Abstract
OBJECTIVES
Decellularized homograft valves (DHVs) have shown promising clinical results, particularly in the treatment of congenital heart disease. However, DHV appears to elicit an immune response in a subset of young patients, indicated by early valve degeneration. As the decellularization process is quality controlled for each DHV, we hypothesized that there may be residual immunogenicity within the extracellular matrix of DHV.
METHODS
A semi-quantitative dot blot analysis was established to screen for preformed recipient antibodies using secondary anti-human antibodies. Fifteen DHV samples (7 aortic, 8 pulmonary) were solubilized and exposed to serum from 20 healthy controls.
RESULTS
The sera from young controls (n = 10, 18–25 years) showed significantly stronger binding of preformed antibodies than sera from older individuals (n = 10, 48–73 years). The difference between the means of arbitrary units was 15.1 ± 6.5 (P = 0.0315). There was high intraindividual variance in the mean amounts of arbitrary units of antibody binding with some healthy controls showing >10 times higher antibody binding towards 2 different DHV. The amount of preformed antibodies bound to DHVs was higher in aortic than in pulmonary DHVs. The mean number of antibody binding (in arbitrary units) was 17.2 ± 4.5 in aortic and 14.5 ± 4.7 in pulmonary DHV (P = 0.27). The amount of preformed antibodies bound to pulmonary DHVs was statistically significantly higher in the sera of healthy males (n = 10) than in the sera of healthy females (n = 10). The mean number of arbitrary units was 17.2 ± 4.2 in male and 11.7 ± 5.3 in female sera (P = 0.036). Antibody binding to aortic DHV was also higher in males, but not significant (18.8 ± 5.0 vs 15.6 ± 4.0). Blood group (ABO) incompatibility between the serum from controls and DHV showed no impact on antibody binding, and there was no age-related impact among DHV donors.
CONCLUSIONS
Residual immunogenicity of decellularized homografts appears to exist despite almost complete cell removal. The established dot blot method allows a semi-quantitative assessment of the individual immune response towards extracellular DHV components and potentially the possibility of preoperative homograft matching.
Keywords: Heart valve replacement, Homograft, Decellularization
INTRODUCTION
The concept of tissue-engineered heart valves initially raised expectations of an unlimited supply of engineered valves in any size or length providing ideal haemodynamic performance and unlimited durability [1].
However, tissue-engineered heart valves currently fall short of these early expectations. Total artificial heart valves, predominantly made from polymers, have shown poor preclinical performance in animal models. Progress has been made recently in terms of valve design, which led to improved mechanical durability in sheep models, and early clinical trials have been initiated in patients undergoing pulmonary valve replacement [2–4].
Tissue-engineered heart (TE) valves based on human tissue were introduced to the clinic ∼15 years ago and are one of the most advanced TE approaches successfully introduced to the clinic to date [5]. These concepts are based on the decellularization of donated cadaver valves with and without subsequent cryopreservation [6]. Cryopreserved decellularized pulmonary homografts, when used for pulmonary valve replacement, have shown better long-term performance than standard cryopreserved homografts [7]. Decellularized fresh homografts, which omit an additional cryopreservation step, have also shown superior results when compared to standard homografts [8–10].
However, early degeneration of decellularized homografts has been observed in a small subset of patients, raising questions of residual immunogenicity. Histological analysis of 1 decellularized homograft explanted 12 months after implantation due to homograft shrinkage showed severe damage in the matrix structure and a high number of infiltrating immune-competent cells without any evidence of bacterial endocarditis. Interestingly, no classic signs of T-cell-mediated graft rejection were evident [11]. This is in line with our analysis of early cellular immune response following implantation of decellularized pulmonary homografts, where no significant changes in cell counts were observed up to 3 years after implantation [12].
These findings lead to the hypothesis that early failure of decellularized homografts may be predominantly antibody mediated on the basis of preformed recipient antibodies, which also has been described for cross-linked conventional xenogenic heart valves [13, 14]. This is further supported by evidence from the analysis of this type of antibody-mediated immune response towards decellularized xenogeneic heart valves and the observed temporal increase in decellularized homograft valve (DHV)-specific IgG levels in 14 patients following decellularized aortic or pulmonary valve replacement [15].
The purpose of this current analysis was therefore to screen for preformed antibodies towards decellularized matrices in healthy controls using a newly developed dot blot method as a first step towards developing potential matching algorithms as a future strategy [16].
MATERIALS AND METHODS
Control subjects
Twenty healthy control subjects were recruited from Hannover Medical School staff and students following ethics approval by the responsible local ethics committee (EC No. 7871_BO_S_2018). All blood serum samples analysed in this study were taken following written informed consent and health status was assessed by a questionnaire. The epidemiological data for all subjects are displayed in Table 1.
Table 1:
Cohort characteristics of healthy controls
| Subject number | Sex | Age (years) | Blood group |
|---|---|---|---|
| Younger | |||
| 1 | Male | 20 | 0+ |
| 2 | Male | 24 | A+ |
| 3 | Male | 18 | A+ |
| 4 | Male | 23 | A+ |
| 5 | Male | 23 | A− |
| 6 | Female | 25 | A+ |
| 7 | Female | 23 | A+ |
| 8 | Female | 23 | 0− |
| 9 | Female | 21 | A+ |
| 10 | Female | 23 | 0− |
| Older | |||
| 11 | Male | 51 | B− |
| 12 | Male | 59 | A+ |
| 13 | Male | 53 | 0+ |
| 14 | Male | 48 | A+ |
| 15 | Male | 55 | A+ |
| 16 | Female | 50 | A+ |
| 17 | Female | 62 | AB+ |
| 18 | Female | 59 | 0+ |
| 19 | Female | 54 | 0+ |
| 20 | Female | 73 | 0− |
Peripheral venous blood was sampled in Monovettes (SARSTEDT, Nümbrecht, Germany), centrifuged at 4°C and 2000 g for 20 min. The supernatant was stored in aliquots at −80°C.
Tissue homogenization
Tissue pieces of 15 non-implanted decellularized aortic and pulmonary homografts (DHV) or DHV reference samples, which were no longer required, were received from corlife oHG (www.corlife.eu), a Hannover Medical School spin-off company responsible for DHV processing. The homografts used in this study were procured by European Homograft Bank, Clinic St. Jean, Brussels (Material Transfer Agreement 180718).
Decellularization comprised ∼30 different steps using a detergent-based, non-cryopreservation approach as previously described [17]. Each homograft was assessed histologically following processing, and the residual dsDNA content was measured before and after processing prior to final release. Reference samples of all homografts were stored in accordance with German law for at least 1 year.
DHV tissues (∼400 mg of each sample) were minced and pulverized in liquid nitrogen and put through a dismembrator (B. Braun, Melsungen, Germany). Subsequently, they were digested: 10 mg of DHV were mixed with 80 µl of tris-hydroxylmethyl-aminomethane (TBS; Merck KGaA, Darmstadt, Germany) and 20 µl of collagenase II solution (5 mg collagenase II with 1 ml of collagenase buffer (260 U/mg). The collagenase buffer consists of 50 µl of 1 M CaCl2 in 50 ml of phosphate-buffered saline (Biochrom, Berlin, Germany).
The solution was diluted to a working concentration of 5 mg/ml with TBS and stored in aliquots of 0.7 ml at −80°C. The collagenase was diluted to the same extent as the heart valve solution and stored in aliquots at −80°C.
Exposure to sera and measurement of antibody levels
A nitrocellulose blotting membrane and filter paper (Sigma-Aldrich, Munich, Germany) were incubated in TBS for 15 min. Both were then placed in the dot blot device (Bio-Rad, Hercules, CA, USA). The membrane was loaded with 10 µg of digested DHV in 200 µl of TBS in every well; each sample was loaded in triplicate.
For the preparation of standards, human serum type AB (Biowhittaker, Walkersville, USA) was diluted to half concentrations with TBS, from 1:16 000 to 1:512 000. Standards S1–S6 were applied twice on the membrane. Two hundred microlitres of diluted collagenase were pipetted into 3 wells. The empty wells were filled with 200 µl of TBS. With a vacuum pump, liquid was sucked through the membrane out of the wells. The nitrocellulose membrane was placed on an orbital shaker at room temperature, incubated in 0.5% glutaraldehyde (Polysciences Inc., Warrington, USA) at 10 rpm for 20 min and washed in TBS 3 times. It was subsequently incubated in 100 ml of phosphate-buffered saline with 5 ml of 1 mM glycine (Carl Roth, Karlsruhe, Germany) for 5 min, followed by incubation in 100 ml of blotting-grade blocker (nonfat dry milk; Bio-Rad) for 1 h. Afterwards, the membrane was incubated in 1 ml of human serum together with 50 ml of the blotting-grade blocker at 4°C for 20 h. This was then washed in TBS with 0.05% Tween 20 (TBST) (PanReac AppliChem, Darmstadt, Germany) 3 times for 5 min and subsequently twice for 10 min. Then, the membrane was incubated in 1.25 µl of goat anti-human IgG, IgA and IgM-HRP secondary antibody and 100 ml of TBST for 1 h.
Finally, the membrane was washed with TBST 3 times for 5 min and 2 times with TBS for 10 min before being incubated in 10 ml of enhanced chemiluminescence substrate (PerkinElmer, Waltham, USA) for 1 min. The membrane was dried between 2 Whatman papers (Whatman International, Maidstone, England) and the chemiluminescence signal was imaged with the ChemiDoc imager (Bio-Rad; applied software settings: ‘chemi Blot’, 30 photos, 70 s exposure time, accumulation signal mode: on).
The amount of bound secondary antibodies determined the intensity of each dot in the images generated with the ChemiDoc imager. This code was transformed densitometrically into a signal intensity (in arbitrary units) with the Image Lab 5.0 software (Bio-Rad).
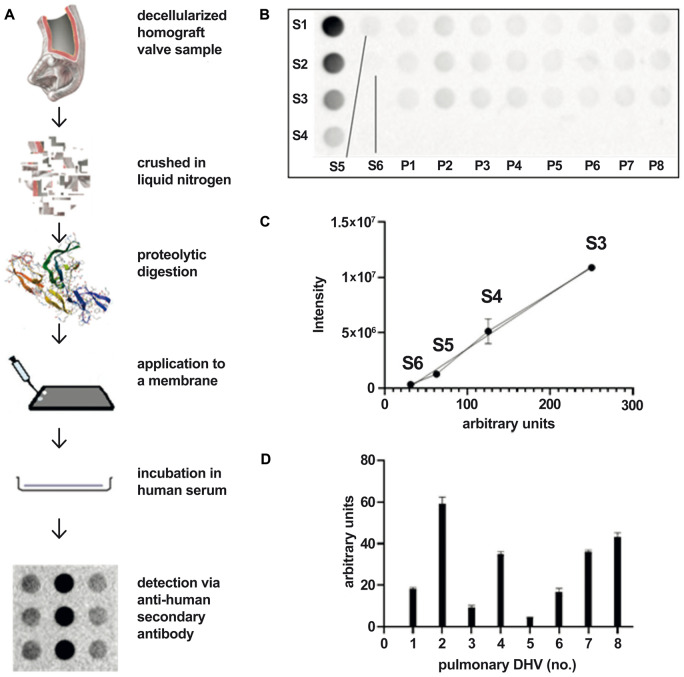
Figure 1 provides a graphical overview of the applied test setting.
Figure 1:
Overview—method to quantify the binding of preformed antibodies to decellularized homograft valve (A). Membrane loading: standards 1–6 and samples of pulmonary decellularized homograft valve (n = 8) (B). Regression of standards. Standards 1 and 2 are excluded because they were oversaturated at the chosen exposure time, which would artificially affect the regression line. A shorter exposure time would not be enough to display standards 5 and 6 (C). Amounts of arbitrary units of preformed antibodies bound to pulmonary decellularized homograft valve (n = 8) in a human serum. Negative values after baseline subtraction that arose in some cases can be explained by steric overlays between remaining donor antibodies and serum antibodies (D). DHV: decellularized homograft valve.
Test reproducibility and variance
The binding of the secondary anti-human antibodies to remaining donor antibodies in DHV after decellularization can lead to false-positive results. As the aim of the study was to assess the immunogenicity of the collagen matrix and extracellular components of DHV, a membrane loaded with DHV, but not exposed to human serum, served as a baseline and the result was subtracted from each specific serum-incubated specimen to eliminate the binding of secondary antibodies to remaining donor antibodies. The negative values after baseline subtraction observed in some cases can be explained by steric overlays between remaining donor antibodies and serum antibodies.
The test robustness and reproducibility of the established dot blot method was extensively tested in decellularized xenogenic heart valves [16]. The resulting inter-assay variance of 9.5% and the intra-assay variance of 9.2% showed that the test is highly reproducible. Technical triplicates were generated for all dot blots. Experimental variance was assessed using Cronbach’s α as an estimate for Tau-equivalent reliability. Cronbach’s α is a function of the number of items in a test, the average covariance between item-pairs, and the variance of the total score. A reliability score of >0.8 generally indicates good internal consistency; a Cronbach’s α of >0.9 indicates excellent consistency.
SPSS 25 (IBM Corporation, Somer, NY, USA), MedCalc (MedCalc Statistical Software version 19.2.0; MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org; 2020) and GraphPad Prism 5.0a (GraphPad Software, San Diego, CA, USA) were used for statistical analyses. Summaries of numeric data are given as means and standard deviation. Student’s t-tests for univariate comparisons and the SPSS procedure MIXED for multivariate comparisons were conducted to analyse statistical significances and factor weights. Differences were considered to be statistically significant for values of P ≤ 0.05.
RESULTS
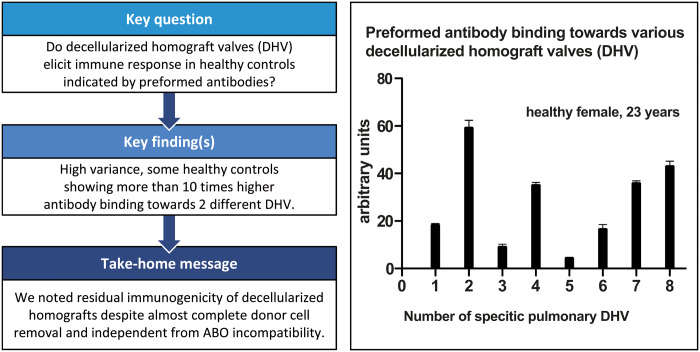
In this study, a dot blot test was used to determine antibody binding to different DHVs. There was considerable heterogeneity in antibody binding among specific control subjects, but also between individual decellularized human specimens. Some healthy controls showed >10 times higher antibody-binding intensities towards 2 different DHVs.
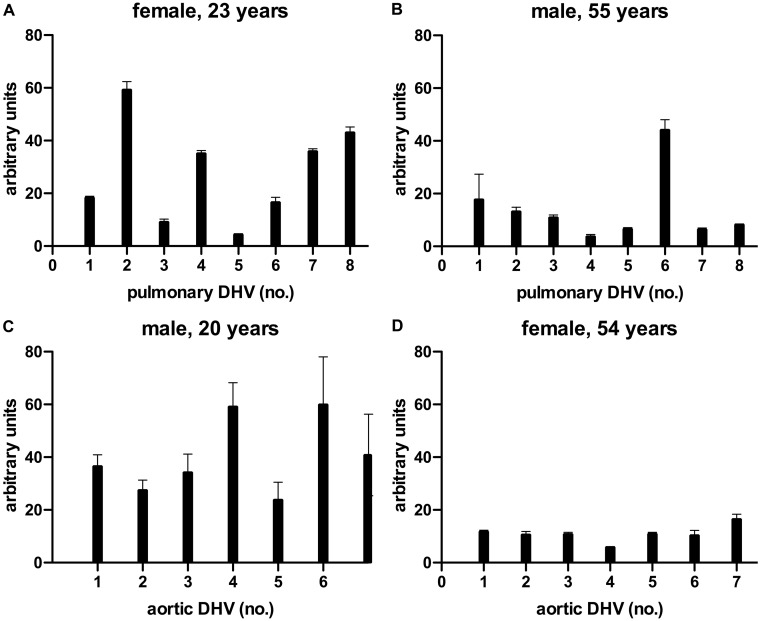
Figure 2 provides examples of the levels of heterogeneity.
Figure 2:
Examples for the binding of preformed antibodies in the serum of a young female (A) and an older male (B) to pulmonary decellularized homograft valve (n = 8) as well as the binding of preformed antibodies in the sera of a young male (C) and an older female (D) to aortic decellularized homograft valve (n = 7). DHV: decellularized homograft valve.
Supplementary Material, Tables SA and SB provide detailed information of the binding of preformed antibodies for all 20 sera of healthy control subjects to all 15 decellularized homografts, grouped by aortic or pulmonary DHV including donor characteristics.
Impact of sex and age
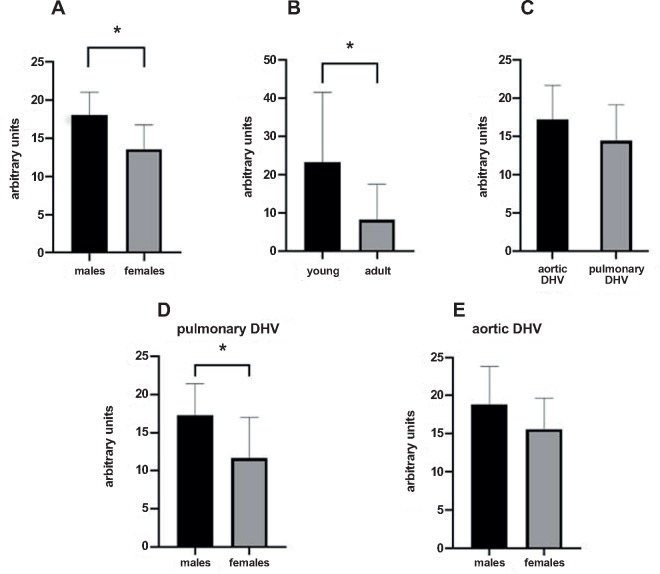
The amount of preformed antibodies, which bound to DHVs, was statistically significantly higher in the sera of healthy males (n = 10) than in the sera of healthy females (n = 10). The mean number of arbitrary units was 18.0 ± 3.0 in male and 13.6 ± 3.2 in female sera (P = 0.0118, Fig. 3A).
Figure 3:
Mean amounts of arbitrary units of preformed antibodies bound to: (A) aortic and pulmonary decellularized homograft valve (n = 15) in the sera of male (n = 10) and female (n = 10) healthy subjects; (B) aortic and pulmonary decellularized homograft valve (n = 15) in the sera of young (n = 10) and old (n = 10) healthy subjects; (C) aortic (n = 7) and pulmonary (n = 8) decellularized homograft valve in the sera of 20 healthy subjects; (D) pulmonary decellularized homograft valve (n = 8) in the sera of male (n = 10) and female (n = 10) healthy subjects; and (E) aortic decellularized homograft valve (n = 7) in the sera of male (n = 10) and female (n = 10) healthy subjects. DHV: decellularized homograft valve.
The binding of preformed antibodies to DHV was higher in the sera of younger subjects (n = 10, 18–25 years) than in the sera of older (n = 10, 48–73 years) healthy subjects. The difference between the means of arbitrary units was 15.1 ± 6.5 and statistically significant (23.3 ± 18.2 vs 8.2 ± 9.4, P = 0.0315, Fig. 3B).
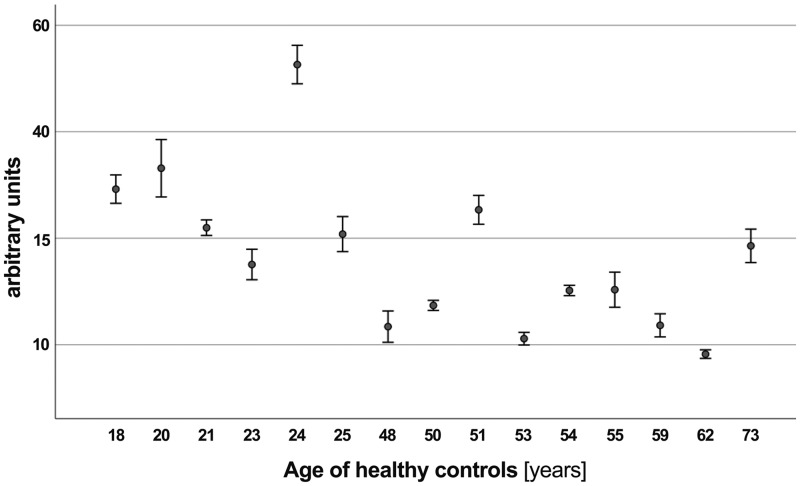
Figure 4 provides an additional graphical overview of preformed antibody binding according to the age of healthy controls, showing less binding in older subjects (R2 linear = 0.150).
Figure 4:
Graphical overview of preformed antibody binding according to age for healthy controls, showing less binding in older subjects (R2 linear = 0.150).
Impact of heart valve type
The amounts of preformed antibodies bound to DHVs were higher in aortic than in pulmonary DHVs. The mean number of antibody binding (in arbitrary units) was 17.2 ± 4.5 in aortic and 14.5 ± 4.7 in pulmonary DHV. This, however, did not reach statistical significance (P = 0.27, Fig. 3C).
The amounts of preformed antibodies bound to pulmonary DHVs were statistically significantly higher in the sera of healthy males (n = 10) than in the sera of healthy females (n = 10). The mean number of arbitrary units was 17.2 ± 4.2 in male and 11.7 ± 5.3 in female sera (P = 0.0360, Fig. 3D).
Antibody binding to aortic DHV was also higher in males, although not statistically significant (18.8 ± 5.0 vs 15.6 ± 4.0, P = 0.21, Fig. 3E).
Impact of DHV donor age and ABO incompatibility
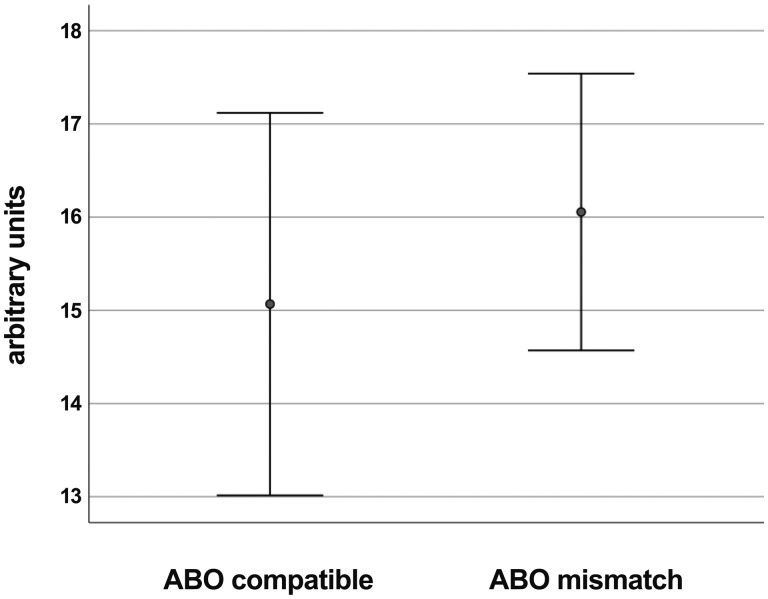
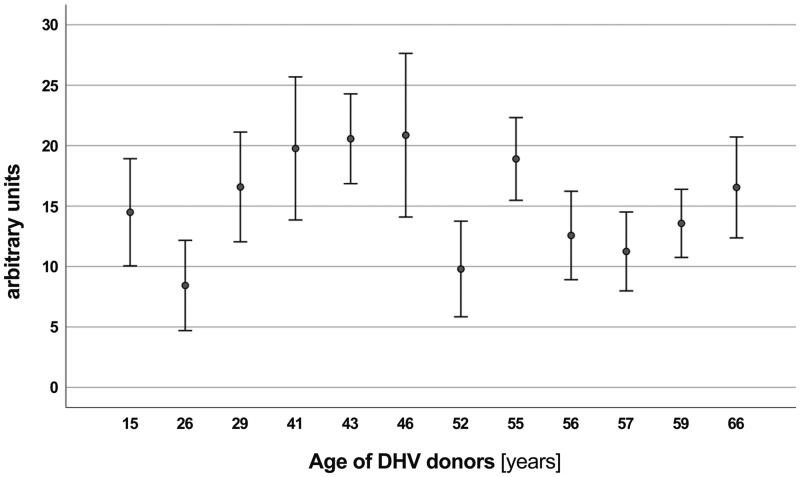
In an univariate analysis, ABO incompatibility between the serum of controls and DHV, as defined for current red blood cell transfusion, showed no impact on antibody binding and there was also no impact based on the age of DHV donors (Figs. 5 and 6).
Figure 5:
Preformed antibody binding differentiated by ABO compatibility between control serum and the decellularized homograft valve donor, showing no difference.
Figure 6:
Graphical overview of preformed antibody binding according to decellularized homograft valve donor age, showing no correlation. DHV: decellularized homograft valve.
Results for multivariate were limited due to the small cohort size and the few events per factor (Supplementary Material, Table SC).
Impact of residual DNA content within DHV
The mean residual DNA content after processing was 5.9 ± 4.6 ng/ml, the upper limit for release being 25 ng/ml. Individual amounts of DNA for each DHV are given within Supplementary Material, Tables SA and SB.
There was no correlation of the residual DNA content within DHV to the amount of preformed antibody binding (Supplementary Material, Fig SA).
Test reproducibility
All tests were performed by 2 experimenters (J.E. and N.M.). Technical triplicates were generated for all dot blots. Experimental variance, as assessed by Cronbach’s α for Tau-equivalent reliability, was 0.973 indicating technical consistency. The interquartile range of the difference to the triplicate mean was −2.02 to +1.48 arbitrary units (Supplementary Material, Fig. SB).
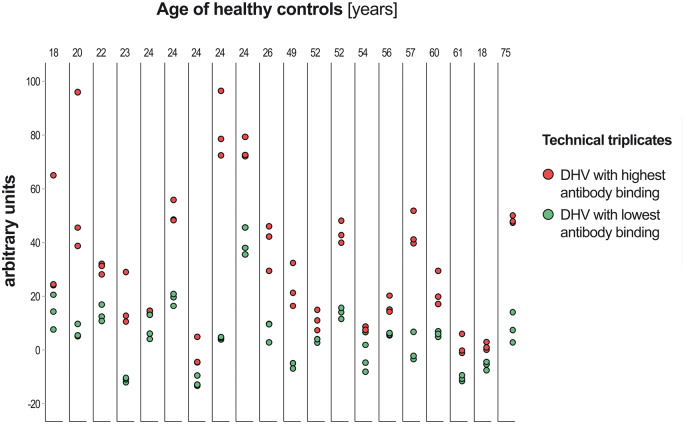
Figure 7 provides a graphical overview of experimental variance and maximum differences in preformed antibody binding between specific DHV according to the age of healthy controls.
Figure 7:
Graphical overview of inter-experimental variance and maximum differences in preformed antibody binding between specific decellularized homograft valve according to the age of healthy controls. DHV: decellularized homograft valve.
DISCUSSION
Decellularization of allogenic heart valves (DHV) has been shown to significantly reduce humoral and cellular immunological response in the recipient [12, 13, 18, 19] and improved clinical durability of decellularized pulmonary homografts has been described by several groups [7, 17, 20]. Despite successful and almost complete decellularization, however, early immune system-mediated failure has been observed in individual patients following implantation of a decellularized pulmonary allograft [11], and some proprietary decellularization methods of aortic allografts in aortic valve replacement have yielded poor results [21].
In this study, we describe for the first time the binding of preformed recipient antibodies to various DHV samples. Such binding showed considerable inter-individual heterogeneity and significant variance towards specific decellularized specimens, with up to ten-fold more binding of preformed antibodies.
Remnants of donor cells, e.g. protein parts of the major histocompatibility complex, are a possible source of potential immunogenicity following decellularization as well as any remnants of the ABO blood group system [22]. Such remnants may persist in the extracellular matrix despite undergoing several washing steps in the detergent-based processing of the allografts. Remains of the detergents itself may be another source of immunization as the high immunogenicity of sodium dodecyl sulphate has been well documented [23]. However, given the in-process controls for complete decellularization and the multiple washing steps during allograft processing, these 3 factors are unlikely to play a key role in the immune system-mediated early degeneration of decellularized allografts. Specific peptides of the extracellular matrix, so-called matrikines, may become visible for the innate and acquired immune system after processing. Matrikines have been shown to modify host cell activity, which may have an impact on the goal of DHV recellularization with non-immunogenic recipient cells [24].
We did not observe any impact arising from ABO compatibility between DHV tissue samples and the serum of healthy controls. This stands in contrast to current results for cryopreserved homografts from Belgium where there was significantly better longevity in ABO-matched pulmonary valve replacements [25]. We also did not find donor age to be a significant risk factor for antibody binding in DHV. Further research is needed to determine potential impact of other donor characteristics, as this may provide a basis for modified allocation algorithms.
As expected, younger subjects had significantly higher levels of preformed antibodies than older subjects. Age-dependent responsiveness of the immune system is a well-described phenomenon [26].
An unexpected finding was the significantly higher preformed antibody binding in male subjects in general and towards decellularized pulmonary allografts in particular. Interestingly, a recent study from the Netherlands on the outcome of standard cryopreserved pulmonary homografts found significantly poorer outcomes in male patients, but male and female patients were not matched for the type of heart defect and number of previous procedures [27]. Sexual dimorphism of the innate immune system is gaining increasingly recognition and has long been documented for autoimmune disorders [28]. Recent research has started to focus on sex differences in general inflammatory pathways, such as the cyclooxygenase-2 signalling and the NLRP3 inflammasome pathway, which promote the release of cytokines IL1ß and IL-18. Sex-specific T-cell response has also been described and in women the influence of exposure to paternal antigens during pregnancy [29]. The impact of sex and gender in the degeneration of heart valve prostheses may play a greater role than previously thought. In vegetarians, predominantly females today, a lower preformed antibody prevalence towards xenogenic heart valves has also been described [15].
As a consequence of this study we have initiated longitudinal analysis of preformed antibody binding in patients before and after operation and the analysis of patients with poor clinical results following DHV implantation. In these analyses, we do benefit from stored reference samples of each homograft before implantation. We also have initiated analysis of explanted DHV using our developed dot blot method. The results of these studies will help to clarify whether current homograft allocation algorhythms will have to be revisited. Prospective allocation of decellularized homografts may include preoperative matching to the specific patient to improve the results especially in the younger patients [30]. For the future, it may also be possible to transfer the established dot blot method, including the matching aspect, to any other biological heart valve prosthesis originating from animal tissue.
Limitations
In this study, we describe our initial experience with preformed antibodies binding to decellularized allografts in healthy controls in a limited sample size. So far, any longitudinal assessment of such antibody binding is lacking adequate analysis of a correlation between the amount of such antibody binding and clinical outcomes in patients receiving decellularized homografts. Respective studies have been initiated and are currently ongoing at Hannover Medical School. To date, there is very scant information on which epitopes preformed antibodies may be directed towards. This information, however, would be highly valuable for specific silencing options to avoid the binding of preformed antibodies.
Furthermore, this study does not add information on the influence of any cross-linking technique, which has been developed for decellularized and non-decellularized xenogeic tissues, on the amount of binding of preformed antibodies.
CONCLUSIONS
Decellularized homografts elicit an immune response leading to the binding of preformed recipient antibodies in healthy controls. There is considerable inter-individual heterogeneity, and also wide variation between specific individual decellularized human specimens. Age plays an important role, as expected, with a higher immune response in young individuals. In male subjects, a significantly higher binding of preformed antibodies was observed. Analysis of preformed recipient antibodies to decellularized human specimens is ongoing in patients to define whether preoperative matching according the amount of preformed antibody binding is reasonable.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank corlife oHG for the supply of decellularized control samples. Furthermore, the excellent technical assistance of Doreen Lenz and Stefanie Reuss is gratefully acknowledged. The authors also thank Nina McGuinness, ELS, for editorial assistance.
Funding
This study was supported in part by a grant from the European Union’s HORIZON 2020 Programme under Grant Agreement No. 643597 (www.arise-clinicaltrial.eu). The funding body had no involvement in study design, data collection or manuscript writing.
Conflict of interest: Axel Haverich holds shares in corlife oHG, the company providing the service of processing decellularized allografts used in this study. Igor Tudorache is a medical consultant for corlife oHG and is involved in the approval of homografts. Ramadan Jashari is a Director of the European Homograft Bank. All other authors declare that there are no conflicts of interest.
Author contributions
Johannes Ebken: Data curation; Formal analysis; Investigation; Methodology; Writing—original draft. Nils Mester: Data curation; Formal analysis; Investigation; Methodology; Writing—original draft. Isabel Smart: Investigation; Methodology; Supervision; Writing—review and editing. Robert Ramm: Investigation; Methodology; Supervision; Writing—review and editing. Tobias Goecke: Investigation; Methodology; Supervision; Writing—review and editing. Ramadan Jashari: Conceptualization; Resources; Writing—review and editing. Dietmar Böthig: Formal analysis; Software; Visualization; Writing—review and editing. Alexander Horke: Data curation; Resources; Supervision; Writing—review and editing. Serghei Cebotari: Conceptualization; Data curation; Resources; Supervision; Writing—review and editing. Igor Tudorache: Conceptualization; Data curation; Resources; Supervision; Writing—review and editing. Murat Avsar: Data curation; Resources; Supervision; Writing—review and editing. Dmitry Bobylev: Data curation; Resources; Supervision; Writing—review and editing. Axel Haverich: Conceptualization; Project administration; Resources; Supervision; Writing—review and editing. Samir Sarikouch: Conceptualization; Funding acquisition; Project administration; Supervision; Writing—review and editing. Andres Hilfiker: Conceptualization; Formal analysis; Methodology; Project administration; Resources; Supervision; Writing—original draft.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Ari Mennander, Marina Radrizzani, Peter Zilla and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- DHVs
Decellularized homograft valves
- TBS
Tris-hydroxylmethyl-aminomethane
- TBST
TBS with 0.05% Tween 20
- TE
Tissue engineering
REFERENCES
- 1. VeDepo MC, Detamore MS, Hopkins RA, Converse GL.. Recellularization of decellularized heart valves: progress toward the tissue-engineered heart valve. J Tissue Eng 2017;8:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nachlas ALY, Li S, Davis ME.. Developing a clinically relevant tissue engineered heart valve—a review of current approaches. Adv Healthc Mater 2017;6: 1–30. [DOI] [PubMed] [Google Scholar]
- 3. Emmert MY, Fioretta ES, Hoerstrup SP.. Translational challenges in cardiovascular tissue engineering. J Cardiovasc Trans Res 2017;10:139–49. [DOI] [PubMed] [Google Scholar]
- 4. Emmert MY, Schmitt BA, Loerakker S, Sanders B, Spriestersbach H, Fioretta ES. et al. Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med 2018;10: 1–13. [DOI] [PubMed] [Google Scholar]
- 5. Cebotari S, Lichtenberg A, Tudorache I, Hilfiker A, Mertsching H, Leyh R. et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation 2006;114:I-132–7. [DOI] [PubMed] [Google Scholar]
- 6. Lisy M, Kalender G, Schenke-Layland K, Brockbank KG, Biermann A, Stock UA.. Allograft heart valves: current aspects and future applications. Biopreserv Biobank 2017;15:148–57. [DOI] [PubMed] [Google Scholar]
- 7. Bibevski S, Ruzmetov M, Fortuna RS, Turrentine MW, Brown JW, Ohye RG.. Performance of SynerGraft decellularized pulmonary allografts compared with standard cryopreserved allografts: results from multiinstitutional data. Ann Thorac Surg 2017;103:869–74. [DOI] [PubMed] [Google Scholar]
- 8. Cebotari S, Tudorache I, Ciubotaru A, Boethig D, Sarikouch S, Goerler A. et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation 2011;124:S115–23. [DOI] [PubMed] [Google Scholar]
- 9. Sarikouch S, Horke A, Tudorache I, Beerbaum P, Westhoff-Bleck M, Boethig D. et al. Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg 2016;50:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tudorache I, Horke A, Cebotari S, Sarikouch S, Boethig D, Breymann T. et al. Decellularized aortic homografts for aortic valve and aorta ascendens replacement. Eur J Cardiothorac Surg 2016;50:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sarikouch S, Theodoridis K, Hilfiker A, Boethig D, Laufer G, Andreas M. et al. Early insight into in vivo recellularization of cell-free allogenic heart valves. Ann Thorac Surg 2019;108:581–9. [DOI] [PubMed] [Google Scholar]
- 12. Neumann A, Sarikouch S, Breymann T, Cebotari S, Boethig D, Horke A. et al. Early systemic cellular immune response in children and young adults receiving decellularized fresh allografts for pulmonary valve replacement. Tissue Eng Part A 2014;20:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boer U, Schridde A, Anssar M, Klingenberg M, Sarikouch S, Dellmann A. et al. The immune response to crosslinked tissue is reduced in decellularized xenogeneic and absent in decellularized allogeneic heart valves. Int J Artif Organs 2015;38:199–209. [DOI] [PubMed] [Google Scholar]
- 14. Smood B, Hara H, Cleveland DC, Cooper DKC.. In search of the ideal valve: optimizing genetic modifications to prevent bioprosthetic degeneration. Ann Thorac Surg 2019;108:624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boer U, Buettner FFR, Schridde A, Klingenberg M, Sarikouch S, Haverich A. et al. Antibody formation towards porcine tissue in patients implanted with crosslinked heart valves is directed to antigenic tissue proteins and alphaGal epitopes and is reduced in healthy vegetarian subjects. Xenotransplantation 2017;24:e12288. [DOI] [PubMed] [Google Scholar]
- 16. Smart I, Goecke T, Ramm R, Petersen B, Lenz D, Haverich A, Niemann H, Hilfiker A.. Dot blots of solubilized extracellular matrix allow quantification of human antibodies bound to epitopes present in decellularized porcine pulmonary valves. Xenotransplantation 2020:e12646. [DOI] [PubMed] [Google Scholar]
- 17. Boethig D, Horke A, Hazekamp M, Meyns B, Rega F, Van Puyvelde J. et al. A European study on decellularized homografts for pulmonary valve replacement: initial results from the prospective ESPOIR Trial and ESPOIR Registry data. Eur J Cardiothorac Surg 2019;56:503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. da Costa FD, Dohmen PM, Duarte D, von Glenn C, Lopes SV, Filho HH. et al. Immunological and echocardiographic evaluation of decellularized versus cryopreserved allografts during the Ross operation. Eur J Cardiothorac Surg 2005;27:572–8. [DOI] [PubMed] [Google Scholar]
- 19. Kneib C, von Glehn CQ, Costa FD, Costa MT, Susin MF.. Evaluation of humoral immune response to donor HLA after implantation of cellularized versus decellularized human heart valve allografts. Tissue Antigens 2012;80:165–74. [DOI] [PubMed] [Google Scholar]
- 20. da Costa FDA, Etnel JRG, Torres R, Balbi Filho EM, Torres R, Calixto A. et al. Decellularized allografts for right ventricular outflow tract reconstruction in children. World J Pediatr Congenit Heart Surg 2017;8:605–12. [DOI] [PubMed] [Google Scholar]
- 21. Helder MR, Kouchoukos NT, Zehr K, Dearani JA, Maleszewski JJ, Leduc C. et al. Late durability of decellularized allografts for aortic valve replacement: a word of caution. J Thorac Cardiovasc Surg 2016;152:1197–9. [DOI] [PubMed] [Google Scholar]
- 22. Baskett RJ, Nanton MA, Warren AE, Ross DB.. Human leukocyte antigen-DR and ABO mismatch are associated with accelerated homograft valve failure in children: implications for therapeutic interventions. J Thorac Cardiovasc Surg 2003;126:232–9. [DOI] [PubMed] [Google Scholar]
- 23. Gibbs S. In vitro irritation models and immune reactions. Skin Pharmacol Physiol 2009;22:103–13. [DOI] [PubMed] [Google Scholar]
- 24. Chakraborty J, Roy S, Ghosh S.. Regulation of decellularized matrix mediated immune response. Biomater Sci 2020;8:1194–215. [DOI] [PubMed] [Google Scholar]
- 25. Dekens E, Van Damme E, Jashari R, Van Hoeck B, François K, Bové T.. Durability of pulmonary homografts for reconstruction of the right ventricular outflow tract: how relevant are donor-related factors? Interact CardioVasc Thorac Surg 2019;28:503–9. [DOI] [PubMed] [Google Scholar]
- 26. Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR. et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol 2009;30:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romeo JLR, Mokhles MM, van de Woestijne P, de Jong P, van den Bosch A, van Beynum IM. et al. Long-term clinical outcome and echocardiographic function of homografts in the right ventricular outflow tract. Eur J Cardiothorac Surg 2019;55:518–26. [DOI] [PubMed] [Google Scholar]
- 28. Jaillon S, Berthenet K, Garlanda C.. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol 2019;56:308–21. [DOI] [PubMed] [Google Scholar]
- 29. Laprise C, Cole K, Sridhar VS, Marenah T, Crimi C, West L. et al. Sex and gender considerations in transplant research: a scoping review. Transplantation 2019;103:e239–e247. [DOI] [PubMed] [Google Scholar]
- 30. Horke A, Bobylev D, Avsar M, Meyns B, Rega F, Hazekamp M. et al. Paediatric aortic valve replacement using decellularized allografts. Eur J Cardiothorac Surg 2020;58:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.