Abstract
Antibiotic use in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients during the COVID-19 pandemic has exceeded the incidence of bacterial coinfections and secondary infections, suggesting inappropriate and excessive prescribing. Even in settings with established antimicrobial stewardship (AMS) programmes, there were weaknesses exposed regarding appropriate antibiotic use in the context of the pandemic. Moreover, antimicrobial resistance (AMR) surveillance and AMS have been deprioritised with diversion of health system resources to the pandemic response. This experience highlights deficiencies in AMR containment and mitigation strategies that require urgent attention from clinical and scientific communities. These include the need to implement diagnostic stewardship to assess the global incidence of coinfections and secondary infections in COVID-19 patients, including those by multidrug-resistant pathogens, to identify patients most likely to benefit from antibiotic treatment and identify when antibiotics can be safely withheld, de-escalated or discontinued. Long-term global surveillance of clinical and societal antibiotic use and resistance trends is required to prepare for subsequent changes in AMR epidemiology, while ensuring uninterrupted supply chains and preventing drug shortages and stock outs. These interventions present implementation challenges in resource-constrained settings, making a case for implementation research on AMR. Knowledge and support for these practices will come from internationally coordinated, targeted research on AMR, supporting the preparation for future challenges from emerging AMR in the context of the current COVID-19 pandemic or future pandemics.
Keywords: antimicrobial resistance, COVID-19, stewardship, surveillance, public health
Introduction
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected all aspects of society, including actions to address antimicrobial resistance (AMR). The pandemic has revealed that increased strain on healthcare systems can lead to increased, often inappropriate antibiotic use1–4 and deprioritisation of AMR surveillance and antimicrobial stewardship (AMS).5
Widespread broad spectrum antibiotic use in patients with COVID-19 has been reported.4 Detailed data are lacking but preliminary data (reviewed below) suggest that outpatient antibiotic use may have decreased in certain settings. This may be due to diminished access to healthcare because of lockdowns and physical distancing, or to decreased community spread of other infections in some settings, or a combination of both. However, treatment of the majority of hospitalised COVID-19 patients with antibiotics early in the COVID-19 pandemic increased antibiotic use compared with the prepandemic period.3,6–8
Globally, there has been a diverse response to SARS-CoV-2 due to differences in the severity of the outbreaks, local and national policies, available resources, cultural aspects and social awareness. However, most countries tried to ensure that their healthcare systems were able to cope with a high predicted number of severe cases but many different practices were employed, ranging from strict, enforced lockdowns to vague and suggestive physical distancing recommendations. Some of the most prominent epicentres were in places where AMR is a challenge9,10 and where many of the factors that are associated with severe SARS-CoV-2 infection, including underlying conditions, advanced age, group housing and residence in a living facility for older people, are also linked to an increased risk of multidrug-resistant infections.11,12
We provide an overview of factors during the pandemic that may influence AMR and provide recommendations for antibiotic use, data collection, surveillance and stewardship, research and policy.
Excessive hospital use of antibiotics did not align with microbiological investigation
The interplay of inappropriate and excessive antibiotic use and (lack of) access to appropriate treatment was challenging in the initial stage of the pandemic. Coinfections are infections caused by pathogens presenting concurrently with COVID-19 upon hospital admission or upon COVID-19 diagnosis, while secondary infections are infections occurring after the start of COVID-19 disease and are usually healthcare-associated and exacerbated by invasive procedures and the use of immunosuppressant drugs.13,14 Suspected coinfections drive empirical therapy at hospital admission, while secondary infections drive antibiotic use after hospital admission and are potentially more preventable through adequate infection prevention and control measures.
Meta-analyses revealed that 7–8% of admitted COVID-19 patients were diagnosed with a bacterial or fungal infection2,15,16; these were more frequent in intensive care unit (ICU) patients (8–14%) than in patients in other wards (4–6%). Coinfections occurred in only 3.5% of patients (95% CI 0.4 to 6.7%), while secondary infections occurred in 14.3% (95% CI 9.6 to 18.9%).16,17 The most commonly reported bacterial co-pathogens were Mycoplasma species, Haemophilus influenzae and Pseudomonas aeruginosa.15,16 In these studies, the criteria for coinfections and sampling for co-pathogens were heterogeneous, and prospective, well-designed studies using appropriate definitions are needed.
Despite low reported levels of bacterial infections, antibiotic use in COVID-19 patients was considerably high: 71.9% (95% CI 56.1 to 87.7%) of COVID-19 patients received antibiotics.16 Importantly, 74% of the antibiotics reportedly prescribed were fluoroquinolones and third-generation cephalosporins. Studies showed an increase in the consumption of amoxicillin-clavulanic acid during the first weeks of the pandemic, mostly to empirically cover coinfections, and a later increase in the consumption of broader spectrum drugs, mostly to cover secondary infections.3,17 At a hospital outside of a COVID-19 epicentre in the USA, antibiotic days of therapy per 1000 bed days of care significantly increased from March to June 2020. Increases were most significant for macrolides and non-antipseudomonal penicillins, agents recommended at the hospital as first-line treatment for community-acquired pneumonia.8 Paradoxically, the empirical and wide-scale antibiotic use in COVID-19 patients may have led to an underestimation of coinfections.18 Data from low- and middle-income countries (LMICs) reporting differences in antibiotic use and the presence of coinfections are currently lacking. The need to conduct studies on the worldwide evolution of AMR during the COVID-19 pandemic with a focus on the challenges of LMICs was recently reviewed by Lucien et al.19
The discrepancy between the proportion of patients with bacterial coinfections or secondary infections and those receiving antibacterial agents has several hypothetical explanations, including the reaction to the medical uncertainties in the best management of COVID-19 patients during the first weeks of the pandemic. The risk of contracting SARS-CoV-2 by medical staff, the strained health resource and supply chain challenges also reduced the collection of samples for microbiological evaluations,20 reducing the opportunities for informed therapy instead of empirical antibiotics. Reduction in antibiotic stewardship activities2,21 is likely related to the redirection of healthcare resources and specialists to the COVID-19 response.
To evaluate the appropriateness of antibiotic use in the first stage of the COVID-19 pandemic it is important to consider specific criteria for different patient populations, such as ventilated critically ill patients, hospitalised but non-ventilated patients, outpatients and the COVID-19-naïve population. Although the incidence of bacterial coinfections in critically ill COVID-19 patients is low, the infections tended to present during the later stages of hospitalisation, and these infections result from microbiota of the respiratory tract or from the nosocomial environment.22 The diagnosis of ventilator-associated pneumonia (VAP) is complex, both from clinical and aetiological perspectives. Starting early treatment with antibiotics after obtaining adequate samples has been recommended in patients with haemodynamic instability or severe hypoxemia, and patients with a high pretest probability of VAP according to clinical criteria.23 However, treatment can be delayed if these criteria are not present.
Further studies using molecular techniques on samples from ventilated and non-ventilated COVID-19 patients are needed, with current experience suggesting that coinfections and secondary infections are likely to reflect local microbiology and resistance patterns.24,25 However, limitations in safe sampling methods on patients with severe COVID-19 inhibit the possibility of diagnosing respiratory infections, which is difficult without pulmonary lavage.
It is currently unknown whether new or evolving antibiotic resistance in areas with low previous rates will emerge in COVID-19 patients, but this should be examined in retrospective and prospective clinical and microbiology studies.
Community consumption of antibiotics is likely to have significantly changed during the pandemic
The vast majority of COVID-19 patients (80%) have uncomplicated illness and are managed on an outpatient basis. Although there is minimal literature on the management of COVID-19 cases in the community, there exists well-documented inappropriate antibiotic use in self-limiting, viral upper respiratory tract infections in non-hospitalised settings.26,27 Inappropriate antibiotic use is thus equally, if not more likely, in community settings, especially where antibiotics are accessible online, without prescription and from informal drug sellers.
Studies have reported decreased antibiotic use in COVID-19 outpatients28 and in hospitals outside of COVID-19 epicentres.29 There is a need to monitor the relationship between changes in antibiotic use in the community and potential future adverse effects. Removal of physical distancing and lockdowns could lead to an increase in the overall incidence of infections and the number of individuals seeking healthcare, which would likely result in an increase in antibiotic prescriptions. An increase in levels of seasonal influenza and other respiratory viruses would further accentuate this. The use of surveillance systems and differential diagnostics that are simple, affordable and timely, combined with support for research projects, would determine where antibiotics are overused and the impact of antibiotic use on the epidemiology of drug-resistant infections. This is particularly needed in LMICs, where existing data on antibiotic use and resistance are limited.
Factors affecting the spread of bacterial pathogens and AMR
The impact of the COVID-19 pandemic on AMR is unknown.21,26–32 An overview of factors, including changes in infection control practices that could affect AMR, are summarised in Table 1. The focus on the importance of hand hygiene and the correct use of personal protective equipment (PPE) would likely have positive impacts, while cohorting due to high patient numbers33 and shortages in the availability of PPE34 probably promote the transmission of multidrug-resistant bacteria. Existing data already available should be used to better inform antibiotic prescription in the COVID-19 pandemic. In a recent bulletin, the WHO recommended that AMS activities should be integrated as a component of the COVID-19 pandemic response across the broader health system through five measures, including implementation of a research agenda to stem the emergence of AMR infections and diseases.35
Table 1.
Interventions implemented for COVID-19 likely to have an impact on antimicrobial resistance (AMR) in the future
| Patient-related factors | COVID-19 management-related factors | Health-system related factors | |
|---|---|---|---|
| Positive impact | • Personal hygiene/hand and respiratory hygiene• Environmental cleaning• Physical distancing | • Hand hygiene by HCW• Use of PPE• Physical distancing• Environmental cleaning | • Implementation of IPC policies• Implementation of AMS policies• Microbiology and pathology laboratory infrastructure with EQA |
| • Altered health-seeking behaviour | • Universal masking | • Isolation wards | |
| • Decreased travel | • Training of personnel on IPC measures | ||
| Negative impact | • Increased susceptibility to bacterial and fungal infections | • Increased antibiotic exposure, specifically broad-spectrum drugs | • Non-compliance/breakdown of IPC and AMS policies |
| • Increased risk of HAI due to invasive interventions and use of immunosuppressive agents | • Deprioritisation of antimicrobial use and resistance surveillance• Overcrowding of patients | ||
| • Reuse of PPE | • Absence of clear guidelines | ||
| • Lack of isolation wards | • Increase in telemedicine | ||
| • Biocide use | • Decreased laboratory capacity on AMR (antimicrobial susceptibility testing, surveillance cultures…) | ||
| • Excess stress of healthcare providers |
Abbreviations: AMS, antimicrobial stewardship; EQA, external quality assessment; HAI, healthcare-associated infections; HCW, healthcare workers; IPC, infection prevention control; PPE, personal protective equipment.
Disruption of surveillance of AMR in hospitals during the COVID-19 pandemic
Personnel, resources and attention were redirected from AMR surveillance to COVID-19 diagnosis, tracking and tracing. The sharing of raw data from surveillance efforts, including by companies, such as GSK's SOAR programme, Merck & Co, Inc.’s SMART programme, Shionogi's SIDERO-WT and the Shionogi Japanese Surveillance Studies Programme, would enable open research and analysis pertaining to global AMR surveillance. These data could help researchers better understand potential disruptions in surveillance efforts, and highlight and analyse any potential resistance patterns that might have emerged because of inappropriate use of antibiotics linked to COVID-19 and secondary infections. While the WHO's Global Antimicrobial Resistance Surveillance System (GLASS) helps to analyse and report global data, the Access to Medicine Foundation's AMR Benchmark found that companies included in its scope of research had data from 38 countries not covered by GLASS, at the time of publication an essential piece of the global AMR surveillance puzzle and important for understanding the impact of COVID-19 on AMR.36
Disruption of research during the COVID-19 pandemic
Research in non-COVID-19 fields including AMR has been deprioritised, delayed and even halted. Delays in research projects limit the ability of scientists to meet contractual grant deadlines and targets within projects. Limitations on international travel have halted the sharing of information at workshops and conferences, and created delays in international networking activities. There have been disruptions in the review, processing and proofing of non-COVID 19 manuscripts. Many funding agencies across the globe have given research grant extensions; however, the long-term impact of the pandemic on AMR research has yet to be understood and factors such as the effect on early-career researchers may take years to manifest. To ensure that AMR research continues to be adequately prioritised and financially supported, it is important to prioritise funds for AMR research at both national and international scales, and it may be necessary to implement targeted AMR fellowship schemes, and new research support mechanisms, to ensure career entry in the field.
The LMICs and resource-constrained settings
COVID-19 has illustrated how vulnerable our healthcare systems are. This is even more noticeable in LMICs and in resource-constrained settings that lack infrastructure and personnel and are not well prepared to deal with pandemics or other emergencies.36–38 In many of these settings, infection rates, antibiotic use and consequent increase or decrease in antibiotic resistance may be very different. In addition, laboratory infrastructure, surveillance and diagnostic capacity for both COVID-19 and AMR are unreliable, and at times are unavailable in many LMIC settings. In addition, infection prevention and control policies, practice and personnel are noticeably suboptimal and, in many instances, unsustainable.39 Expectations for regulated antibiotic use are difficult to enforce in those settings where there is poor access to antibiotics without prescription and substandard and counterfeit medicines are frequently available. Adding to this, sociobehavioural interventions such as physical distancing and hand hygiene are limited, and large proportions of the population are living hand to mouth, especially in areas with high population densities, such as informal settlements, where there is suboptimal access to clean water and sanitation services. In addition, co-managing multiple infectious disease threats simultaneously is a further challenge for LMICs. Initial reports show a more severe disease and higher death rate in patients coinfected with TB and COVID-19.40 Unless current trends are reversed, the increasing levels of MDR TB will likely have a similar impact on LMICs in the future, with estimates suggesting that by 2050 drug-resistant TB will be responsible for 2.6 million of the total 10 million annual deaths associated with AMR.40,41 An exit strategy from COVID-19 for many LMICs may not be pharmacologically based in the short term and more community-based strategies are currently being explored.42
Key recommendations for the future
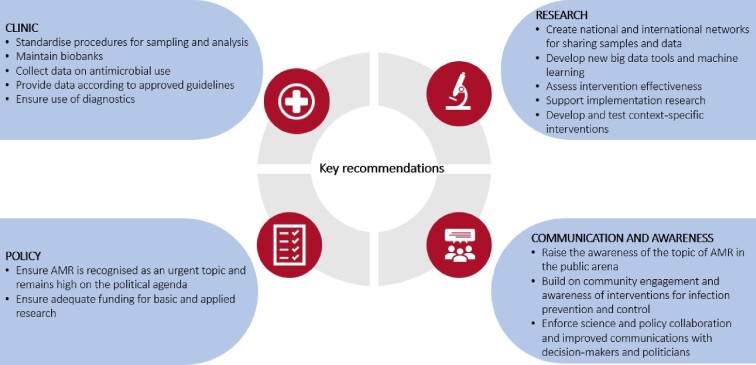
The COVID-19 pandemic has magnified weaknesses in fighting AMR and there are many lessons that can be learnt from the current situation that will affect the ways in which AMR can be addressed. Knight et al. recently published an overview of the impact of the COVID-19 pandemic on AMR and made a call for action for the AMR community to consider the global perspective when dealing with this global health challenge.43 Below, we describe recommendations to define a strong research agenda to facilitate this knowledge and enhance the understanding of beneficial and detrimental practices that drive or limit the spread of AMR. These recommendations are summarised in Figure 1.
Figure 1.
Key recommendations for continued support for antimicrobial resistance (AMR) research for clinical, research and policy stakeholders.
Microbiological diagnosis of coinfections and secondary infections
To study the impact of the COVID-19 pandemic on AMR, it is critical to maintain existing screening and diagnostic systems, and to ensure appropriate collection of microbiological samples for guiding the individual management of patients. Research and innovation focused on developing diagnostic tests differentiating between bacterial infection and SARS-CoV-2 infection, or the use of multiplex diagnostic tests targeting viruses and bacterial pathogens, would prevent the overuse of antibiotics.
Collection of microbiological samples should be guided by clinical presentation and microbiological diagnostics. Practices to collect samples should guarantee the safety of healthcare workers to the best degree possible. An important limitation of the available data is the heterogeneity in microbiological sampling, laboratory microbiological procedures and interpretation of results. Specifically, designed studies with routine collection of samples, appropriate microbiological procedures and adequate criteria for aetiological diagnosis should be performed. The development of standardised protocols by the WHO or a network of interested investigators for microbiological diagnosis of COVID-19-associated coinfections and secondary infections would be useful to ensure data integrity and comparability across study centres.
Samples for biobanks should be collected, using the appropriate informed consent, and many institutions are already collecting samples from COVID-19 patients for future studies. Networking will be important for the greater good of AMR research, with high quality data needed to link clinical case data to microbiology and ensure comparability between hospitals within and between countries.
Early in the COVID-19 pandemic, autopsies were infrequently conducted in patients who died with COVID-19 due to the risk of infection to pathologists and ancillary personnel.44 As a result, limited microbiological information is available in individual hospitals. A minority of autopsies showed inflammatory changes using histopathology that is potentially consistent with bacterial or fungal bronchopneumonia,45 but in the absence of microbiological studies, it is impossible to interpret these findings. Pooling of data and samples is needed to draw accurate conclusions regarding the ultimate causes of death, and to the role of bacterial coinfections.
Predictors (clinical, biomarkers) for bacterial coinfection and secondary infections
Research on the predictive ability of diverse clinical and laboratory investigations at hospital admission for coinfections is needed. Collection of comprehensive data must be standardised in prospective studies and in different profiles of patients, in association with appropriate microbiological sampling. This is critical for any retrospective research, including big data analysis; however, prospective collection of data with the objective of development and validation of predictive scores for bacterial coinfections is needed. Such scores might be useful in deciding which patients might be considered for antibacterial use at admission. Biomarkers such as C-reactive protein and procalcitonin (PCT) that have been used by clinicians to help in diagnosing bacterial infections may be elevated in severe COVID-19 patients,31 which limits their use in defining the proper use and duration of antibiotic therapy. The clinical usefulness of PCT alone or in combination with other biomarkers should be optimised in prospective studies to develop protocols guiding antibiotic treatment of COVID-19 patients. For secondary infections, the usual protocols for nosocomial infections may be used. In addition, studies addressing the impact on outcome of empirical antibacterial coverage in different groups of patients must be designed and performed, as not all patients with coinfection need empirical antibacterial treatment.
Collection and analysis of global data on the use of antibiotics during the COVID-19 pandemic
Collection of antibiotic use data in COVID-19 and non-COVID-19 wards, as well as in the community, is needed. Recommended methods and indicators, as well as stratification for specific families and drugs, and for type of wards, are to be followed.46–52 Analysis according to the AWaRE categorisation of antibiotics would provide invaluable information on whether the choice and/or quantities of antibiotics prescribed could potentially escalate antibiotic resistance. To detect variations in the consumption of some antimicrobials, monthly consumption comparisons with data from preceding years is needed.
When analysing changes in antimicrobial use, the confounding effect of changes in the case-mix occurring in hospitals, particularly during periods of high rates of COVID-19, must be considered. Therefore, collection of data allowing the control of confounders is also needed. Adequate statistical management of the data including the use of time series analyses is required to understand changes in antibiotic consumption among different healthcare and industrial sectors.
Data on the appropriateness of prescribing practices must also be considered, both in the context of treatment guidelines and stewardship principles. To evaluate the quality of prescription, national, regional or local guidelines must exist as a reference. Such guidelines would be subject to change as new evidence becomes available. In terms of AMS, diagnosis confirming non-viral aetiology, antibiotic choice, dose, dosing frequency, route, duration and de-escalation are important considerations.
Surveillance of AMR
Surveillance of resistance must continue and be reinforced, in both COVID-19 and non-COVID-19 patients. The key bacteria, resistance and specific mechanisms of resistance must be recorded with appropriate indicators (incidence density) on a regular basis.48–52 Epidemiological data from patients colonised or infected with high-risk or emerging multidrug-resistant bacteria must be collected. Active surveillance for colonisation must be continued in high-risk areas (ICUs, haematological wards) and in high-risk patients, particularly when cohorting is used for COVID-19 patients.
Investigating the correlation between changes in antibiotic use during the pandemic and evolution and/or escalation of antibiotic resistance must be conducted considering information on hospital structural changes, variations in the composition of the case-mix of the wards, and adjusted by changes in diagnostic procedures and clinical algorithms. Carefully analysing global AMR surveillance data, particularly from surveillance programmes in LMICs where national efforts to monitor resistance may be limited, will be critical to understanding potential emerging patterns of resistance and the contributing factors behind them. All partners collecting data, including pharmaceutical companies, should be encouraged to share the raw data of their programmes on open data platforms.
AMS interventions
It is critical to ensure that AMS programmes remain active. Due to the multidisciplinary approach implemented in many hospitals for the management of COVID-19 patients, stewardship programmes should be reinforced, with activities directed to non-infectious disease physicians, including educational activities, prospective audits of prescriptions and feedback of data.
Of particular importance is diagnostic stewardship of community-acquired pneumonia by general practitioners to control inappropriate usage of antibiotics, not only in patients not infected by SARS-CoV-2, but even more importantly in those suffering from mild COVID-19 and treated at home. Measuring the impact of the interventions should be performed using suitable methods.53 The endpoints may be antibiotic consumption, appropriateness of use, rates of adverse events of antibiotics, overall mortality, duration of hospital stay, rates of antibiotic resistance and need for readmission after discharge. It would be most suitable to design multicentre, cluster randomised trials or well-executed quasi-experimental designs with time series analyses.
Using a multidisciplinary approach to support AMR stewardship and surveillance
Sharing of data and samples is strained by the academically driven weakness of ‘publish or perish’. Although there might have been a disruption to some AMR surveillance activities, the new multidisciplinary networks of infectious disease clinicians, microbiologists and other healthcare workers, formed to attend to COVID-19 patients, have the potential to continue working together to address AMR in the future. Large multinational registries that have been built to support COVID-19 research could be leveraged for AMR research.
Continuing public and political engagement on infectious diseases and promotion of research
Communication between governments, healthcare professionals, scientists, the media and the public has been a key component in the pandemic response.54 The AMR research community is in an ideal position to work with the media and policymakers to raise awareness of the topic of AMR in the public arena and build on community engagement and awareness of the importance of interventions in sanitary infrastructures,55 handwashing, disinfection, social distancing when ill or avoiding unnecessary use of antibiotics. Harnessing the public understanding of the relevance of infectious diseases towards the long-term pandemic of AMR could have major implications for promoting good practices about AMR and the control of transmission.
A critical lesson from the COVID-19 pandemic is the importance of embedding research in the response, which is particularly challenging to perform in the pandemic situation. Supporting good quality implementation research could help to understand how and why an intervention has been successful. As global solidarity efforts and pledges emerge to address COVID-19, so too must efforts to openly share research and data. Cooperation and coordination on behalf of the private sector could contribute to a more fulsome picture of potential changes in antibiotic use in all corners of the world. When it comes to AMR, a research agenda that can generate context-specific solutions for decision-makers will be key to successful preparedness.
Conclusions
The COVID-19 pandemic has demonstrated the economic and societal impact of an uncontrolled infectious disease, an impact that is similar to what has been predicted for AMR in multiple reports. Among the many consequences of the COVID-19 pandemic, there is the important potential impact on AMR through the change in antibiotic use and health-seeking behaviour, as well as infection prevention and control practices. Determining these effects on AMR is critical for both promoting good practices and prioritising research. Being proactive, in the context of predictable AMR, will allow us the luxury of not having to be reactive in the future, as we currently have to be with COVID-19. However, if not addressed, AMR will likely have similar consequences but over a longer timescale.
Contributor Information
Jesús Rodríguez-Baño, Unidad Clínica de Enfermedades Infecciosas, Microbiología y Medicina Preventiva, Hospital Universitario Virgen Macarena, Sevilla, Spain; Departamento de Medicina, Universidad de Sevilla, Sevilla, Spain; Instituto de Biomedicina de Sevilla (IBiS), Sevilla, Spain.
Gian Maria Rossolini, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Clinical Microbiology and Virology Unit, Florence Careggi University Hospital, Florence, Italy.
Constance Schultsz, Department of Global Health - AIGHD Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Evelina Tacconelli, Division of Infectious Diseases, Department of Diagnostic and Public Health, University of Verona, Verona, Italy.
Srinivas Murthy, BC Children's Hospital, University of British Columbia, Vancouver, Canada.
Norio Ohmagari, Disease Control and Prevention Center, National Center for Global Health and Medicine, Tokyo, Japan.
Alison Holmes, Department of Medicine, Faculty of Medicine, Imperial College London, London, UK.
Till Bachmann, The University of Edinburgh, Edinburgh Medical School, Division of Infection and Pathway Medicine, UK.
Herman Goossens, Laboratory of Medical Microbiology, Vaccine & Infectious Disease Institute, University of Antwerp, Antwerp, Belgium.
Rafael Canton, Servicio de Microbiología. Hospital Universitario Ramón y Cajal and Instituto Ramón y Cajal de Investigación Sanitaria, Madrid, Spain; Red Española de Investigación en Patología Infecciosa (REIPI), Instituto de Salud Carlos III, Madrid, Spain.
Adam P Roberts, Department of Tropical Disease Biology, Liverpool School of Tropical Medicine, Liverpool, UK.
Birgitta Henriques-Normark, Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Stockholm, Sweden; Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden.
Cornelius J Clancy, University of Pittsburgh, Pittsburgh, PA, USA.
Benedikt Huttner, Division of Infectious Diseases, Geneva, University Hospitals, Faculty of Medicine, University of Geneva, Geneva, Switzerland.
Patriq Fagerstedt, JPIAMR Secretariat, Swedish Research Council, Stockholm, Sweden.
Shawon Lahiri, JPIAMR Secretariat, Swedish Research Council, Stockholm, Sweden.
Charu Kaushic, Institute of Infection and Immunity, Canadian Institutes of Health Research; McMaster Immunology Research Center, Dept Pathology and Mol. Medicine, McMaster University, Hamilton, Ontario, Canada.
Steven J Hoffman, Global Strategy Lab, Dahdaleh Institute for Global Health Research, Faculty of Health and Osgoode Hall Law School, York University, Toronto, Canada.
Margo Warren, Access to Medicine Foundation, Amsterdam, the Netherlands.
Ghada Zoubiane, International Centre for Antimicrobial Resistance Solutions (ICARS), Copenhagen, Denmark.
Sabiha Essack, International Centre for Antimicrobial Resistance Solutions (ICARS), Copenhagen, Denmark; Antimicrobial Research Unit, University of KwaZulu-Natal, Durban, South Africa.
Ramanan Laxminarayan, Center for Disease Dynamics, Economics & Policy, New Delhi, India.
Laura Plant, Institute of Infection and Immunity, Canadian Institutes of Health Research.
Authors’ contributions
All the authors contributed to conceiving and writing this manuscript.
Acknowledgements
This work was founded on the Joint Programming Initiative on Antimicrobial Resistance webinar series ‘AMR in a post-pandemic world’.
Funding
This work was supported by the Medical Research Council, UK Research and Innovation [grant numbers MR/S004793/1 and MR/S037640/1 to AR], the National Institute for Health Research [grant number NIHR200632 to AR], Plan Nacional de I+D+i 2013–2016 and the Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades [grant numbers REIPI RD16/0016/0001 and RD16/0016/0011 to JRB and RC], the Instituto de Salud Carlos III [grant number AC16/00076 to JRB], the German Federal Ministry of Education and Research [grant number 01KI1830 to ET], Innovative Medicines Initiative 1 and 2 Joint Undertaking [grant numbers 115737, 115523 and 820755 to ET] and the Global Antibiotic Research and Development Partnership (GARDP) to ET. LP, PF and SL would like to acknowledge funding to the JPIAMR from the European Commission (EXEDRA grant no 733296, JPI-EC-AMR grant no 681055 and JPIAMR-ACTION grant no 963864).
Competing interests
SE is chairperson of the Global Respiratory Partnership and member of the Global Hygiene Council both sponsored by unrestricted educational grants from Reckitt and Benckiser Ltd., UK.
Ethical approval
Not required.
Data availability
None.
References
- 1.Yang X, Yu Y, Xu Jet al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;2600(20):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawson TM, Moore LSP, Zhu Net al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020:ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abelenda-Alonso G, Padullés A, Rombauts Aet al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol. 2020;1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beović B, Doušak M, Ferreira-Coimbra Jet al. Antibiotic use in patients with COVID-19: a ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J Antimicrob Chemother. 2020;75(11):3386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch C, Mahida N, Gray J. Antimicrobial stewardship: a COVID casualty? J Hosp Infect. 2020;106(3):401–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velasco-Arnaiz E, López-Ramos MG, Simó-Nebot Set al. Kids Corona Project . Pediatric 205 antimicrobial stewardship in the COVID-19 outbreak. Infect Control Hosp Epidemiol. 2020;206:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nestler M, Godbout E, Lee Ket al. Impact of COVID-19 on pneumonia-focused antibiotic use at an 209 academic medical center. Infect Control Hosp Epidemiol. 2020;1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buehrle DJ, Decker BK, Wagener MMet al. Antibiotic consumption and stewardship at a hospital outside of an early Coronavirus disease 2019 epicentre. Antimicrob Agents Chemother. 2020;64(11):e01011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy CJ, Nguyen MH. COVID-19, superinfections and antimicrobial development: what can we expect? Clin Infect Dis. 2020:ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control . Surveillance of antimicrobial resistance in Europe 2018. Stockholm: ECDC; 2019. [Google Scholar]
- 11.Berenguer J, Ryan P, Rodríguez-Baño Jet al. COVID-19@Spain Study Group. Characteristics and predictors of death among 4,035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26(11):1525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136(11):834–44. [DOI] [PubMed] [Google Scholar]
- 13.Bengoechea JA, Bamford CGG. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol Med. 2020;12:e12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansbury L, Lim B, Baskaran Vet al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020:S0163-4453(20)30323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langford BJ, So M, Raybardhan Set al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Vidal C, Sanjuan G, Moreno-García Eet al. COVID19-researchers group. Incidence of co-infections and superinfections in hospitalised patients with COVID-19: a Retrospective Cohort Study. Clin Microbiol Infect. 2021;27(1):83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaughn V, Gandhi T, Petty LAet al. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clin Infect Dis. 2020:ciaa1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang C-Y, Chan K-G. Underestimation of co-infections in COVID-19 due to non-discriminatory use of antibiotics. J Infect. 2020;81:e29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucien MAB, Canarie MF, Kilgore PEet al. Antibiotics and antimicrobial resistance in the COVID-19 era: perspective from resource-limited settings. Int J Infect Dis. 2021;104:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes S, Troise O, Donaldson Het al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huttner BD, Catho G, Pano-Pardo JRet al. COVID-19: don't neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26(7):808–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Y, Yang Q, Xu Met al. Secondary bacterial infections in critical Ill patients with Coronavirus Disease 2019. Open Forum Infect Dis. 2020;7(6):ofaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.François B, Laterre PF, Luyt CEet al. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Version 2. Crit Care. 2020;24(1):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantón R, Gijón D, Ruiz-Garbajosa P. Antimicrobial resistance in ICUs: an update in the light of the COVID-19 pandemic. Curr Opin Crit Care. 2020;26(5):433–41. [DOI] [PubMed] [Google Scholar]
- 25.Porretta AD, Baggiani A, Arzilli Get al. Increased risk of acquisition of New Delhi metallo-beta-lactamase-producing carbapenem-resistant enterobacterales (NDM-CRE) among a cohort of COVID-19 patients in a teaching hospital in Tuscany, Italy. Pathogens 2020;9(8):E635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekker AR, Verheij TJ, van der Velden AW. Inappropriate antibiotic prescription for respiratory tract indications: most prominent in adult patients. Family Practice. 2015;32(4):401–7. [DOI] [PubMed] [Google Scholar]
- 27.Gulliford MC, Dregan A, Moore MVet al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open. 2014;4(10):e006245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens RW, Jensen K, O'Horo JCet al. Antimicrobial prescribing practices at a tertiary care center in patients diagnosed with COVID-19 across the continuum of care. Infect Control Hosp Epidemiol. 2021;42(1):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaduganathan M, van Meijgaard J, Mehra MRet al. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA. 2020;323(24):2524–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spernovasilis NA, Kofteridis DP. COVID-19 and antimicrobial stewardship: what is the interplay? Infect Control Hosp Epidemiol. 2020:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawson TM, Moore LSP, Castro-Sanchez Eet al. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75(7):1681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawson TM, Ming D, Ahmad Ret al. Antimicrobial use, drug-resistant infections and COVID-19. Nat Rev Microbiol. 2020;18(8):409–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Condes E, Arribas JR; COVID19 MADRID-S.P.P.M. group . Impact of COVID-19 on Madrid hospital system. Enferm Infecc Microbiol Clin. 2020;2020;S0213-005X(20)30236-6. doi:10.1016/j.eimc.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartari E, Hopman J, Allegranzi Bet al. International Society of Antimicrobial Chemotherapy Infection Prevention Control Working Group, (ISAC-IPC) . Perceived challenges of COVID-19 infection prevention and control preparedness: a multinational survey. J Glob Antimicrob Resist. 2020;22:779–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Getahun H, Smith I, Trivedi Ket al. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ. 2020;98:442–A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antimicrobial Resistance Benchmark 2020. Available at https://accesstomedicinefoundation.org/media/uploads/downloads/5e270aa36821a_Antimicrobial_Resistance_Benchmark_2020.pdf. [Google Scholar]
- 37.Lai CC, Wang CY, Wang YHet al. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55(4):105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bong CL, Brasher C, Chikumba Eet al. The COVID-19 pandemic: effects on low- and middle-income countries. Anesth Analg. 2020;131(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubbink JH, Branco TM, Kamara KBet al. COVID-19 treatment in sub-Saharan Africa: if the best is not available, the available becomes the best. Travel Med Infect Dis. 2020;37:101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Neill J. Tackling drug-resistant infections globally: final report and recommendations. London: Her Majesty's Government and Welcome Trust, 2016. [Google Scholar]
- 41.The urgent threat of TB drug resistance. Drug-resistant TB threatens to erase decades of progress. Atlanta: Centers for Disease Control and Prevention. https://www.cdc.gov/globalhivtb/images/dght_mdr-tb_factsheet_10-14-2016_ck_v3.pdf. [Google Scholar]
- 42.Walker PGT, Whittaker C, Watson OJet al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020;369(6502):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight GM, Glover RE, McQuaid CFet al. Antimicrobial resistance and COVID-19: intersections and implications. ELife. 2021;10:e64139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanley B, Lucas SB, Youd Eet al. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73(5):239–42. [DOI] [PubMed] [Google Scholar]
- 45.Grosse C, Grosse A, Salzer HJFet al. Analysis of cardiopulmonary findings in COVID-19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;49:107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng F, Huang Y, Guo Yet al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brotherton AL. Metrics of antimicrobial stewardship programs. Med Clin North Am. 2018;102(5):965–76. [DOI] [PubMed] [Google Scholar]
- 48.Barlam TF, Cosgrove SE, Abbo LMet al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.British Society for Antimicrobial Chemotherapy . Antimicrobial Stewardship. From principles to practice. 2018. Available at http://www.bsac.org.uk/antimicrobialstewardshipebook/BSAC-AntimicrobialStewardship-FromPrinciplestoPractice-eBook.pdf
- 50.Rodríguez-Baño J, Paño-Pardo JR, Alvarez-Rocha Let al. Programs for optimizing the use of antibiotics (PROA) in Spanish hospitals: GEIH-SEIMC, SEFH and SEMPSPH consensus document. Enferm Infec Microbiol Clin. 2012;30(1):22.e1–23. [DOI] [PubMed] [Google Scholar]
- 51.Kritsotakis EI, Gikas A. Surveillance of antibiotic use in hospitals: methods, trends and targets. Clin Microbiol Infect. 2006;12(8):701–4. [DOI] [PubMed] [Google Scholar]
- 52.Tacconelli E, Sifakis F, Harbarth Set al. EPI-Net COMBACTE-MAGNET Group . Surveillance for control of antimicrobial resistance. Lancet Infect Dis. 2018;18(3):e99–106. [DOI] [PubMed] [Google Scholar]
- 53.Schweitzer VA, van Werkhoven CH, Rodríguez Baño Jet al. Optimizing design of research to evaluate antibiotic stewardship interventions: consensus recommendations of a multinational working group. Clin Microbiol Infect. 2020;26(1):41–50. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Cleary PD, Little Jet al. Communicating in a public health crisis. Lancet. 2020;2(10):e503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collignon P, Beggs JJ, Walsh TRet al. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. 2018;2(9):e398–405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.