Abstract
Background
Bone cement implantation syndrome (BCIS) is characterized by hypoxia, hypotension, and the loss of consciousness during cemented arthroplasty; it may result in death. Its incidence has only been explored for hemiarthroplasty and THA after fracture or cancer. To our knowledge, there are no studies that comprehensively explore and compare the incidence of BCIS in other arthroplasty procedures.
Questions/purposes
(1) To report the incidence of BCIS in TKA, unicondylar knee arthroplasty, hip hemiarthroplasty, THA, shoulder arthroplasty, TKA, and revision THA and TKA; (2) to determine whether severe BCIS is associated with an increased risk of death within 30 days of surgery; and (3) to identify factors associated with the development of severe BCIS.
Methods
All patients undergoing cemented arthroplasty for any reason (TKA [11% cemented, 766 of 7293], unicondylar knee arthroplasty [100% cemented, 562 procedures], hip hemiarthroplasty for femur fractures [100% cemented, 969 procedures], THA [8% cemented, 683 of 8447], shoulder arthroplasty [84% cemented, 185 of 219], and revision arthroplasty of the hip and knee [36% cemented, 240 of 660]) between January 2008 and August 2019 were considered for inclusion in the current retrospective observational study. Fixation choice was dependent on surgeon preference (THA and TKA), prosthesis design (shoulder arthroplasty), or bone quality (revision arthroplasty). The following procedures were excluded because of insufficient data: < 1% (1 of 766) of TKAs, 1% (4 of 562) of unicondylar knee arthroplasties, 6% (54 of 969) of hip hemiarthroplasties, 1% (6 of 683) of THAs, 6% (12 of 185) of shoulder arthroplasties, and 14% (34 of 240) of revision procedures. This resulted in a final inclusion of 3294 procedures (765 TKAs [23%], 558 unicondylar knee arthroplasties [17%], 915 hip hemiarthroplasties [28%], 677 THA [21%], 173 shoulder arthroplasties [5%], and 206 revision arthroplasties [6%]), of which 28% (930 of 3294) had an emergent indication for surgery. Of the patients, 68% (2240 of 3294) were females, with a mean age of 75 ± 11 years. All anesthetic records were extracted from our hospital’s database, and the severity of BCIS was retrospectively scored (Grade 0 [no BCIS], Grade 1 [O2% < 94% or fall in systolic blood pressure of 20% to 40%], Grade 2 [O2% < 88% or fall in systolic blood pressure of > 40%], and Grade 3 [cardiovascular collapse requiring CPR]). Procedures were dichotomized into no or moderate BCIS (Grades 0 and 1) and severe BCIS (Grades 2 and 3). The adjusted 30-day mortality of patients with severe BCIS was assessed with a multivariate Cox regression analysis. A multivariate logistic regression analysis was performed to identify factors associated with the development of severe BCIS.
Results
BCIS occurred in 26% (845 of 3294) of arthoplasty procedures. The incidence was 31% (282 of 915) in hip hemiarthroplasty, 28% (210 of 765) in TKA, 24% (165 of 677) in THA, 23% (47 of 206) in revision arthroplasty, 20% (113 of 558) in unicondylar knee arthroplasty, and 16% (28 of 173) in shoulder arthroplasty. Patients with severe BCIS were more likely (hazard ratio 3.46 [95% confidence interval 2.07 to 5.77]; p < 0.001) to die within 30 days of the index procedure than were patients with less severe or no BCIS. Factors independently associated with the development of severe BCIS were age older than 75 years (odds ratio 1.57 [95% CI 1.09 to 2.27]; p = 0.02), American Society of Anesthesiologists Class III or IV (OR 1.58 [95% CI 1.09 to 2.30]; p = 0.02), and renal impairment (OR 3.32 [95% CI 1.45 to 7.46]; p = 0.004).
Conclusion
BCIS is common during cemented arthroplasty; severe BCIS is uncommon, but it is associated with an increased risk of death within 30 days of surgery. Medically complex patients undergoing hip hemiarthroplasty may be at particular risk. Patients at high risk for severe BCIS (renal impairment, ASA III/IV, and age older than 75 years) should be identified and preventive measures such as medullary lavage before cementation, femoral venting, and avoidance of excessive pressurization of implants should be taken to reduce the likelihood and consequences of BCIS. Because of the increased risk of periprosthetic fractures in uncemented hip stems, factors associated with the development of BCIS should be weighed against the risk factors for sustaining periprosthetic fractures (poor bone quality, female sex) to balance the risks of fixation method against those of BCIS for each patient.
Level of Evidence
Level III, therapeutic study.
Introduction
Bone cement implantation syndrome (BCIS) is a complication associated with the implantation of polymethyl methacrylate bone cement [8]. Hypoxia, hypotension, and/or unexpected loss of consciousness often occur because of cementation, prosthesis insertion, joint reduction, or tourniquet deflation [8]. BCIS may lead to cardiac arrest or death because of massive pulmonary emboli [8]. Pressurization and expansion of the cement between the bone and implant during prosthesis insertion might be responsible for the formation and release of emboli that cause the array of symptoms that are observed in patients with BCIS [8, 23]. Postmortem investigations showed that these emboli originate from fat, polymethyl methacrylate, and bone dust [7, 12, 23, 25]. Hypersensitivity (with histamine release), mediator release from the formed emboli, and complement activation are also thought to play a substantial role in the pathophysiology of BCIS [2, 9, 13, 15, 18, 19, 31].
BCIS is mainly known for its association with hip hemiarthroplasty, THA, and vertebroplasty, but is also seen during TKA [8]. The incidence and associated mortality of BCIS have only been investigated in cemented hemiarthroplasty after displaced femoral neck fractures and in cemented THA and hemiarthroplasty in patients with cancer [22, 27]. To our knowledge, the incidence (compared with hemiarthroplasty or THA), associated factors, and mortality associated with BCIS are not yet known for other hip, knee, or shoulder arthroplasties.
We therefore sought (1) to report the incidence of BCIS in TKA, unicondylar knee arthroplasty, hip hemiarthroplasty, THA, shoulder arthroplasty, TKA, and revision THA and TKA; (2) to determine whether severe BCIS is associated with an increased risk of death within 30 days of surgery; and (3) to identify factors associated with the development of severe BCIS.
Patients and Methods
Patient Selection
In this study, we retrospectively analyzed anesthesia records; consequently, it was considered exempt from review by our hospital’s ethics committee. All patients undergoing cemented arthroplasty at our institution since the inception of structured electronic anesthetic data collection (January 1, 2008) until the date of review (August 1, 2019) were selected. All patients who underwent TKA (always fully cemented primary components; no stemmed components were used), unicondylar knee arthroplasty (always fully cemented), cemented hip hemiarthroplasty, THA (either fully cemented or hybrid), shoulder arthroplasty (all anatomic, reverse, and stemless designs were performed using cemented glenoid and uncemented humeral components), and revision hip or knee arthroplasty (always fully cemented) were identified. During this same time period, there were no uncemented unicondylar knee arthroplasties or hemiarthroplasties performed at our institution; 89% (6527 of 7293) were uncemented TKAs, 92% (7764 of 8447) were uncemented THAs, 21% (46 of 219) were uncemented shoulder arthroplasties, and 69% (454 of 660) were uncemented revision hip and knee arthroplasties. Fixation choice was based on surgeon preference (TKA, THA), prosthesis design (shoulder arthroplasty), and bone quality at the time of surgery (revision arthroplasties). The only inclusion criterion was the use of polymethyl methacrylate bone cement to fix the implant or components (including hybrid fixation). Patients with insufficient or missing anesthetic data were excluded. All patients were operated on at our center by an experienced surgeon or a surgeon who was supervised by an experienced surgeon. Cement was always mixed in a vacuum, and a cement gun was used in all procedures (no finger packing was used).
Patient Population
A total of 3294 procedures in 3010 patients were included in the final analysis (Table 1). We excluded the following procedures because of insufficient data: < 1% (1 of 766) of TKAs, 1% (4 of 562) of unicondylar knee arthroplasties, 6% (54 of 969) of hip hemiarthroplasties, 1% (6 of 683) of THAs, 6% (12 of 185) of shoulder arthroplasties, and 14% (34 of 240) of revision procedures. These excluded patients were similar with regard to age, sex, American Society of Anesthesiologists (ASA) class, and smoking to the included patients. The study group consisted of 765 TKA procedures (median age 72 years [range 45 to 93], 64% [486] females, all elective), 558 unicondylar knee arthroplasties (median age 65 years [range 39 to 89], 54% [301] females, all elective), 915 hip hemiarthroplasties (median age 85 years [range 51 to 103], 66% [607] females, 92% [845] emergent indication), 677 THA procedures (median age 79 years [range 48 to 96], 82% [553] females, 8% [56] emergent indication), and 173 shoulder arthroplasties (median age 73 years [range 46 to 90], 80% [139] females, 4% [7] emergent indication). The median (range) follow-up time was 916 days (174 to 4377) for all patients. The overall 30-day and 3-year survival of all included patients was 98% (95% confidence interval 97 to 98) and 86% (95% CI 85 to 88), respectively. Thirty-day survival was 98% (95% CI 97 to 99), 98% (95% CI 97 to 99), 92% (95% CI 88 to 96), and 0% in patients with BCIS Grade 0 (no BCIS), Grade 1, Grade 2, and Grade 3, respectively (Fig. 1), to be explained in Outcome Measures section. Thirty-day mortality occurred in 96% (80 of 83) of patients who underwent hip hemiarthroplasty, 2% (2 of 83) of patients who had THA, and 1% (1 of 83) of patients who underwent revision arthroplasty; mortality did not occur within 30 days in patients with TKA, unicondylar knee arthroplasty, or shoulder arthroplasty. Three-year survival was 87% (95% CI 85 to 89), 87% (95% CI 84 to 90), 79% (95% CI 73 to 86), and 0% in BCIS Grades 0, 1, 2, and 3, respectively (Fig. 2).
Table 1.
Clinical characteristics of all included patients who underwent arthroplasty
| Factor | Grades 0 and 1 (n = 3105) | Grades 2 and 3 (n = 189) | Total (n = 3294) |
| Age in years, mean ± SD | 75 ± 10 | 79 ± 11 | 75 ± 11 |
| Female, % (n) | 68 (2107) | 70 (133) | 68 (2240) |
| BMI in kg/m2, mean ± SD | 27 ± 5 | 27 ± 6 | 27 ± 5 |
| Cigarette smokers, % (n)a | 12 (293) | 12 (17) | 12 (310) |
| ASA classification, % (n)a | |||
| I | 13 (318) | 6 (9) | 12 (327) |
| II | 61 (1506) | 51 (73) | 60 (1579) |
| III | 25 (632) | 35 (50) | 26 (682) |
| IV | 1 (30) | 7 (10) | 2 (40) |
| Spinal anesthesia, % (n)ab | 90 (2062) | 89 (121) | 90 (2183) |
| Elective, % (n)c | 73 (2256) | 57 (108) | 72 (2364) |
| Serum hemoglobin level in g/dL, mean ± SD | 8 ± 1 | 8 ± 1 | 8 ± 1 |
| Serum creatinine level in µmol/L, mean ± SD | 80 ± 36 | 89 ± 42 | 81 ± 37 |
| Medication use, % (n) | |||
| Antihypertensive agents | 55 (1716) | 62 (117) | 56 (1833) |
| β-blockers | 26 (804) | 35 (67) | 26 (871) |
| Diuretics | 20 (632) | 25 (47) | 21 (679) |
| Calcium antagonists | 16 (510) | 17 (32) | 17 (542) |
| Organic nitrates | 7 (206) | 10 (18) | 7 (224) |
| Anticoagulants | 34 (1066) | 43 (81) | 35 (1147) |
| Vitamin K antagonists | 9 (263) | 13 (24) | 9 (287) |
| Antiplatelet drugs | 23 (699) | 25 (48) | 23 (747) |
| Glucose lowering agents | 13 (403) | 12 (23) | 13 (426) |
| Statins | 32 (990) | 32 (60) | 32 (1050) |
Numbers do not add up due to missing data.
The remainder of patients had general anesthesia.
The remainder of patients had an emergent indication for surgery.
Fig. 1.
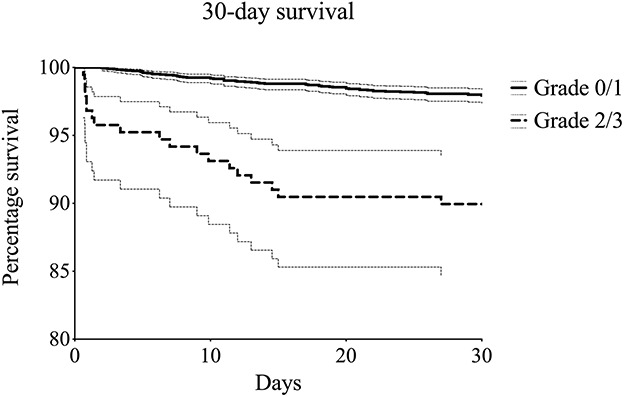
Kaplan-Meier survival function curves (with 95% confidence intervals) were used for an exploratory comparison of severe (Grade 2/3) and no or moderate BCIS (Grade 0/1), with mortality after 30 days as the endpoint.
Fig. 2.
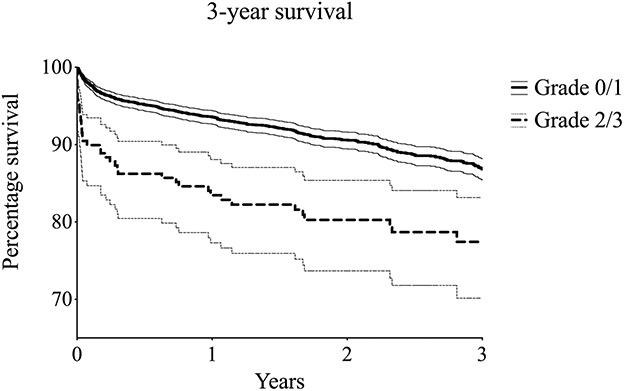
Kaplan-Meier survival function curves (with 95% confidence intervals) were used for an exploratory comparison of severe (Grade 2/3) and no or moderate BCIS (Grade 0/1), with mortality after 3 years as the endpoint.
Baseline Parameters
Baseline parameters included age, sex, BMI, history of smoking, ASA classification, operative time, preoperative hemoglobin levels, preoperative creatinine levels, indication for surgery (elective or emergency), and type of anesthesia (general or spinal).
According to the Anatomical Therapeutic Chemical classification system recommended by the WHO [35], the use of the following medication was extracted from the hospital database: antihypertensive agents (C02 + 03 + 07 + 08 + 09), β-blockers (C07), diuretics (C03), organic nitrates (C01DA), calcium antagonists (C08), ACE inhibitors (C09A + B), anticoagulants (B01), vitamin K antagonists (B01AA), antiplatelet drugs (B01AC), glucose-lowering agents (A10A + 10B), and statins (C10AA + B).
Outcome Measures
The primary study outcome was the incidence of BCIS after cemented arthroplasty.
All anesthetic records were retrospectively assessed for systolic blood pressure and arterial oxygenation, which were measured directly after induction of anesthesia and every 5 minutes thereafter. These measurements were recorded after induction (T1), within 15 minutes before prosthesis insertion (T2), within 5 minutes after the application of bone cement (T3), and in the postanesthesia recovery unit (T4). Based on the criteria of Donaldson et al. [8], BCIS was then retrospectively classified into a grade of severity based on the lowest systolic blood pressure and arterial oxygenation after application of cement (that is, the difference between T3 or T4 and T2) according to Olsen et al. [22]: Grade 0: no hypoxia (O2% > 94%) and no fall in systolic blood pressure (decrease of < 20%); Grade 1: moderate hypoxia (O2% < 94%) or moderate fall in systolic blood pressure (decrease of 20% to 40%); Grade 2: severe hypoxia (O2% < 88%) or severe fall in systolic blood pressure (decrease of > 40%); and Grade 3: cardiovascular collapse resulting in cardiopulmonary resuscitation.
According to Donaldson et al. [8], an unexpected loss of consciousness is also a symptom of Grade 3 BCIS. Because this was not reported consistently, we classified procedures according to the fall in systolic blood pressure or hypoxia after cement implantation.
The secondary outcome was all-cause mortality within 30 days postoperatively. Information on mortality has been extracted from the Dutch population register (Basisregistratie personen; date accessed of January 22, 2020), which is based on citizen service number and therefore represents accurate mortality data. To assess the association between severe BCIS and the risk of death within 30 days, we performed a multivariate Cox regression analysis (with adjustments for potential confounders such as age, procedure type, and ASA classification). To assess the association between patient factors and the development of severe BCIS, we constructed a logistic regression model in which we univariately tested all parameters summarized below and backward selected these variables to assess which parameters were independently associated with the development of severe BCIS.
The following parameters were assessed as potential patient factors associated with the development of severe BCIS: age (older than 75 years versus 75 years and younger), sex, obesity (BMI > 30 kg/m2 versus BMI ≤ 30 kg/m2) [34], smoking, ASA classification (III or IV versus I or II), anesthesia type (general or spinal), indication for surgery (emergent [within 48 hours] or elective), anemia according to the WHO definition (< 8.1 mmol/L in men and < 7.5 mmol/L in women) [33], renal impairment (serum creatinine level > 150 μmol/L), procedure type, and use of medication. All patient factors were determined a priori.
Statistical Analysis
All data were entered into Microsoft Excel (Redmond, WA, USA). After the inclusion and exclusion criteria were applied and patients were selected, the severity of BCIS was scored. All data were then exported into SPSS version 26.0 (IBM SPSS, Armonk, NY, USA) for statistical analysis. The incidence of BCIS and its severities are expressed as percentages for each type of joint arthroplasty. Continuous baseline values are reported as means and SDs (or medians and interquartile ranges in case of non-normality). Categorical, nominal, and dichotomous values are expressed as percentages and frequencies. The severity of BCIS was dichotomized into no or moderate BCIS (Grades 0 and 1) and severe BCIS (Grades 2 and 3) for survival analyses and construction of a multivariate logistic regression model [22].
An association model was constructed using a multivariate Cox regression analysis to calculate the hazard ratio of severe BCIS for 30-day mortality, with correction for possible confounders (age, smoking, sex, renal impairment, emergent surgery, and ASA classification). To identify possible factors associated with the development of severe BCIS, we selected statistically significant variables (with an adjusted significance level of p < 0.10) by performing univariate logistic regression analyses, which revealed that age older than 75 years, ASA Class III or IV, emergent surgery, renal impairment, procedure type, and use of certain types of medication (antihypertensive agents, β-blockers, anticoagulants, and vitamin K antagonists) were potentially associated with severe BCIS. A multivariate logistic regression model was then constructed using stepwise backward selection to determine the odds ratio of each factor for the development of BCIS. A p value of < 0.05 was considered statistically significant.
Results
Incidence of BCIS
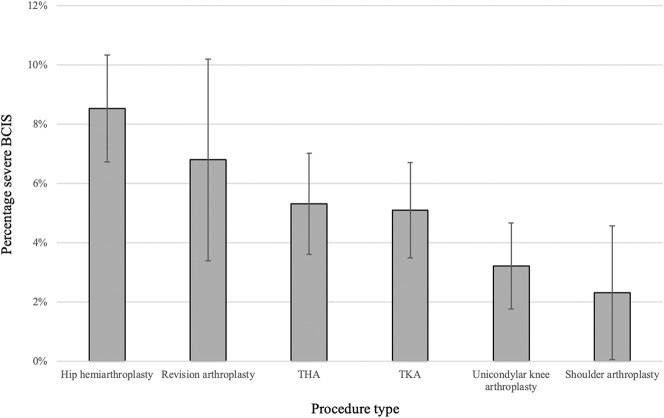
The overall incidence of BCIS among all included arthroplasties was 26% (845 of 3294) (Table 2). The incidence was 31% (282 of 915) in hemiarthroplasty of the hip, 28% (210 of 765) in TKA, 24% (165 of 677) in THA, 23% (47 of 206) in revision arthroplasty, 20% (113 of 558) in unicondylar knee arthroplasty, and 16% (28 of 173) in shoulder arthroplasty. The incidence of severe BCIS (Grades 2 or 3) was 9% (78 of 915) in patients undergoing hip hemiarthroplasty, 7% (14 of 206) in those undergoing revision arthroplasty, 5% (36 of 677) in those with THA, 5% (39 of 765) in those with TKA, 3% (18 of 558) in those with unicondylar knee arthroplasty, and 2% (4 of 173) in those undergoing shoulder arthroplasty (Fig. 3). Less than 1% (4 of 915) of patients who underwent cemented hip hemiarthroplasty had Grade 3 BCIS; all of those were immediately resuscitated but did not survive the procedure (Table 2).
Table 2.
Incidence of BCIS for different arthroplasties
| Procedure | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Total |
| Hemiarthroplasty of the hip, % (n) | 69 (633) | 22 (204) | 8 (74) | 0.4 (4) | 915 |
| Revision arthroplasty, % (n) | 77 (159) | 16 (33) | 7 (14) | 0 (0) | 206 |
| THA, % (n) | 76 (512) | 19 (129) | 5 (36) | 0 (0) | 677 |
| TKA, % (n) | 73 (555) | 22 (171) | 5 (39) | 0 (0) | 765 |
| Unicondylar knee arthroplasty, % (n) | 80 (445) | 17 (95) | 3 (18) | 0 (0) | 558 |
| Shoulder arthroplasty, % (n) | 84 (145) | 14 (24) | 2 (4) | 0 (0) | 173 |
| Total, % (n) | 74 (2449) | 20 (656) | 6 (185) | 0.1 (4) | 3294 |
BCIS = bone cement implantation syndrome.
Fig. 3.
Incidence and 95% CIs of severe BCIS (Grades 2 and 3) and the distribution among all included arthroplasties.
Increased Risk of Death in Patients with Severe BCIS
After controlling for the confounding variables of age and emergency (rather than elective) surgery, we found that patients with severe BCIS were more likely (HR 3.46 [95% CI 2.07 to 5.77]; p < 0.001) to die within 30 days after the index procedure than patients with less severe or no BCIS.
Factors Associated with Severe BCIS
Three factors were independently associated with the development of severe BCIS: age older than 75 years (OR 1.57; [95% CI 1.09 to 2.27]; p = 0.02), ASA Class III or IV (OR 1.58; [95% CI 1.09 to 2.30]; p = 0.02), and renal impairment (OR 3.32; [95% CI 1.45 to 7.46]; p = 0.004) (Table 3).
Table 3.
Results of the multivariate logistic regression analysis
| Factor | OR (95% CI) | p value |
| Age older than 75 years | 1.57 (1.09 to 2.27) | 0.02 |
| ASA class III or IV | 1.58 (1.09 to 2.30) | 0.02 |
| Serum creatinine level greater than 150 µmol/L | 3.32 (1.45 to 7.46) | 0.004 |
ORs reflect the odds of developing severe BCIS after cement implantation.
Discussion
BCIS is an underappreciated complication of cemented arthroplasty procedures, but it can have devastating consequences [8]. Most of what we know about it in the clinical setting comes from studies of hip hemiarthroplasty in patients with femoral neck fractures or cancer [22, 27], but its incidence (and its implications on mortality) is unknown for other cemented arthroplasty procedures [8]. The current analysis showed BCIS to be relatively common, but severe BCIS, which was associated with 30-day mortality, was rare in most procedures and mostly occurred in hip hemiarthroplasty and revision arthroplasty. Factors that were associated with the development of severe BCIS were age older than 75 years, ASA class III/IV, and renal impairment. Surgeons and anesthetists can use this information to identify high-risk procedures and patients to take preventive measures and be vigilant for the possible development of BCIS and its implications.
Limitations
This study has several limitations. Because the study was retrospective, we could not report on loss of consciousness (sign of BCIS Grade 2) as it was inconsistently reported in medical records. Even though this may result in an underestimation of the occurrence of BCIS Grade 2, we still had complete information on the systolic blood pressure and O2% and have a similar incidence of Grade 2 BCIS as a previous investigation [22]. Moreover, details on different cementing techniques and implant designs were not explored and can influence BCIS risk (for example, cementing a hip stem versus cementing the tibial component of a TKA). We did, however, consider procedure type in both our regression analyses, and most procedure groups (TKA, UKA, hip hemiarthroplasty, and THA) were homogenous regarding implant design and cementing technique. Furthermore, we dichotomized BCIS (Grade 0/1 versus Grade 2/3) to increase statistical power; this approach was similar to that used in a previous study [22]. This does, however, result in an incapability to resolve smaller differences between BCIS grades. Unfortunately, there were no structured data regarding previous comorbidities in the patients’ medical records, introducing the possibility of potential unexplored factors that may be associated with the development of severe BCIS. Fortunately, this does not disqualify our findings but rather calls for further research on factors that may also be associated with the development of severe BCIS. Further, the indication for surgery was not available, only whether a procedure was emergent. Even though not conclusive, an operation’s level of urgency provides at least some information about its circumstances and can help readers to infer surgical indications (for example, fracture surgery is mostly emergent in this setting, and osteoarthritis typically was elective). Finally, missing data introduces the possibility of selection bias, which we believe is minor because the dataset was relatively complete; only a small percentage of procedures were excluded because of missing data (3% [111 of 3405]), and characteristics were not different from the included patients in terms of age, sex, BMI, and ASA class.
Incidence of BCIS
We found that BCIS was common after arthroplasty, but severe BCIS was uncommon; severe BCIS (Grades 2 and 3) was more common in patients undergoing hemiarthroplasty of the hip and revision arthroplasty of the hip and knee, while it was less common in those undergoing unicondylar knee arthroplasty and shoulder arthroplasty. This information helps surgeons and anesthetists to stay vigilant for this relatively unknown complication and identify high-risk procedures. To our knowledge, the incidence of BCIS has only been investigated for hip hemiarthroplasty for femoral fractures by Olsen et al. [22] and hemi-/THA for cancer by Schwarzkopf et al. [27]. We found a similar incidence of BCIS in hip hemiarthroplasty patients as Olsen et al. (31% versus 28%) [22], but there was a discrepancy compared with the findings of Schwarzkopf et al. (24% to 31% versus 75%) [27]. Taking the proposed pathophysiologic mechanism of BCIS into account (embolic showers due to excessive pressurization) [8], this inconsistency is likely explained by the increased tendency of cancer patients to develop emboli [1].
Increased Risk of Death in Patients with Severe BCIS
Severe BCIS was associated with an increased risk of death within 1 month of surgery; most of this finding was driven by patients undergoing hemiarthroplasty for hip fractures. This is a potentially important finding because it points to a trade-off that is on surgeons’ minds: Considerable evidence suggests that cementless hemiarthroplasties increase the risk of postoperative periprosthetic femoral fracture [4, 16, 24]. However, we found that (even after controlling for age, smoking, sex, renal impairment, emergent surgery, and ASA classification) the occurrence of severe BCIS in this series may have increased patients’ risk of postoperative death. Available evidence comparing overall mortality between cemented and uncemented hemiarthroplasty for hip fractures shows an increased short-term mortality in cemented implants [6, 21, 28], but long-term studies tend to show similar mortality from higher reoperation rates in uncemented implants subsequent to periprosthetic fractures [20, 26]. Factors associated with the development of BCIS in cemented stems (older age, ASA class III/IV, and renal impairment) should be weighed against risk factors for sustaining periprosthetic fractures in uncemented stems (poor bone quality, female sex [11]) to choose the right implant for the individual patient undergoing hip hemiarthroplasty and improve both short- and long-term mortality. Especially for older patients (older than 75 years) the use of uncemented stems is associated with an increased risk of periprosthetic femoral fractures and, therefore, reoperation [3, 5, 14, 29]. Future studies using large numbers of patients, which explore this trade-off by comparing the short-term risks of BCIS in cemented implants with the longer-term risks of periprosthetic fractures and reoperation in uncemented implants, are needed to further inform surgeons on the right choice of fixation in hip hemiarthroplasty.
Factors Associated with Severe BCIS
Factors independently associated with severe BCIS were increasing age, more severe ASA classification, and renal impairment. Other previously established risk factors include chronic obstructive pulmonary disease, cancer and lung metastases, and the use of diuretics or warfarin [22, 27]. These factors help surgeons and anesthetists in identifying patients who may have an increased chance of developing BCIS and take precautions, including extensive medullary lavage before cementation [32], finger packing rather than cement gun usage [17], femoral venting [30], avoidance of excessive pressurization of implants [10], and vigilance of the anesthetist through adequate communication with the surgeon [10]. Further research, preferably designed as randomized prospective studies, is needed to further explore the efficacy and effectiveness of these preventive measures in a clinical setting.
Conclusion
BCIS is common among patients undergoing all types of arthroplasty, and severe BCIS (Grades 2 and 3), though much less common, is still not rare, and it is associated with an increased risk of 30-day mortality; this finding was mostly driven by patients undergoing hip hemiarthroplasty. High-risk patients (older age, ASA class III/IV, and renal impairment) should be identified before high-risk procedures (such as hemiarthroplasty of the hip and revision arthroplasty). Preventive measures like medullary lavage before cementation, femoral venting, and avoidance of excessive pressurization of implants should be taken. Because of the increased risk of periprosthetic fractures in uncemented hip stems, factors associated with the development of BCIS should be weighed against the risk factors for sustaining periprosthetic fractures (poor bone quality, female sex) to balance the risks of fixation method against those of BCIS for each patient. Now that we have identified the high-risk procedures, future research should further explore the efficacy and effectiveness of prophylactic measures for BCIS in randomized clinical trials, further identify high-risk patients and procedures, and address differences in short-term mortality between patients undergoing cemented procedures and those undergoing uncemented procedures in large prospective studies.
Acknowledgments
We thank Marjolein Schager RN, MDiv, for her help with English-language proofreading. We thank Quirijn Duchatteau MSc, for his help with data acquisition and processing.
Footnotes
Each author certifies that he nor she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers (Basel). 2018;10:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengtson A, Larsson M, Gammer W, Heideman M. Anaphylatoxin release in association with methylmethacrylate fixation of hip prostheses. J Bone Joint Surg Am. 1987;69:46-49. [PubMed] [Google Scholar]
- 3.Bunyoz KI, Malchau E, Malchau H, Troelsen A. Has the use of fixation techniques in THA changed in this decade? The uncemented paradox revisited. Clin Orthop Relat Res. 2020;478:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chammout G, Muren O, Laurencikas E, et al. More complications with uncemented than cemented femoral stems in total hip replacement for displaced femoral neck fractures in the elderly: a single-blinded, randomized controlled trial with 69 patients. Acta Orthop. 2017;88:145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornell CN. Guest editorial: An appeal for evidenced-based care and adoption of best practices in the management of displaced femoral neck fractures. Clin Orthop Relat Res. 2019;477:913-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costain DJ, Whitehouse SL, Pratt NL, Graves SE, Ryan P, Crawford RW. Perioperative mortality after hemiarthroplasty related to fixation method: a study based on the Australian Orthopaedic Association national joint replacement registry. Acta Orthop. 2011;82:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Froidmont S, Bonetti LR, Villaverde RV, del Mar Lesta M, Palmiere C. Postmortem findings in bone cement implantation syndrome-related deaths. Am J Forensic Med Pathol. 2014;35:206-211. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson AJ, Thomson HE, Harper NJ, Kenny NW. Bone cement implantation syndrome. Br J Anaesth. 2009;102:12-22. [DOI] [PubMed] [Google Scholar]
- 9.Gammer W, Bengtson A, Heideman M. Inhibition of complement activation by high-dose corticosteroids in total hip arthroplasty. Clin Orthop Relat Res. 1988:205-209. [PubMed] [Google Scholar]
- 10.Griffiths R, White S, Moppett I, et al. Safety guideline: reducing the risk from cemented hemiarthroplasty for hip fracture 2015: Association of Anaesthetists of Great Britain and Ireland British Orthopaedic Association, British Geriatric Society. Anaesthesia. 2015;70:623-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gromov K, Bersang A, Nielsen CS, Kallemose T, Husted H, Troelsen A. Risk factors for post-operative periprosthetic fractures following primary total hip arthroplasty with a proximally coated double-tapered cementless femoral component. Bone Joint J. 2017;99:451-457. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa M, Fujioka Y, Morimoto Y, Okamura A, Kemmotsu O. Pathological evaluation of venous emboli during total hip arthroplasty. Anaesthesia. 2001;56:571-575. [PubMed] [Google Scholar]
- 13.Lamade WR, Friedl W, Schmid B, Meeder PJ. Bone cement implantation syndrome. A prospective randomised trial for use of antihistamine blockade. Arch Orthop Trauma Surg. 1995;114:335-339. [DOI] [PubMed] [Google Scholar]
- 14.Leopold SS. Editor's Spotlight/Take 5: Has the use of fixation techniques in THA changed in this decade? The uncemented paradox revisited. Clin Orthop Relat Res. 2020;478:694-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis RN. Some studies of the complement system during total hip replacement using bone cement under general anaesthesia. Eur J Anaesthesiol. 1997;14:35-39. [DOI] [PubMed] [Google Scholar]
- 16.Lin FF, Chen YF, Chen B, Lin CH, Zheng K. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a meta-analysis of randomized controlled trails. Medicine (Baltimore). 2019;98:e14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCaskie A, Barnes M, Lin E, Harper W, Gregg P. Cement pressurisation during hip replacement. J Bone Joint Surg Br. 1997;79:379-384. [DOI] [PubMed] [Google Scholar]
- 18.Modig J, Busch C, Olerud S, Saldeen T, Waernbaum G. Arterial hypotension and hypoxaemia during total hip replacement: the importance of thromboplastic products, fat embolism and acrylic monomers. Acta Anaesthesiol Scand. 1975;19:28-43. [DOI] [PubMed] [Google Scholar]
- 19.Modig J, Busch C, Waernbaum G. Effects of graded infusions of monomethylmethacrylate on coagulation, blood lipids, respiration and circulation. An experimental study in dogs. Clin Orthop Relat Res. 1975;113:187-197. [DOI] [PubMed] [Google Scholar]
- 20.Okike K, Chan PH, Prentice HA, Paxton EW, Burri RA. Association between uncemented vs cemented hemiarthroplasty and revision surgery among patients with hip fracture. JAMA. 2020;323:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen F, Hård Af Segerstad M, Nellgård B, Houltz E, Ricksten SE. The role of bone cement for the development of intraoperative hypotension and hypoxia and its impact on mortality in hemiarthroplasty for femoral neck fractures. Acta Orthop. 2020;91:293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen F, Kotyra M, Houltz E, Ricksten SE. Bone cement implantation syndrome in cemented hemiarthroplasty for femoral neck fracture: incidence, risk factors, and effect on outcome. Br J Anaesth. 2014;113:800-806. [DOI] [PubMed] [Google Scholar]
- 23.Orsini EC, Byrick RJ, Mullen JB, Kay JC, Waddell JP. Cardiopulmonary function and pulmonary microemboli during arthroplasty using cemented or non-cemented components. The role of intramedullary pressure. J Bone Joint Surg Am. 1987;69:822-832. [PubMed] [Google Scholar]
- 24.Parker MJ, Cawley S. Cemented or uncemented hemiarthroplasty for displaced intracapsular fractures of the hip: a randomized trial of 400 patients. Bone Joint J. 2020;102:11-16. [DOI] [PubMed] [Google Scholar]
- 25.Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;369:39-48. [DOI] [PubMed] [Google Scholar]
- 26.Rogmark C, Leonardsson O. Hip arthroplasty for the treatment of displaced fractures of the femoral neck in elderly patients. Bone Joint J. 2016;98:291-297. [DOI] [PubMed] [Google Scholar]
- 27.Schwarzkopf E, Sachdev R, Flynn J, Boddapati V, Padilla RE, Prince DE. Occurrence, risk factors, and outcomes of bone cement implantation syndrome after hemi and total hip arthroplasty in cancer patients. J Surg Oncol. 2019;120:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talsnes O, Vinje T, Gjertsen JE, et al. Perioperative mortality in hip fracture patients treated with cemented and uncemented hemiprosthesis: a register study of 11,210 patients. Int Orthop. 2013;37:1135-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanzer M, Graves SE, Peng A, Shimmin AJ. Is cemented or cementless femoral stem fixation more durable in patients older than 75 years of age? A comparison of the best-performing stems. Clin Orthop Relat Res. 2018;476:1428-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tronzo RG, Kallos T, WYCHE MQ. Elevation of intramedullary pressure when methylmethacrylate is inserted in total hip arthroplasty. JBJS. 1974;56:714-718. [PubMed] [Google Scholar]
- 31.Tryba M, Linde I, Voshage G, Zenz M. Histamine release and cardiovascular reactions to implantation of bone cement during total hip replacement. Article in German. Der Anaesthesist. 1991;40:25-32. [PubMed] [Google Scholar]
- 32.Wheelwright EF, Byrick RJ, Wigglesworth DF, et al. Hypotension during cemented arthroplasty. Relationship to cardiac output and fat embolism. J Bone J Surg Br. 1993;75:715-723. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Nutritional anaemias: report of a WHO scientific group [meeting held in Geneva from 13 to 17 March 1967]. 1968. Available at: https://apps.who.int/iris/handle/10665/40707. Accessed August 6, 2019.
- 34.World Health Organization. Physical status: the use of and interpretation of anthropometry, report of a WHO expert committee. 1995. Available at: https://www.who.int/childgrowth/publications/physical_status/en/. Accessed August 6, 2019. [PubMed]
- 35.World Health Organization. WHOCC–ATC/DDD Index. 2016. Available at: https://www.whocc.no/atc_ddd_index/. Accessed August 6, 2019.