Biobank and the Indiana Clinical and Translational Sciences Institute funded by the NIH (grant number UL1TR002529); and the National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020; 77:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395:1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med 2020; 173:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guervilly C, Burtey S, Sabatier F, et al. Circulating endothelial cells as a marker of endothelial injury in severe COVID -19. J Infect Dis 2020; 222:1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blann AD, Woywodt A, Bertolini F, et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost 2005; 93:228–35. [DOI] [PubMed] [Google Scholar]
- 6. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr 2020; 14:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alvarado-Vasquez N. Could a family history of type 2 diabetes be a risk factor to the endothelial damage in the patient with COVID-19? Med Hypotheses 2021; 146:110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau SK, Woo PC, Li KS, et al. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A 2005; 102:14040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ge XY, Li JL, Yang XL, et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013; 503:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu B, Zeng LP, Yang XL, et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog 2017; 13:e1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020; 368:473–4. [DOI] [PubMed] [Google Scholar]
- 12. Huang KJ, Su IJ, Theron M, et al. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol 2005; 75:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA 2020; 324:1565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scherger S, Henao-Martínez A, Franco-Paredes C, Shapiro L. Rethinking interleukin-6 blockade for treatment of COVID-19. Med Hypotheses 2020; 144:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institutes of Health. COVID-19 treatment guidelines. Interleukin-6 inhibitors.https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/interleukin-6-inhibitors/. Accessed 18 May 2021. [PubMed]
- 19. Mudd PA, Crawford JC, Turner JS, et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv 2020; 6:eabe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181:1016–35.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020; 370:856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sungnak W, Huang N, Bécavin C, et al. ; HCA Lung Biological Network . SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020; 26:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bertram S, Heurich A, Lavender H, et al. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One 2012; 7:e35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem 1998; 273:15099–103. [DOI] [PubMed] [Google Scholar]
- 27. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li W, Amet T, Xing Y, et al. Alcohol abstinence ameliorates the dysregulated immune profiles in patients with alcoholic hepatitis: a prospective observational study. Hepatology 2017; 66:575–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li W, Lin EL, Liangpunsakul S, et al. Alcohol abstinence does not fully reverse abnormalities of mucosal-associated invariant T cells in the blood of patients with alcoholic hepatitis. Clin Transl Gastroenterol 2019; 10:e00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev 2012; 76:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ueland T, Holter JC, Holten AR, et al. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J Infect 2020; 81:e41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kokesova A, Coufal S, Frybova B, Kverka M, Rygl M. The intestinal fatty acid-binding protein as a marker for intestinal damage in gastroschisis. PLoS One 2019; 14:e0210797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abhyankar S, Gilliland DG, Ferrara JL. Interleukin-1 is a critical effector molecule during cytokine dysregulation in graft versus host disease to minor histocompatibility antigens. Transplantation 1993; 56:1518–23. [DOI] [PubMed] [Google Scholar]
- 34. Barrett DM, Teachey DT, Grupp SA. Toxicity management for patients receiving novel T-cell engaging therapies. Curr Opin Pediatr 2014; 26:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013; 27:669–84. [PMC free article] [PubMed] [Google Scholar]
- 36. Guo XJ, Thomas PG. New fronts emerge in the influenza cytokine storm. Semin Immunopathol 2017; 39:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chien JY, Hsueh PR, Cheng WC, Yu CJ, Yang PC. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology 2006; 11:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang CH, Liu CY, Wan YL, et al. Persistence of lung inflammation and lung cytokines with high-resolution CT abnormalities during recovery from SARS. Respir Res 2005; 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim ES, Choe PG, Park WB, et al. Clinical progression and cytokine profiles of middle east respiratory syndrome coronavirus infection. J Korean Med Sci 2016; 31:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med 2011; 9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maude S, Barrett DM. Current status of chimeric antigen receptor therapy for haematological malignancies. Br J Haematol 2016; 172:11–22. [DOI] [PubMed] [Google Scholar]
- 42. Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020; 8:1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Francescangeli F, De Angelis ML, Baiocchi M, Rossi R, Biffoni M, Zeuner A. COVID-19-induced modifications in the tumor microenvironment: do they affect cancer reawakening and metastatic relapse? Front Oncol 2020; 10:592891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khalil BA, Elemam NM, Maghazachi AA. Chemokines and chemokine receptors during COVID-19 infection. Comput Struct Biotechnol J 2021; 19:976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koenig LM, Boehmer DFR, Metzger P, Schnurr M, Endres S, Rothenfusser S. Blocking inflammation on the way: rationale for CXCR2 antagonists for the treatment of COVID-19. J Exp Med 2020; 217:e20201342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 2008; 75:346–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Austin KM, Covic L, Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood 2013; 121:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mazor R, Alsaigh T, Shaked H, et al. Matrix metalloproteinase-1-mediated up-regulation of vascular endothelial growth factor-2 in endothelial cells. J Biol Chem 2013; 288:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mallapaty S. The coronavirus is most deadly if you are older and male—new data reveal the risks. Nature 2020; 585:16–7. [DOI] [PubMed] [Google Scholar]
- 50. Fraser DD, Cepinskas G, Slessarev M, et al. Inflammation profiling of critically ill coronavirus disease 2019 patients. Crit Care Explor 2020; 2:e0144. [DOI] [PMC free article] [PubMed] [Google Scholar]