Abstract
Background and objectives
COVID-19 is responsible for the 2019 novel coronavirus disease pandemic. Despite the vast research about the adult population, there has been little data collected on acute kidney injury (AKI) epidemiology, associated risk factors, treatments, and mortality in pediatric COVID-19 patients admitted to the ICU. AKI is a severe complication of COVID-19 among children and adolescents.
Methods
A comprehensive literature search was conducted in PubMed/MEDLINE and Cochrane Center Trials to find all published literature related to AKI in COVID-19 patients, including incidence and outcomes.
Results
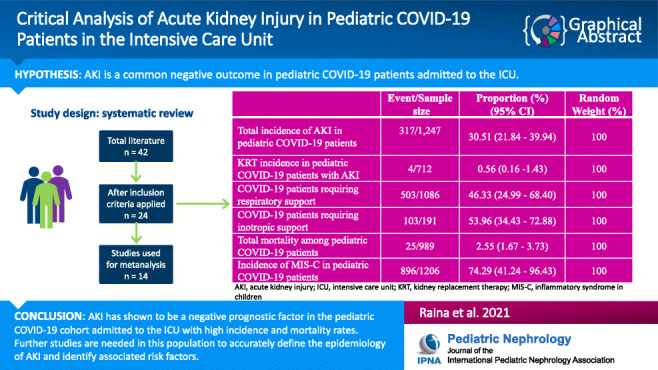
Twenty-four studies reporting the outcomes of interest were included. Across all studies, the overall sample size of COVID positive children was 1,247 and the median age of this population was 9.1 years old. Among COVID positive pediatric patients, there was an AKI incidence of 30.51%, with only 0.56% of these patients receiving KRT. The mortality was 2.55% among all COVID positive pediatric patients. The incidence of multisystem inflammatory syndrome in children (MIS-C) among COVID positive patients was 74.29%.
Conclusion
AKI has shown to be a negative prognostic factor in adult patients with COVID-19 and now also in the pediatric cohort with high incidence and mortality rates. Additionally, our findings show a strong comparison in epidemiology between adult and pediatric COVID-19 patients; however, they need to be confirmed with additional data and studies.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00467-021-05084-x.
Keywords: COVID-19, Acute kidney injury, Kidney replacement therapy, Adult, Pediatric, MIS-C
Introduction
Since January 30, 2020, the virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or COVID-19 was declared a global health emergency of international concern by the World Health Organizations (WHO) and has continued to evolve into a devastating pandemic [1]. COVID-19 has infected more than 103 million people of all ages and has a mortality of 2.3 million people globally as of February 2020 (John Hopkins Coronavirus Tracker). We have extensively developed our knowledge on the pathophysiology, diagnosis, management, and post infection complications of COVID-19 in the past year; however, this knowledge continues to evolve. While the majority (approximately 80%) of adult patients with COVID-19 have mild symptoms, the virus may produce severe illness, multiorgan failure, and death in a small portion of the population [2]. Kidney manifestations of COVID-19, such as acute kidney injury (AKI), are typically considered negative prognostic factors and may appear in up to 25% of adult patients. The disease is most severe among those who have comorbidities or those who are elderly [3, 4].
Limited research on the epidemiology and manifestations of COVID-19 among pediatric patients has been reported to be much different than that of adults. As reported recently, children only comprise about 1–2% of COVID-19 cases documented worldwide [5]. In a recent meta-analysis of 9,335 pediatric COVID-19 patients, 13.1% of the patients presented asymptomatically through contact exposure in family clusters [6]. Overall, 57.4% of patients were hospitalized and at least one underlying comorbidity was reported in 27.1% of cases [6]. The neonatal age group, male gender, lower respiratory tract disease, and pre-existing medical conditions have all been associated with severe disease among children [5]. The most common clinical features of COVID-19 in pediatric patients are fever, dry cough, and pneumonia along with an increasing prevalence of multisystem involvement, including AKI among other kidney manifestations [7]. Thus far, among pediatric COVID cases presenting with the same severity of illness as adults, a similar clinical picture of kidney involvement may be expected [8].
Furthermore, multisystem inflammatory syndrome in children (MIS-C) is a life-threatening complication of the virus specifically among children. MIS-C is associated with SARS-CoV-2 and has been shown to occur within 2–4 weeks, most commonly after an infection [9, 10]. Children with MIS-C have been reported to exhibit symptoms similar to those of Kawasaki disease including fever, toxic shock syndrome, macrophage activation syndrome, secondary hemophagocytic lymphohistiocytosis, and multisystem organ involvement, which commonly includes AKI [9, 11, 12].
Despite this data, there is a lack of large-scale studies analyzing the extent of AKI in pediatric patients. Thus, this systematic review aims to evaluate the incidence of AKI, associated mortality, the need for kidney replacement therapy (KRT), and outcomes in the pediatric COVID-19 population through the analysis of published literature.
Method
Database sources and search strategy
This study was registered with the International Prospective Register for Systematic Reviews (PROSPERO: CRD42021237695). In this study, a systemic search was performed in PubMed/MEDLINE and Cochrane Center Trials databases to find all published literature related to AKI in COVID-19 patients, including incidence and outcomes. The search terms for the pediatric population included “COVID-19”, “Coronavirus”, “Betacoronavirus”, “kidney”, “renal”, “pediatric”, “acute kidney injury”, and “MISC” in the search criteria (Appendix A). All literature from the search strategy was then imported into Rayyan QCRI using EndNote. In addition, the reference lists of the selected studies were further reviewed for any additional publications that were relevant to the search criteria. The search for the pediatric literature spanned from November 1, 2019 to December 24, 2020. Language restriction of only English studies was applied. This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses “PRISMA” checklist (Appendix B).
Literature selection
The pediatric literature was independently reviewed by two reviewers using the inclusion and exclusion criteria with a third reviewer to settle any disputes. The inclusion criteria for the publications of pediatric populations included prospective, retrospective, and case series studies assessing AKI in COVID-19 positive patients. All included studies needed to contain laboratory-confirmed SARS-CoV-2 diagnosis (via detection of SARS-CoV-2 nucleic acid via polymerase-chain-reaction), demographic and epidemiological data (incidence of AKI, severity, and comorbidities), and outcome. Males and females ≤ 24 years were the criteria for the pediatric population. Studies exclusively on kidney transplant patients or a negative SARS-CoV-2 status were excluded along with systematic reviews, meta-analyses, animal studies, and letters not presenting any original data (Table 1).
Table 1.
PICO table
| Criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Any pediatrics population (< 24 years) | |
| Intervention/input exposure | SARS-CoV-2 diagnostic test | Negative SARS-CoV-2 test |
| Comparison | AKI incidence in COVID positive children population. | |
| Outcomes | Pediatric patients who are COVID + and have AKI | |
| Study types | Prospective/retrospective studies, case studies | Systematic review, meta analyses, animal studies |
Quality assessment
All included studies were evaluated for study quality independently using a quality assessment tool from the National Heart Lung and Brain Institute (NHLBI) (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). This tool is designed to validate the internal studies designed for critical appraisal. The study quality had eight questions: study objective, study population, case definition, consecutive cases, outcome validity, adequate length of follow-up, statistical methods, and results. Studies were scored out of 8 points, where 6–8 points were considered good quality, 4–5 as fair quality, and < 4 were regarded as poor quality.
Data extraction and statistical analysis
Data regarding patient demographics, the presence of comorbidities, AKI incidence, mortality rates, need for KRT, and outcomes were recorded electronically. The pooled proportions for these outcomes and their respective 95% confidence intervals (95% CI) were assessed for each study or calculated when not reported. The degree of between-study statistical heterogeneity was assessed using the I2 test, where I2 ≥ 50% indicated high heterogeneity. A meta-analysis of the outcomes was performed with overall pooled estimates calculated with a random effects model and fixed effects model to assess for high and low heterogeneity, respectively. Forest plots were utilized to visualize the outcomes of each study, including the combined estimated outcomes with their 95% CI. Funnel plots were created to graphically assess publication bias in each study. A p value ≤ 0.05 was used to consider statistical significance. All statistical analyses were performed with R software version 3.1.0.
Results
Study selection and details
The database search for the pediatric population yielded a total of 42 studies with 24 studies included that reported the outcomes of interest (Fig. 1). Of these studies, 10 were retrospective cohort studies, 7 were case reports, 4 were case series, 2 were cross-sectional studies, and one was a prospective cohort study [12–35]. There were 23 studies that were determined to be of good quality while one study was of fair quality [S. Table 1]. However, only a total of 14 pediatric studies were used for our meta-analysis as the other studies had total sample sizes less than 10. The overall sample size of COVID positive children across the studies was 1,247 (ranging from 17 to 570 across 14 different studies). The pooled median age of children was 9.1 years (median of 6.5–14.5 years across all studies) and 56.2% of patients were male. The values pertaining to the distribution of race among the pediatric patients was reported to be African American (29.1%), Caucasian (16.4%), Asian (3.4%), and a category for unknown/missing patients (51.1%). A total of 26.7% of children had at least 1 pre-existing co-morbidity (based on 20 studies). The data for various lab parameters among these patients were very limited including glomerular filtration rate (1 study), serum creatinine (median: 62.5 μmol/L (0.71 mg/dL); 6 studies), blood urea nitrogen, D-dimer (median 2.7 mg/L (0.27 mg/dL); 8 studies), sodium (median 132.5 mEq/L; 3 studies), and potassium (1 study). Also, only one study reported the data for uremia, proteinuria, and hematuria. The details of the studies are provided in Tables 2 and 3.
Fig. 1.
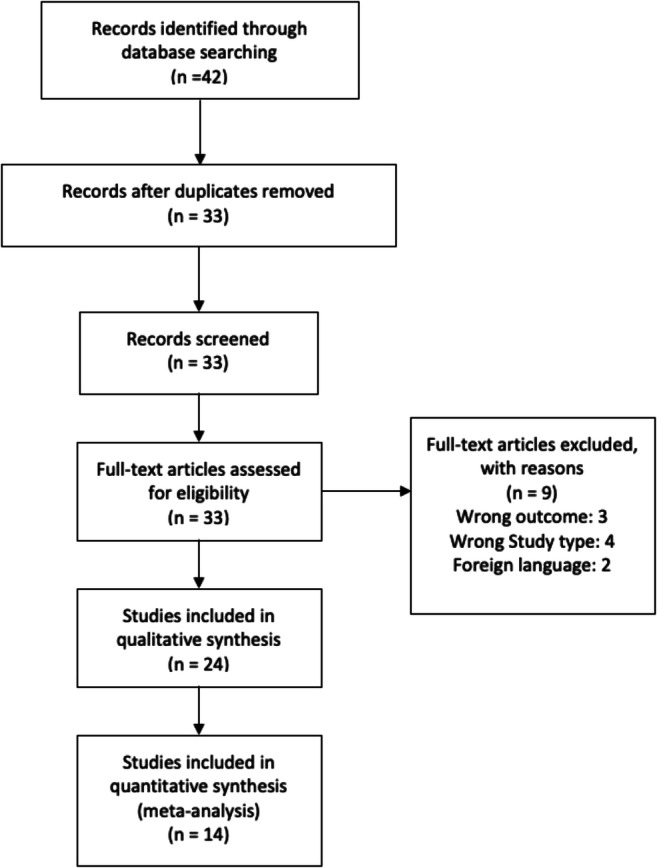
Prisma flowchart for pediatric studies
Table 2.
Summary table for pediatric studies
| Study | Study design | Location | Age (years) (mean ± SD) | COVID+ with AKI | Treatment |
|---|---|---|---|---|---|
| *González-Dambrauskas S et al. | Retrospective observational multicenter | Chile, Colombia, Italy, Spain, United States | 6.52 ± 2.99 | 3 | Antibiotics, 15 (88%); remdesivir, 4 (24%); lopinavir and/or rionavir, 1 (6%); corticosteroids, 9 (53%); tocilizumab, 7 (41%); hydroxychloroquine, 8 (47%) |
| *Whittaker E et al. | Uncontrolled case series multicenter | England | 10 ± 1.3 | 11 | IVIG, 33 (75%); corticosteroids, 33 (75%); anakinra, 2 (5%); infliximab, 7 (16%) |
| *Dufort EM et al. | Retrospective observational multicenter | New York, USA | 9.33 ± 1 | 10 | Systemic glucocorticoids, 63 (64%); IVIG, 69 (70%) |
| *Lee PY et al. | Retrospective single center | Boston, USA | 8.77 ± 2.82 | 6 | Immunomodulary therapy, 22 (79%); antimicrobial therapy, 22 (79%); anticoagulation therapy, 24 (86%) |
| *Capone CA et al. | Case series, single center | New York, USA | 8.83 ± 1.183 | 23 | IVIG, 33 (100%); second dose IVIG, 11 (33); methylprednisolone, 23 (70%); aspirin, 29 (88%); anakinra, 4 (12); tocilizumab, 3 (9%); infliximab, 1 (3); enoxaparin, 14 (42) |
| Wang X et al. | Retrospective observational | Wuhan, China | 1.08 | 3 | Meropenem + linezolid, 3 (100%); oseltamivir, 1 (33%); ribavirin, 2 (67%); corticosteroids, 3 (100%); IVIG, 3 (100%) |
| Tram N et al. | Case report | Brussels, Belgium | 15 | 1 | N/A |
| Samies NL et al. | Case report | Alabama, USA | 16 | 1 | IV fluids and hemodialysis for 5 days |
| Repper DC et al. | Case report | California, USA | 15 | 1 | IVIG, corticosteroids |
| Qiu L et al. | Case report | China | 0.67 | 1 | IVIG, methylprednisolone, fluid and electrolyte therapy, low dose diuretics and dopamine |
| Niño-Taravilla C et al. | Case report | Chile | 8 | 1 | Cefotaxime, fluid resuscitation, tocilizumab, |
| *Mamishi S et al. | Retrospective multicenter | Iran | 6.98 ± 0.98 | 13 | IVIG, 18 (48%); steroids, 27 (60) |
| Lee M et al. | Case report | California, USA | 15 | 1 | Normal saline, clindamycin, ceftriaxone, doxycycline, IVIG, methylprednisolone |
| *Grimaud M et al. | Retrospective observational | France | N/A | 14 | All participants (20) received IVIG with adjuvant corticosteroids, IL-1 receptor antagonist, or a monoclonal antibody against IL-6 |
| Oberweis ML et al. | Case report | Belgium | 8 | 1 | IVIG, dobutamine, milrinone |
| *Toubiana J et al. | Prospective observational | Paris, France | 9.03 ± 2.15 | 11 | IVIG, 21 (100%), 10 (48%) corticosteroids |
| Shahbaznejad L et al. | Case series | Iran | 5.32 ± 4.10 | 2 | Antibiotics (7); hydroxychloroquine (6); packed cell (6); albumin (6); IVIG 1g/kg (5); IVIG 2 g/kg (1); steroids (2); vasoactive drugs (4); infliximab (1) |
| *Godfred-Cato S et al. | Retrospective report | District of Columbia and New York, USA | 9.01 ± 3.33 | 105 | IVIG (424), steroids (334), antiplatelet meds (309), anticoagulation meds (233), vasoactive (221), immune modulators (119) |
| *Dionne A et al. | Retrospective cohort, single center | Boston, USA | 9.28 ± 2.05 | 2 | IVIG (16, 64%), steroids (13, 52%), anakinra (4, 16%), aspirin (14, 56%), remdesivir (9, 36%) |
| *Derespina KR et al. | Retrospective observational, multicenter | New York, USA | 14.5 ± 1.67 | 9 | Hydroxychloroquine (27, 38.6%), azithromycin (23, 32.9%), remdesivir (13, 18.6%), corticosteroids (23,32.9%), antibiotics (48,68.6%) |
| *Stewart DJ et al. | Retrospective observational, single center | London, UK | 9.33 ± 1.22 | 15 | N/A |
| *Bjornstad EC et al. | Multicenter cross-sectional analysis | United States, Western Europe, Eastern Europe/Russia | 9.98 ± 2.95 | 47 | N/A |
| Deep A et al. | Multicenter observational study | United Kingdom | N/A | N/A | N/A |
| Joshi K et al. | Case series | New York, USA | 11.25 ± 1.17 | 2 | Tocilizumab (2), remdesivir (1), IVIG (1), steroids (1), hydroxychloroquine (2) |
N/A Not applicable. * The studies included in the meta-analysis
Table 3.
Quality criteria checklist assessment for the studies
| Study | Inclusion and exclusion specified | Study type | Sample size specified | COVID test mentioned | AKI related labs | Management mentioned |
|---|---|---|---|---|---|---|
| *González-Dambrauskas S et al. | Y | R | Y | Y | N | Y |
| *Whittaker E et al. | N/A | CS | Y | Y | Y | Y |
| *Dufort EM et al. | Y | R | Y | Y | N | Y |
| *Lee PY et al. | Y | R | Y | Y | Y | Y |
| Capone CA et al. | N/A | CS | Y | Y | N | Y |
| Wang X et al. | R | Y | Y | Y | Y | |
| Tram N et al. | N/A | CR | Y | Y | Y | Y |
| Samies NL et al. | N/A | CR | Y | Y | Y | Y |
| Repper DC et al. | N/A | CR | Y | Y | Y | Y |
| Qiu L et al. | N/A | CR | Y | Y | Y | Y |
| Niño-Taravilla C et al. | N/A | CR | Y | Y | Y | Y |
| *Mamishi S et al. | Y | R | Y | Y | Y | Y |
| Lee M et al. | N/A | CR | Y | Y | Y | Y |
| Grimaud M et al. | Y | R | N | Y | Y | Y |
| Oberweis ML et al. | N/A | CR | Y | Y | Y | Y |
| *Toubiana J et al. | Y | P | Y | Y | Y | Y |
| Shahbaznejad, L et al. | N/A | CS | Y | Y | N | Y |
| *Godfred-Cato S et al. | Y | R | Y | Y | N | Y |
| *Dionne A et al. | Y | R | Y | Y | N | Y |
| *Derespina KR et al. | Y | R | Y | Y | Y | Y |
| Stewart DJ et al. | Y | R | Y | Y | Y | N |
| *Bjornstad EC et al. | Y | CrS | Y | Y | Y | N |
| Deep A et al. | Y | R | N | N | N | N |
| Joshi K et al. | N/A | CS | Y | Y | N | Y |
Y yes, N no, R retrospective, P prospective, CS case series, CR case report, CrS cross sectional, N/A not applicable
*The studies included in the meta-analysis
Data analysis
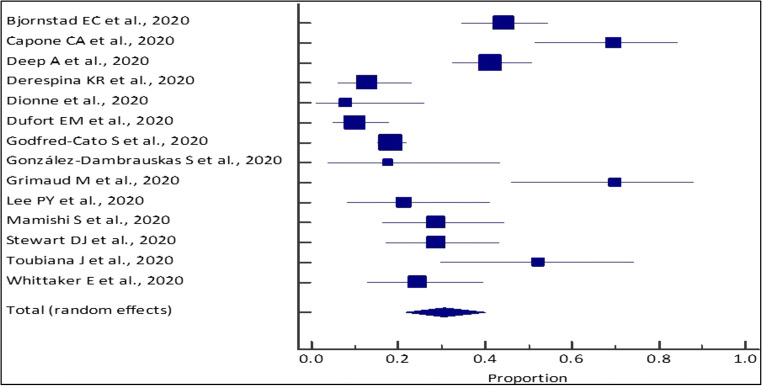
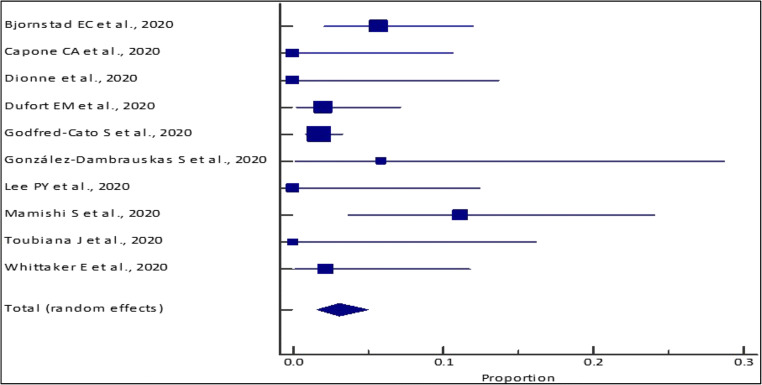
This meta-analysis for the outcomes of interest was conducted for studies with total sample sizes ≥ 10 (14 studies; n = 1,247). Through our analysis, the pooled (95% CI) AKI incidence in COVID positive pediatric patients was 30.51% (21.84–39.94%) [I2 = 89.8% (84.65 to 93.21%); p < 0.0001; random effects; 14 studies; n = 1,247] [Table 4; Figs. 2 and 3]. Additionally, our analysis showed a pooled mortality (95% CI) of 2.55% (1.67–3.73%) [I2 = 38.72% (0.00 to 70.77%); p = 0.0999; fixed effects; 10 studies; n = 989] in COVID positive patients [Table 5; Figs. 4 and 5]. Only four studies reported the use of KRT in COVID pediatric patients with AKI. The pooled proportion (95% CI) of AKI COVID positive patients receiving KRT was 0.56% (0.16–1.43%) [I2 = 0% (0.00 to 76.45%); p = 0.6493; fixed effects; 4 studies; n = 712].
Table 4.
Meta-analysis of pediatric AKI incidence among COVID positive patients across different studies
| Study | Event/sample size | Proportion (%) (95% CI) |
Random weight (%) |
|---|---|---|---|
| Bjornstad EC et al., 2020 | 47/106 | 44.34 (34.69–54.31) | 7.95 |
| Capone CA et al., 2020 | 23/33 | 69.70 (51.29–84.41) | 6.87 |
| Deep A et al., 2020 | 48/116 | 41.38 (32.31–50.90) | 8.00 |
| Derespina KR et al., 2020 | 9/70 | 12.86 (6.05–23.01) | 7.66 |
| Dionne et al., 2020 | 2/25 | 8.00 (0.98–26.03) | 6.48 |
| Dufort EM et al., 2020 | 10/99 | 10.10 (4.95–17.79) | 7.91 |
| Godfred-Cato S et al., 2020 | 105/570 | 18.42 (15.32–21.85) | 8.45 |
| González-Dambrauskas S et al., 2020 | 3/17 | 17.65 (3.80–43.43) | 5.84 |
| Grimaud M et al., 2020 | 14/20 | 70.00 (45.72–88.11) | 6.12 |
| Lee PY et al., 2020 | 6/28 | 21.43 (8.30–40.95) | 6.64 |
| Mamishi S et al., 2020 | 13/45 | 28.89 (16.37–44.32) | 7.25 |
| Stewart DJ et al., 2020 | 15/52 | 28.85 (17.13–43.08) | 7.40 |
| Toubiana J et al., 2020 | 11/21 | 52.38 (29.78–74.29) | 6.20 |
| Whittaker E et al., 2020 | 11/45 | 24.44 (12.88–39.54) | 7.25 |
| Total (random effects) | 317/1,247 | 30.51 (21.84–39.94) | 100 |
Fig. 2.
Forest plot of the meta-analysis of pediatric AKI incidence among COVID-positive patients across different studies. The lower diamond in the graph represents the pooled estimate
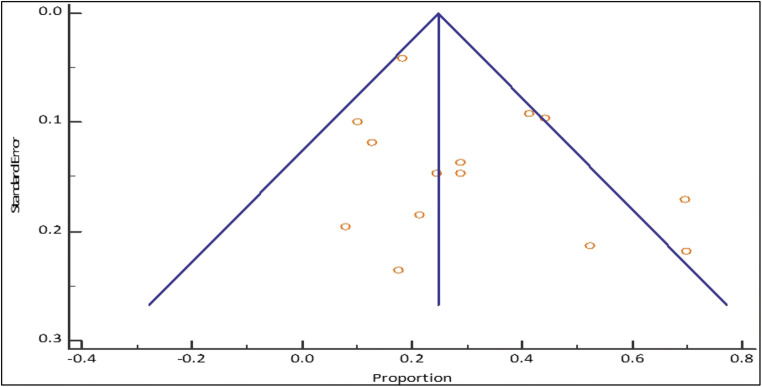
Fig. 3.
Funnel plot for pediatric AKI incidence among COVID-positive patients
Table 5.
Meta-analysis of pediatric mortality among COVID positive patients across different studies
| Study | Event/sample size | Proportion (%) (95% CI) |
Fixed weight (%) |
|---|---|---|---|
| Bjornstad EC et al., 2020 | 6/106 | 5.66 (2.11–11.91) | 10.71 |
| Capone CA et al., 2020 | 0/33 | 0 (0–10.58) | 3.40 |
| Dionne et al., 2020 | 0/25 | 0 (0–13.72) | 2.60 |
| Dufort EM et al., 2020 | 2/99 | 2.02 (0.25–7.11) | 10.01 |
| Godfred-Cato S et al., 2020 | 10/570 | 1.75 (0.84–3.2) | 57.16 |
| González-Dambrauskas S et al., 2020 | 1/17 | 5.88 (0.15–28.69) | 1.80 |
| Lee PY et al., 2020 | 0/28 | 0 (0–12.34) | 2.90 |
| Mamishi S et al., 2020 | 5/45 | 11.11 (3.71–24.05) | 4.60 |
| Toubiana J et al., 2020 | 0/21 | 0 (0–16.11) | 2.20 |
| Whittaker E et al., 2020 | 1/45 | 2.22 (0.06–11.77) | 4.60 |
| Total (fixed effects) | 25/989 | 2.55 (1.67–3.73) | 100 |
Fig. 4.
Forest plot of the meta-analysis of pediatric mortality among COVID-positive patients across different studies. The lower diamond in the graph represents the pooled estimate
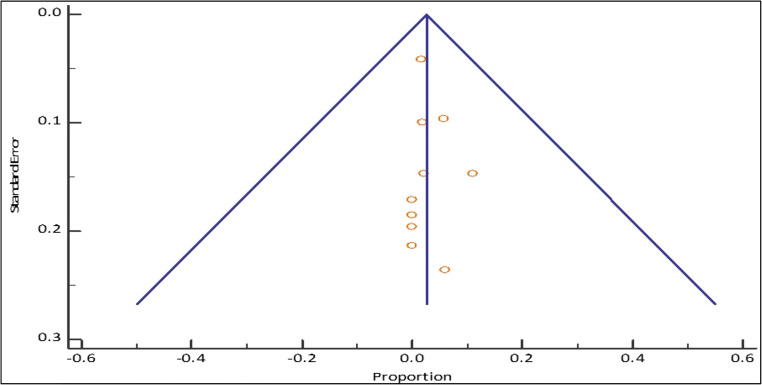
Fig. 5.
Funnel plot for pediatric mortality among COVID-positive patients
The distribution of various treatments and intervention modalities was also assessed in this study. The analysis showed that the pooled proportion (95% CI) of COVID positive patients receiving respiratory support was 46.33% (24.99–68.40%) [p < 0.0001; random effects; 12 studies; n = 1,086] while 53.96% (34.43–72.88%) of patients received inotropic support [p < 0.0001; random effects; 6 studies; n = 191]. The other supportive treatments are described in Table 2. It was evaluated that the median duration of hospital stay among COVID positive patients was 6 days (median of 4–8 days across 7 studies providing the data). Lastly, this meta-analysis evaluated the incidence of MIS-C among COVID-positive patients and found a pooled incidence (95% CI) of 74.29% (41.24–96.43%) [I2 = 99.16% (98.98 to 99.32%); p < 0.0001; random effect; 12 studies; n = 1,206].
Discussion
A multitude of studies have made it clear that COVID-19 has significantly affected individuals of all ages. Although the virus is known to particularly target the lungs, it is not uncommon for the disease to manifest in other organ systems, especially in severe cases. More specifically, various pathological studies have particularly highlighted the kidneys as a target of COVID-19 with the development of AKI in critically ill COVID-19 adult patients. There have also been instances of AKI occurrence in pediatric COVID-19 patients. However, there has been no consensus regarding the accurate incidence, outcomes, and associated mortality among this population. Therefore, our systematic review and meta-analysis was performed to specifically address the incidence, mortality, outcomes, and various treatment modalities utilized in COVID-positive pediatric patients with AKI.
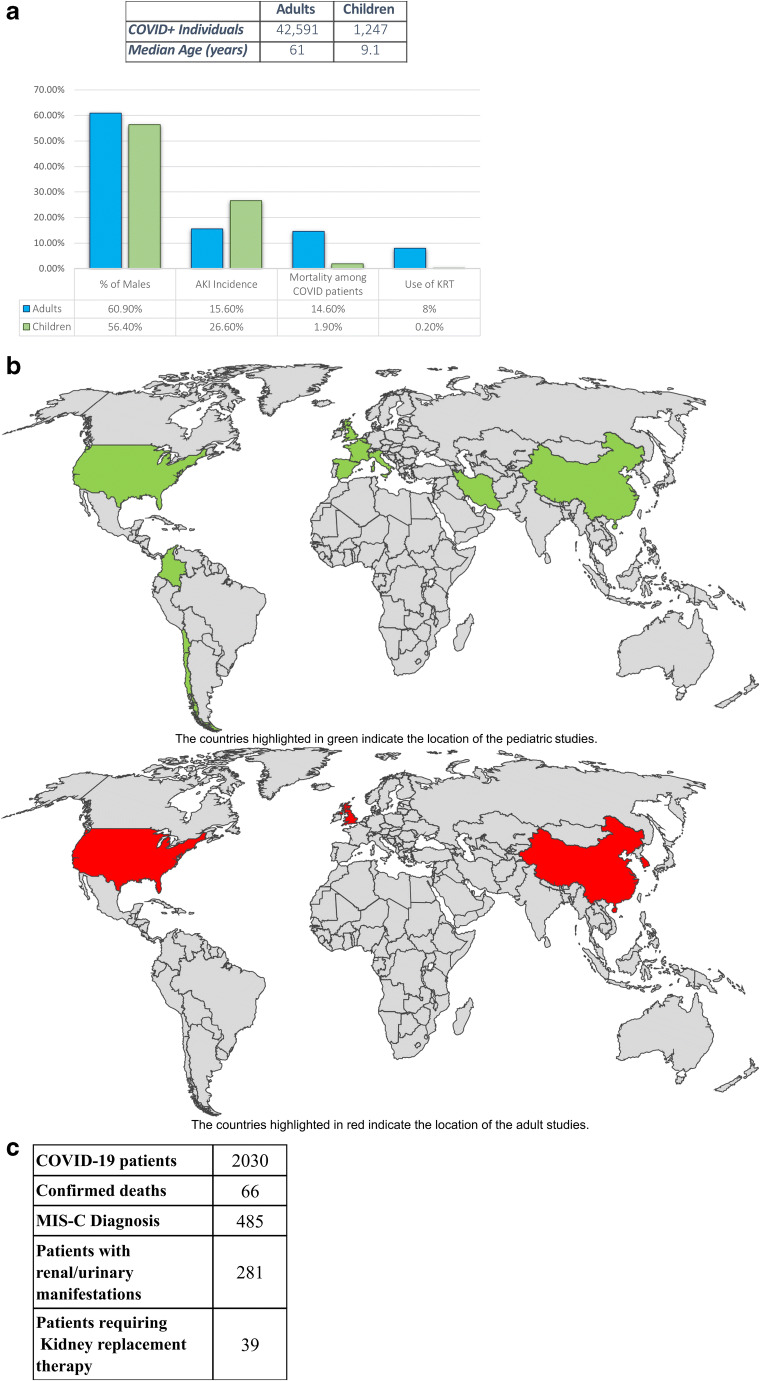
In our meta-analysis, we found an AKI incidence of 30.51% (21.84–39.94%). The higher incidence among pediatric patients was higher than we expected, however, in the multicenter cohort study (the Critical Coronavirus and Kids Epidemiologic [CAKE] study) of critically ill children with COVID-19, the incidence of AKI was reported to be 18% [13]. Additionally, in a multicenter AKI study among critically ill children with COVID-19 by Bjornstad et al. (n = 106), AKI was found to occur in 44% (n = 47) of patients [14]. We also found a mortality of 2.55% (1.67–3.73%) in COVID AKI patients with 0.56% of patients requiring KRT (0.16–1.43%). This is similar to the data reported by the Virtual Pediatric Systems (VPS) from North American pediatric ICUs as of February 2020. Based on the 2,030 COVID-19 positive patients reported by VPS, there was a mortality rate of 3.3% (Fig. 6c). Additionally, in the 281 (13.8%) of COVID-19 patients with kidney systems involved, there was a mortality rate of 10.3% (n = 29) with 13.9% (n = 39) requiring organ support via KRT. Lastly, it had been reported by various studies that some pediatric patients uniquely exhibited MIS-C. A recent systematic review by Abrams et al. analyzing the clinical features of 440 children with MIS-C revealed that the proportion of patients who received a positive SARS-CoV-2 RT-PCR test ranged from 13 to 60% while the proportion of positive serology tests ranged from 75 to 100% [36]. Additionally, Feldstein et al. reported that out of 186 children with MIS-C, 70% were COVID-positive by RT-PCR or antibody testing [10]. Based on our review, we found that the pooled incidence (95% CI) of MIS-C among COVID-positive patients was 74.29% (41.24–96.43%) [p < 0.0001; random effect; 12 studies; n = 1,206]. This is much higher than reported by VPS, which reported a total of 23.9% of pediatric patients with MIS-C (485 out of 2,030 patients). These differences may potentially be attributed to variations in the definition and clinical knowledge of the syndrome due to its novelty [7].
Fig. 6.
(a) Comparison of the adult vs. pediatric COVID-19 population. (b) World map of pediatric vs. adult COVID-19 studies. (c) COVID-19 clinical data from North American pediatric intensive care units (https://www.covid19.myvps.org)
To bring our pediatric data into comparison with adult data regarding AKI in COVID-positive patients, a sub-analysis was performed with our systematic review which assessed AKI incidence in the majority of adult COVID patients. The parameters of our adult systematic review are highlighted in Appendix C. Our comparison analysis was performed by calculating medians and utilizing the Mann–Whitney U-test with a p value ≤ 0.05 considered for statistical significance (Fig. 6a). The pooled median age of children was 9.1 years (ranging from median of 6.5–14.5 years across all studies) and adults was 61 years (median of 45.6–73 years). The proportion of males was almost equal for children (56.2%) and adult (60.9%) studies. However, there was a lower proportion of children (26.7%) with at least 1 pre-existing co-morbidity than that of adults (40.9%).
The median (IQR) AKI incidence was calculated for both populations and was found to be higher among pediatric patients [26.6% (17.8–43.6%); n = 14] compared to the adult population [15.6% (5.7–31.7%); n = 53] (p = 0.07). Mortality was also compared and the median (IQR) mortality among COVID-positive patients was observed to be significantly lower among pediatric patients [1.9% (0.0–4.8%); n = 10] compared to adults [14.6% (5.4–39.3%); n = 25] (p < 0.001) (Fig. 6b). In respect to the utilization of KRT among COVID-positive patients, the median utilization was significantly lower among pediatric patients [0.2% (0.0–0.6%); n = 4] when compared to adults [8.0% (3.3–16.7%); n = 33] (p < 0.001) (Fig. 6b).
Overall, this suggests that children may have less severe symptoms from SARS-CoV-2 as compared to adults. The observed outcomes among children may be attributed to a more active innate immune response, generally being overprotected by parents, engaging in fewer outdoor activities, undertaking less international travel, and having less comorbidities. Additionally, children have a difference in the distribution, maturation, and functioning of viral receptors. Differences in the receptors in the renin–angiotensin system (RAS) and altered inflammatory responses to pathogens could also account for age-induced disease differences. Angiotensin II receptors (ACE2) have been shown to have a strong interaction with the receptor-binding domain of the COVID-19 spike-protein. Children have fewer ACE2 receptors than adults, which may potentially contribute to the reduced susceptibility to severe disease in children [37–40]. Despite these reasons providing a case why children are less likely to progress to severe disease, increasing pediatric cases and newer reports are starting to show the opposite. In the study by Bjornstad et al., the investigators reported a mortality of 6% and suggested the possibility of severe disease within the pediatric population [14]. Furthermore, there is a significant difference among the outcomes of children with COVID-19 receiving care in high-income countries versus low- and middle-income countries. In comparison to high-income countries, low- and middle-income countries report lower proportions of admissions to the ICU (9.9% vs. 26.0%), yet higher numbers of deaths (relative risk 2.14, 95% CI: 1.43 to 3.20) [9]. This suggests that more large-scale global studies are required to fully assess the extent of AKI in pediatric COVID patients.
Although our study was able to accurately determine outcomes in COVID patients, one of the main limitations in our study was the sample size, as COVID and AKI incidence reports are still new. Due to a paucity of literature, it restricted us from doing a detailed analysis. Additionally, some studies presented insufficient data, forcing us to exclude them from our analysis despite meeting our inclusion criteria. It is also important to note that in our analysis, only studies with a sample size greater than 10 were included to remove the influence on outcome by studies with small sample sizes.
Conclusion
Overall, AKI has shown to be a negative prognostic factor in adult patients with AKI and now also in the pediatric cohort with high incidence and mortality rates. Additionally, our findings do show a strong comparison in epidemiology between adult and pediatric COVID-19 patients; however, they need to be confirmed with additional data and studies as the direction of the relationships cannot be ascertained and still remain unclear in the literature. With the prevalence of cases worldwide, it is crucial for clinicians to be informed about the prevalence of AKI in both adult and pediatric populations.
Supplementary Information
(DOCX 18 kb)
(DOCX 13 kb)
(DOCX 18 kb)
(DOCX 15 kb)
Acknowledgements
We would like to thank Dr. Kirsten Kusumi from the Akron Children’s Hospital for medically editing the manuscript. We would also like to thank Ms. Simonson from the Cleveland Clinic Foundation Library and the Akron Children’s Library for her aid in the data search and Dr. Abhishek Tibrewal for his contribution with the data review and statistical analysis.
Author contribution
RR conceptualized the study. RR, RC, IM, SS, and MF drafted the study protocol, conducted the literature search, study screening, selection and data extraction, and drafted the manuscript. All authors critically reviewed the manuscript to ensure intellectual content and approved the final manuscript.
Funding
There is none to report.
Declarations
Patient consent for publication
Not required.
Conflict of interest
The authors declare no competing interest.
Footnotes
Rupesh Raina and Ronith Chakraborty are Co-first author
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (2020) Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-thesecond-meeting-of-the-internationalhealth-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- 2.Raina R, Chakraborty R, Sethi SK, Bunchman T. Kidney replacement therapy in COVID-19 induced kidney failure and septic shock: a pediatric continuous renal replacement therapy [PCRRT] position on emergency preparedness with resource allocation. Front Pediatr. 2020;8:413. doi: 10.3389/fped.2020.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinowicz S, Leshem E, Pessach IM. COVID-19 in the pediatric population-review and current evidence. Curr Infect Dis Rep. 2020;22:29. doi: 10.1007/s11908-020-00739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. 2021;5:133–141. doi: 10.1016/S2352-4642(20)30304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deep A, Bansal M, Ricci Z. Acute kidney injury and special considerations during renal replacement therapy in children with coronavirus disease-19: perspective from the Critical Care Nephrology Section of the European Society of Paediatric and Neonatal Intensive Care. Blood Purif. 2021;50:150–160. doi: 10.1159/000509677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z (2021) Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child. 10.1136/archdischild-2020-321385 [DOI] [PMC free article] [PubMed]
- 10.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin M. Childhood multisystem inflammatory syndrome - a new challenge in the pandemic. N Engl J Med. 2020;383:393–395. doi: 10.1056/NEJMe2023158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K et al (2020) COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 20:e276–e288. 10.1016/S1473-3099(20)30651-4 [DOI] [PMC free article] [PubMed]
- 13.González-Dambrauskas S, Vásquez-Hoyos P, Camporesi A, Díaz-Rubio F, Piñeres-Olave BE, Fernández-Sarmiento J, et al. Pediatric critical care and COVID-19. Pediatrics. 2020;146:e20201766. doi: 10.1542/peds.2020-1766. [DOI] [PubMed] [Google Scholar]
- 14.Bjornstad EC, Krallman KA, Askenazi D, Zappitelli M, Goldstein SL, Basu RK et al (2020) Preliminary assessment of acute kidney injury in critically ill children associated with SARS-CoV-2 infection: a multicenter cross-sectional analysis. Clin J Am Soc Nephrol 16:446–448. 10.2215/CJN.11470720 [DOI] [PMC free article] [PubMed]
- 15.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee PY, Day-Lewis M, Henderson LA, Friedman K, Lo J, Roberts JE. Distinct clinical and immunological features of SARS-COV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Lorry R, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 Infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Chen X, Tang F, Luo W, Fang J, Chang Q, et al. Be aware of acute kidney injury in critically ill children with COVID-19. Pediatr Nephrol. 2021;36:163–169. doi: 10.1007/s00467-020-04715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tram N, Chiodini B, Montesinos I, Vicinanaza A, Piccoli XB, Gubbelmans N, et al. Rhabdomyolysis and acute kidney injury as leading COVID-19 presentation in an adolescent. Pediatr Infect Dis J. 2020;39:e314–e315. doi: 10.1097/INF.0000000000002853. [DOI] [PubMed] [Google Scholar]
- 21.Samies NL, Pinninti S, James SH. Rhabdomyolysis and acute renal failure in an adolescent with coronavirus disease 2019. J Pediatric Infect Dis Soc. 2020;9:507–509. doi: 10.1093/jpids/piaa083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Repper DC, Arrieta AC, Cook JE, Renella P. A case of Lemierre syndrome in the era of COVID-19: all that glitters is not gold. Pediatr Infect Dis J. 2020;39:e445–e447. doi: 10.1097/INF.0000000000002939. [DOI] [PubMed] [Google Scholar]
- 23.Qiu L, Jiao R, Zhang A, Chen X, Ning Q, Fang F, et al. A case of critically ill infant of coronavirus disease 2019 with persistent reduction of T lymphocytes. Pediatr Infect Dis J. 2020;39:e87–e90. doi: 10.1097/INF.0000000000002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niño-Taravilla C, Espinosa-Vielma YP, Otaola-Arca H, Poli-Harlowe C, Tapia LI, Ortiz-Fritz P. Pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 treated with tocilizumab. Pediatr Rep. 2020;12:142–148. doi: 10.3390/pediatric12030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamishi S, Movahedi Z, Mohammadi M, Ziaee V, Khodabandeh M, Abdolsalehi MR, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect. 2020;148:e196. doi: 10.1017/S095026882000196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M, Hilado M, Sotelo S, Opas LM, Im DD (2020) Acute kidney injury in Multisystem Inflammatory Syndrome in Children (MIS-C): a case report. SN Compr Clin Med 1–4. 10.1007/s42399-020-00647-9 [DOI] [PMC free article] [PubMed]
- 27.Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69–68. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oberweis ML, Codreanu A, Boehm W, Olivier D, Pierron C, Tsobo C, et al. Pediatric life-threatening coronavirus disease 2019 with myocarditis. Pediatr Infect Dis J. 2020;39:e147–e149. doi: 10.1097/INF.0000000000002744. [DOI] [PubMed] [Google Scholar]
- 29.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahbaznejad L, Navaeifar M, Abbaskhanian A, Hosseinzadeh F, Rahimzadeh G, Rezai MS, et al. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr. 2020;20:513. doi: 10.1186/s12887-020-02415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dionne A, Mah DY, Son M, Lee PY, Henderson L, Baker AL, et al. Atrioventricular block in children with multisystem inflammatory syndrome. Pediatrics. 2020;146:e2020009704. doi: 10.1542/peds.2020-009704. [DOI] [PubMed] [Google Scholar]
- 33.Derespina KR, Kaushik S, Plichta A, Nadkarni VM, Agus MSD, Medar SS et al (2020) Clinical manifestations and outcomes of critically ill children and adolescents with coronavirus disease 2019 in New York City. J Pediatr 226:55–63.e2. 10.1016/j.jpeds.2020.07.039 [DOI] [PMC free article] [PubMed]
- 34.Stewart DJ, Hartley JC, Johnson M, Marks SD, du Pré P, Stojanovic J. Renal dysfunction in hospitalised children with COVID-19. Lancet Child Adolesc Health. 2020;4:e28–e29. doi: 10.1016/S2352-4642(20)30178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deep A, Upadhyay G, du Pré P, Lillie J, Pan D, Mudalige N, et al. Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 pandemic: experience from PICUs across United Kingdom. Crit Care Med. 2020;48:1809–1818. doi: 10.1097/CCM.0000000000004662. [DOI] [PubMed] [Google Scholar]
- 36.Abrams JY, Godfred-Cato SE, Oster ME, Chow EJ, Koumans EH, Bryant B et al (2020) Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr 226:45–54.e1. 10.1016/j.jpeds.2020.08.003 [DOI] [PMC free article] [PubMed]
- 37.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 38.South AM, Brady TM, Flynn JT. ACE2 (angiotensin-converting enzyme 2), COVID-19, and ACE inhibitor and Ang II (Angiotensin II) receptor blocker use during the pandemic: the pediatric perspective. Hypertension. 2020;76:16–22. doi: 10.1161/HYPERTENSIONAHA.120.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53:371–372. doi: 10.1016/j.jmii.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)
(DOCX 13 kb)
(DOCX 18 kb)
(DOCX 15 kb)