Abstract
Antibody responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in serum and cerebrospinal fluid (CSF) samples from 16 patients with coronavirus disease 2019 and neurological symptoms were assessed using 2 independent methods. Immunoglobulin G (IgG) specific for the virus spike protein was found in 81% of patients in serum and in 56% in CSF. SARS-CoV-2 IgG in CSF was observed in 2 patients with negative serological findings. Levels of IgG in both serum and CSF were associated with disease severity (P < .05). All patients with elevated markers of central nervous system damage in CSF also had CSF antibodies (P = .002), and CSF antibodies had the highest predictive value for neuronal damage markers of all tested clinical variables.
Keywords: COVID-19, SARS-CoV-2, serology, CSF, IgG, neurological symptoms
Patients with coronavirus disease 2019 and neurological symptoms had immunoglobulin G against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S/S1 protein in both serum (81%) and cerebrospinal fluid (56%). All patients with elevated markers of central nervous system damage also had immunoglobulin G against SARS-CoV-2 in cerebrospinal fluid.
Coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), are associated with neurological manifestations including cerebrovascular disorder, encephalopathy, autoimmune and neuropsychiatric complications [1]. Results of polymerase chain reaction (PCR) for SARS-CoV-2 in cerebrospinal fluid (CSF) are typically negative [2], but a small number of positive cases have been described [3–6]. Results of CSF workup are often normal, although markers of central nervous system (CNS) injury can be elevated [5–8].
Benameur et al [9] investigated and found immunoglobulin G antibodies (IgG) against subunit 1 (S1) of the SARS-CoV-2 spike protein (S) in CSF samples from 3 patients with coronavirus disease 2019 (COVID-19) in whom encephalopathy and encephalitis developed. High titers of anti–SARS-CoV-2 IgG were also detected in CSF samples from 8 patients with neurological symptoms, 1 of whom also showed signs of intrathecal production of anti–SARS-CoV-2 IgG, indicated by a high IgG index [10]. These reports provide important initial findings, but the data are scarce, and immune responses in patients with COVID-19 and neurological symptoms require further characterization. Here, we describe IgG responses against the SARS-CoV-2 S and S1 in serum and CSF samples from patients with mild to severe CNS symptoms.
METHODS
Patients and Study Design
The National Ethical Review Authority approved this study (nos. 2020-01883 and 2014/148). Informed consent was obtained from each patient, or the next of kin if a patient was unable give consent. Patients with confirmed COVID-19 and ≥1 new-onset neurological symptom—including altered mental status, cranial nerve symptoms, paresis, and/or extrapyramidal, cerebellar, and sensory symptoms—were prospectively included from April until September 2020. Patients had either a positive PCR test result for SARS-CoV-2 in nasopharynx samples (n = 15) or SARS-CoV-2–specific IgG against nucleoprotein-based antigen in a serum sample (n = 1). Matched CSF and serum samples were analyzed serologically alongside their routine workup.
Lumbar puncture was carried out in conjunction with or after the period of most severe symptoms. Five patients were sampled >2 months after COVID-19 debut owing to residual cognitive and/or neurological symptoms. Four of these patients had previously been treated in the intensive care unit (ICU) at some stage of their disease. The patients were examined by a neurologist. National Institutes of Health criteria for COVID-19 severity were used to classify patient clinical status as mild, moderate, severe, or critical [11].
Detection of IgG in Serum and CSF Samples
The levels of IgG in serum and in CSF samples against SARS-CoV-2 S or S1 were assessed in 2 independent laboratories using an enzyme-linked immunosorbent assay (ELISA) and a suspension immunoassay (SIA), respectively.
SARS-CoV-2 Spike Timer-Based ELISA
A SARS-CoV-2 ELISA based on nativelike spike trimers was used, as described elsewhere [12]. Briefly, 96-well ELISA plates (Nunc MaxiSorp) were coated with spike trimers (100 μL of 1 ng/μL) in phosphate-buffered saline (PBS) overnight at 4°C. Plates were washed 6 times with 300 mL of PBS–Tween 20 (PBST; 0.05%) and blocked using PBS-5% no-fat milk (Sigma). Thawed serum was diluted 1:100 and CSF diluted 33.3:100 blocking buffer, and samples were incubated overnight at 4°C, before being washed as before. Wells were then incubated for 1 hour at room temperature with secondary horseradish peroxidase–conjugated anti-human goat anti-human IgG (Southern Biotech; 2014-05), diluted 1:10 000 in blocking, and washed with TMB Stabilized Chromogen (Invitrogen) and the reaction was stopped using 1-mol/L sulfuric acid. Results are expressed as optical density values measured at 450 nm, using an Asys Expert 96 ELISA reader (Biochrom). The cutoff for the assay was set at 6 standard deviations above the mean value for negative control samples obtained on the same day (from blood donors in spring 2019).
SARS-CoV-2 S1-Based SIA
Assays were performed using the MagPix system (Luminex), as described elsewhere [13]. First, 10 µg of SARS-CoV-2 S1 protein (40591-V08H, Sino Biological) was covalently coupled to 2.5 × 106 carboxylated paramagnetic beads (MagPlex microspheres; Luminex), using sulfo-N-hydroxysulfosuccinimide and 1-ethyl-3-[3 dimethylaminopropyl]carbodiimide hydrochloride (Pierce Biotechnology–ThermoFisher). Aliquots of 50 µL of bead mix (25 beads per microliter of PBST [0.05% Tween 20]) and 50 µL of diluted serum (1:25 in PBST) or 50 µL of diluted CSF (1:10 in PBST) were mixed and incubated in a 96-well plate for 60 minutes in the dark at room temperature on a plate shaker (at 600 rpm).
Beads were then washed with 100 µL of PBST using a magnet plate separator (Life Technologies–ThermoFisher). Next, 100 µL of biotinylated protein G (Pierce) was added (with 2 µg/mL PBST). The beads were incubated for 30 minutes, as described above, and washed once, followed by 15-minute incubation with 100 µL of phycoerythrin-conjugated streptavidin (Invitrogen-ThermoFisher) (in 2 µg/mL PBST). After resuspension in 100 µL of PBST, the beads were analyzed by measuring the fluorescence of 50 beads per sample at default settings, using xPONENT software (Luminex). Results were expressed as median fluorescence intensity. The cutoff for the assay was set at 6 standard deviations from the mean value for negative controls (blood donors from 2018), plus 10%.
CSF Biomarkers for CNS Injury, Blood-Brain Barrier Injury and Intrathecal IgG Production
Markers for neurological damage were measured in CSF samples. Total tau protein was measured using Lumipulse technology, according to the manufacturer’s instructions (Fujirebio). Neurofilament light chain and glial fibrillary acidic protein were measured using in-house ELISAs, as described elsewhere [14]. Albumin and IgG levels in serum and CSF samples were measured on a Roche Cobas Analyzer (Roche Diagnostics).
Statistical Analysis
Correlation analysis was performed using Spearman rank correlation for variables with skewed distribution. Antibody levels above the cutoff in either or both methods were classified as positive, while those negative with both methods were classified as negative. These binary variables were compared with categories for clinical variables using a likelihood ratio. Student t tests were used for groupwise comparisons of age. To assess the strength of possible predictors in relation to each other, a forward binary logistic regression model was used. The statistical analysis was performed using SPSS software, version 27 (IBM).
RESULTS
Patients Demographics and Characteristics
Clinical characteristics and demographics are presented in Table 1. Routine CSF workup showed that only 2 had increased numbers of cells/pleocytosis. All patients had negative PCR findings for SARS-CoV-2 in CSF at the time point measured in this study (Table 1). The median (interquartile range [IQR]) CSF-serum albumin ratio in the whole sample of patients was 6.1 (4.7–7.4) and the median IgG index was 0.43 (0.40–0.46).
Table 1.
Characteristics and Cerebrospinal Fluid (CSF) Findings in Patients Positive or Negative for Anti-S/S1 Immunoglobulin G in CSF. Neurological Symptoms and Respiratory Support at some stage in the disease course Before Lumbar Puncture
| Finding | Patients by CSF Anti-S/S1 IgG Results, No. (%)a | |
|---|---|---|
| Positive (n = 9) | Negative (n = 7) | |
| Time since symptom onset, median (IQR), d | 30 (21–74) | 43 (4–124) |
| Age, median (IQR), y | 64 (48–73) | 43 (36–54) |
| Male sex, no. (%) | 5 (56) | 3 (43) |
| Severity of COVID-19 | ||
| Mild | 0 (0) | 3 (43) |
| Moderate | 3 (33) | 1 (14) |
| Severe | 1 (11) | 2 (29) |
| Critical | 5 (56) | 1 (14) |
| ICU care | 7 (78) | 3 (33) |
| Duration of ICU stay, median (IQR), d | 10 (2–27) | 0 (0–2) |
| GCS ≤12 | 6 (67) | 1 (14) |
| High-flow oxygen | 2 (22) | 3 (43) |
| Invasive ventilation | 6 (67) | 1 (14) |
| Altered mental status | 8 (89) | 5 (71) |
| Cranial nerve symptoms | 2 (22) | 0 (0) |
| Anosmia or ageusia | 3 (33) | 3 (43) |
| Vertigo | 3 (33) | 4 (57) |
| Headache | 3 (33) | 7 (100) |
| Peripheral paralysis | 2 (22) | 1 (14) |
| Central paralysis | 3 (33) | 3 (43) |
| Sensory symptoms | 1 (11) | 3 (43) |
| Pleocytosis | 0 (0) | 2 (29) |
| CSF-serum albumin ratio,b median, (IQR), ×103 | 7.4 (6.5–12.5) | 4.5 (3.8–5.3) |
| IgG index,b median (IQR) | 0.44 (0.42–0.49) | 0.42 (0.38–0.44) |
| OCBs | 1 (11) | 1 (14)c |
| Elevated IgG in CSFd,e | 3 (33) | 0 (0) |
| Elevated T-taud | 5 (56) | 1 (14)f |
| Elevated NfLd | 7 (78) | 0 (0)g |
| Elevated GFApd | 2 (22) | 0 (0)g |
Abbreviations: COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; GCS, Glasgow Coma Scale; GFAp, glial fibrillary acidic protein; ICU, intensive care unit; IgG, immunoglobulin G; IQR, interquartile range; NfL, neurofilament light chain; OCB, oligoclonal bands; T-tau, total tau protein.
aData represent no. (%) of patients unless otherwise specified.
bThe CSF-serum albumin ratio was calculated as CSF albumin/serum albumin, a measure of blood-brain barrier function (reference ranges: age 15–45 years, <6.8; age >45 years, <10.2), while the IgG index was calculated as (CSF IgG/serum IgG), as a measure of intrathecal IgG production (reference range: age >15 years, <0.63).
cUnique for CSF.
dIncreases in the T-tau, NfL, and GFAp biomarkers were determined in relation to age-related normal reference limits. The reference ranges for these assays were as follows: T-tau, <360 ng/L for age <50 and <479 ng/L for age >50 years; NfL, <560, <890, and <1850 ng/L for ages 30–40, 40–60, and >60 years, respectively; and GFAp, <750 and <1250 ng/L for ages 20–60 and >60 years, respectively.
eNonspecific increase in CSF IgG.
fData missing for 3 individuals.
gData missing for 1 individual.
Comparison of The Karolinska Institute and Uppsala University Methods for IgG Serology
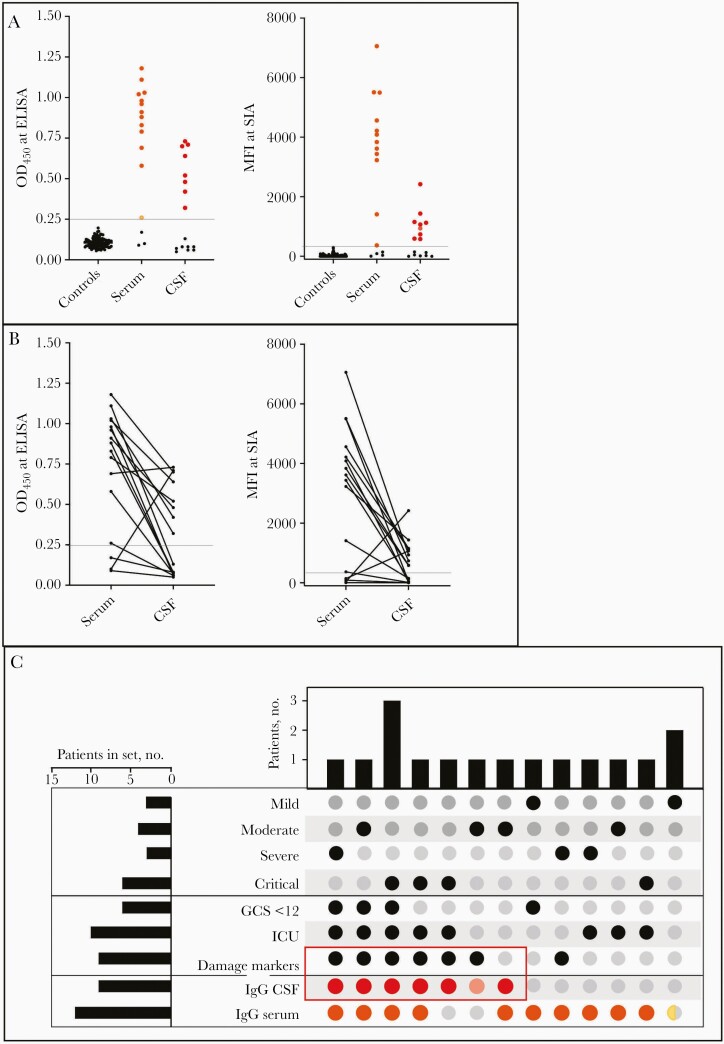
The correlation between IgG against the SARS-CoV-2 S and S1, using ELISA based on S-trimers or SIA based on the S1 subunit of S, was high in both serum (ρ = 0.659; P = .006) and CSF (ρ = 0.867; P < .001) samples (Figure 1A). In total, 13 patients (81%) were classified as seropositive for serum and 9 (56%) as seropositive for CSF samples by either or both methods (Figure 1B). Both methods identified 12 patients as positive for anti-S/S1 IgG in serum and 8 for anti-S/S1 IgG in CSFsamples. Individual results with either method are presented in Figure 1C.
Figure 1.
A, Anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein immunoglobulin G (anti-S/S1 IgG) in serum and cerebrospinal fluid (CSF) samples, determined using enzyme-linked immunosorbent assay (ELISA) and suspension immunoassay (SIA). Cutoffs are indicated: 0.25 for optical density at 450 nm (OD450) and 300 for median fluorescence intensity (MFI). Twelve cases with anti-S/S1 IgG values over the cutoff in serum samples (orange) and 8 in CSF samples (red) were identified with both methods; 1 case over the cutoff with only 1 method was noted for serum (yellow) and 1 case for CSF (pink). B, Relationship between serum and CSF levels of anti-S/S1 IgG in individual cases for SIA and ELISA. Both methods detected a case with higher levels in CSF than in serum, and an additional case was identified with SIA. C, UpSet plot showing the distribution of patients in the cohort with different combinations of variables. Red rectangular box indicates overlap in anti-S/S1 IgG over cutoff in CSF, using both methods (red) or 1 method (pink), with damage markers (high levels of central nervous system injury markers, ie, neurofilament light chain, glial fibrillary acidic protein, and total tau protein) in 8 cases. The other clinical variables (sets), based on the time from debut to sample collection, include the severity of coronavirus disease 2019 (mild, moderate, severe, or critical) Glasgow Coma Scale (GCS) <12, and requirement for intensive care unit (ICU) care. Serum anti-S/S1 IgG levels over the cutoff are indicated, seen with both methods (orange) or 1 method (yellow). One patient with mild disease did not have an IgG level above the cutoff.
SARS-CoV-2 S and S1 Antibodies in CSF
There was no correlation between anti-S/S1 IgG in serum and CSF using either method (ELISA, ρ = 0.271 and P = .31; SIA, ρ = 0.235 and P = .38). Two patients were found to have anti-S/S1 IgG in CSF but not in serum; 1 of them, sampled 12 days after symptom debut, had a positive finding only with the SIA method. Both patients presented with altered mental state and fever and had elevated levels of neurofilament light chain in CSF. One of them recovered within days, while the other required ICU care. Neuroimaging findings were negative in both patients.
The CSF-serum albumin ratio was correlated strongly with anti-S/S1 IgG in CSF for both methods (ELISA, ρ = 0.694 and P = .004; SIA ρ = 0.798 and P < .001), but no correlation with IgG index could be observed. Neither was the IgG index correlated with anti-S/S1 IgG in CSF detected using any of the methods. Patients with positive findings of SARS-CoV-2 S and S1 IgG in CSF samples had significantly higher median CSF-serum albumin ratios (7.4 [IQR: 6.5–12.5]) than those with negative findings (4.5 [IQR: 3.8–5.3]; P = .004); no significant differences could be seen in IgG index between these groups.
SARS-CoV-2 S/S1 Antibodies in CSF Samples in Relation to Clinical Variables
SARS-CoV-2 S/ S1 IgG levels in CSF samples were explored for possible correlation to clinical presentation and/or outcome. The distribution of symptoms in patients with or without IgG in CSF is shown in Table 1 and in Figure 1D. There was a positive correlation between National Institutes of Health criteria for COVID-19 severity and the levels of anti-S/S1 IgG in serum (P < .05) or CSF (P < .05) samples. COVID-19 severity was not correlated with CSF-serum albumin ratio. Seven patients with anti-S/S1 IgG in CSF required ICU care, 6 required invasive ventilation, and 6 had a moderate to severe GCS (≤12) at some point before lumbar puncture. Importantly, all 7 patients with ≥1 elevated biomarker for neurological damage had anti-S/S1 IgG in CSF samples (P = .002).
With a forward binary logistic regression model, the presence of anti-S/S1 IgG in CSF was the only factor that predicted elevated markers of CNS damage, with an odds ratio of 40 (95% confidence interval, 2–794; P = .02). The other factors tested in the model were not significant, including COVID-19 severity, age group, invasive ventilation, sex, ICU care, and days since symptoms onset. Restricting the IgG-positive group to only the 8 patients identified using both methods in CSF did not change the results.
Discussion
Patients with COVID-19 and neurological symptoms had IgG against the SARS-CoV-2 S/S1 protein in both serum (81%) and CSF (56%) samples. CSF antibody–positive patients were overrepresented among patients requiring invasive ventilation. Furthermore, all patients with elevated markers of CNS damage also had IgG against SARS-CoV-2 in CSF. The results support previous findings of CSF IgG in individuals with severe disease and encephalopathy [10].
It remains uncertain whether SARS-CoV-2–specific antibodies in CSF indicate intrathecal production or a passive diffusion due to blood-brain barrier impairment that could be linked to COVID-19 severity. We found a strong significant correlation between detection of SARS-CoV-2–specific IgG in CSF and COVID-19 severity, level of consciousness, and respiratory symptoms. Increased CSF-serum albumin ratios indicate disturbance of the blood-brain barrier, and values above the reference range were found in 3 patients, all with SARS-CoV-2–specific antibodies in CSF. Those 3 patients were treated in the ICU and had critical COVID-19. No correlation was seen between IgG index and the SARS-CoV-2 antibodies in CSF. There was no correlation between serum and CSF levels of IgG against SARS-CoV-2. We also found SARS-CoV-2 IgG in CSF in 2 patients with negative serological findings. The choroid plexus is lacking tight junctions and might enable an easier breach of barrier.
Our findings support and extend the results in a previous report of 3 patients with encephalopathy or encephalitis, elevated levels of cytokines, and laboratory-confirmed coronavirus disease with increased CSF levels of anti-S1 IgM but negative PCR findings [9] and in a study of 2 patients with COVID-19 encephalopathy, who had elevated antibody titers against SARS-CoV-2 S1, S2, and nucleocapsid in CSF [15]. A strength of our study is the use of 2 methods for IgG analysis (solid phase and suspension), which gave mainly concordant results. Differences in detected IgG responses between the 2 methods may be due to the use of different S antigen preparations and/or different detection methods. Further analysis of antibody responses in CSF samples from patients with neurological symptoms during or after COVID-19 may help explain disease sequelae after SARS-CoV-2 infection.
Our population, although larger than in previous publications, represents few patients with heterogeneous clinical presentations and a wide span in sampling dates, which may have affected the IgG levels. The measurement of IgG was semiquantitative, and binary categorization of the data was used in the main analysis for this reason. In 2 patients who were anti-S/S1 IgG negative, lumbar puncture was performed only 4 days after symptom onset, which was probably too early for formation of IgG. The current study identified patients with severe COVID-19 who displayed SARS-CoV-2–specific IgG in CSF and were at high risk of CNS damage detectable by CSF biomarkers. These patients may benefit from early detection and early treatment interventions. Negative serological findings did not exclude CSF positivity for SARS-CoV-2 IgG, as these was observed in 2 patients. Further studies are needed to investigate whether detection of anti–SARS-CoV-2 IgG in CSF is a result of intrathecal production. Future work should establish the effects of the virus on the CNS and examine the relationship between CSF positivity and treatment response and prognosis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants; Uppsala Biobank for sample management; and Svante Berg, Mats Rydén, and Mikaela Magnusson for excellent research assistance.
Financial support. This work is supported by the Swedish Research Council (including grants 2018–02532 to H. Z., 2014-02569 and 2014-07606 to R. F., 2017-00968 to G. B. K. H., and 2018-02569 to Å. L.), the Open Medicine Foundation, SciLife/KWA, the ALF agreement between the Swedish government and the county councils (J. L. C.), the Swedish Society for Medical Research. (J. V.), the Wallenberg Foundations (Wallenberg clinical fellowship to E. R. and. Wallenberg scholarship to H. Z.), the Swedish Research Council (grant 2018–02532 to H. Z.), the European Research Council (grant 681712 to H. Z.), Swedish State Support for Clinical Research (grant ALFGBG-720931 to H. Z.), the Alzheimer Drug Discovery Foundation (grant 201809-2016862 to H. Z.), the European Union’s Horizon 2020 research and innovation program (Marie Skłodowska-Curie grant 860197 to H. Z.), the UK Dementia Research Institute at UCL (H. Z.), the Knut and Alice Wallenberg Foundation and Science for Life Laboratory Uppsala (projects “Nevermore Covid” and “SiCoV” to Å. L.), and the European Union’s Horizon 2020 research and innovation program (grant 874735 to Å. L.; versatile emerging infectious disease observatory).
Potential conflicts of interest. H. Z. has served on scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a cofounder of Brain Biomarker Solutions in Gothenburg, part of the GU Ventures Incubator Program. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell 2020; 183:16–27.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bellon M, Schweblin C, Lambeng N, et al. Cerebrospinal fluid features in SARS-CoV-2 RT-PCR positive patients. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Virhammar J, Kumlien E, Fällmar D, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 2020; 95:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 2020; 94:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Espíndola OM, Brandão CO, Gomes YCP, et al. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int J Infect Dis 2021; 102:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edén A, Kanberg N, Gostner J, et al. CSF biomarkers in patients with COVID-19 and neurologic symptoms: a case series. Neurology 2021; 96:e294–300. [DOI] [PubMed] [Google Scholar]
- 7. Kanberg N, Ashton NJ, Andersson LM, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology 2020; 95:e1754–9. [DOI] [PubMed] [Google Scholar]
- 8. Virhammar J, Naas A, Fallmar D, et al. Biomarkers for CNS injury in CSF are elevated in COVID-19 and associated with neurological symptoms and disease severity. Eur J Neurol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benameur K, Agarwal A, Auld SC, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis 2020; 26: 2016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alexopoulos H, Magira E, Bitzogli K, et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm 2020; 7:e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available from: www.covid19treatmentguidelines.nih.gov. [PubMed] [Google Scholar]
- 12. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367:1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dopico XC, Hanke L, Sheward DJ, et al. Antibody responses to SARS-CoV-2 train machine learning to assign likelihood of past infection during virus emergence in Sweden. medRxiv [Preprint: not peer reviewed]. 19 October 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.07.17.20155937v3. [Google Scholar]
- 14. Gaetani L, Höglund K, Parnetti L, et al. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther 2018; 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andriuta D, Roger PA, Thibault W, et al. COVID-19 encephalopathy: detection of antibodies against SARS-CoV-2 in CSF. J Neurol 2020; 267:2810–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.