Keywords: diabetes mellitus, fibrosis, gadolinium, NADPH oxidase 4, nephrogenic fibrosing dermopathy
Abstract
Dozens of millions of people are exposed to gadolinium-based contrast agents annually for enhanced magnetic resonance imaging. Gadolinium-based contrast agents are known nephrotoxins and can trigger the potentially fatal condition of systemic fibrosis. Risk factors are practically entirely undefined. We examined the role of NADPH oxidase 4 (Nox4) in gadolinium-induced systemic disease. Age- and weight-matched mice were randomized to experimental diabetes (streptozotocin) and control groups followed by systemic gadolinium-based contrast agent treatment. Nox4-deficient mice were randomized to experimental diabetes and gadolinium-based contrast agent treatment. Skin fibrosis and cellular infiltration were apparent in both gadolinium-based contrast agent-treated and experimental diabetes groups. Similarly, both groups demonstrated renal pathologies with evidence of reactive oxygen species generation. Deletion of Nox4 abrogated both skin and renal pathology, whether from diabetes or gadolinium-based contrast agent treatment. These discoveries demonstrate the importance of Nox4 in gadolinium-based contrast agent- and diabetes-induced fibrosis.
NEW & NOTEWORTHY A mouse model of gadolinium-based contrast agent- and diabetes-induced fibrosis was used to demonstrate the role of NADPH oxidase 4 (Nox4) in gadolinium-induced systemic disease. Using these models, we established the role of Nox4 as a mediator of reactive oxygen species generation and subsequent skin and kidney fibrosis. These novel findings have defined Nox-4-mediated mechanisms by which gadolinium-based contrast agents induce systemic diseases.
INTRODUCTION
Gadolinium-based contrast agents stimulate bone marrow-derived cells to induce aberrant fibrosis (1). There are multiple different brands of magnetic resonance imaging (MRI) contrast (none of them, are technically “new” as these all debuted in the 1990s). Every gadolinium-based contrast agent undergoes some degree of renal elimination (most of them entirely). Because of contrast-enhanced MRI scans, gadolinium concentrations in surface waters have polluted ecosystems surrounding municipalities, with the metal entering tap water and the human food chain (2). The long-term retention and accumulation of gadolinium in every vital organ (including the brain) is of universal concern to patients. This is a new and growing area of research (3).
Regardless of chemical structure or thermodynamic stability, these agents induce profibrotic signals in the skin and ravage the kidneys (4). Gadolinium imparts a memory into bone marrow; repeat exposures of contrast redoubles the fibrosis (5). Therefore, repeated exposure amplifies the severity of fibrosis. Gadolinium-based contrast agent treatment leads to the formation of gadolinium-rich nanoparticles in skin multinucleated giant cells and the vacuoles of renal proximal tubule cells (6). Gadolinium-based contrast agents, far from inert, induce many factors of metabolic syndrome, i.e., hypercholesterolemia, hypertriglyceridemia, and insulin resistance (7).
Other than acute, chronic, or end-stage renal impairment, the risk factors for gadolinium-induced systemic fibrosis are entirely undefined (8). Experimental obesity augments the systemic impact of gadolinium-based contrast agents (e.g., worsened hypertriglyceridemia and cholesterol and increased insulin resistance). Both experimental obesity and gadolinium promote the infiltration of bone marrow-derived cells to the kidney with an increase in fibrosis (7). Diabetes mellitus is also a profibrotic state. Herein, we examined the overlap of experimental diabetes and gadolinium-based contrast agent on systemic fibrosis. NADPH oxidase 4 (Nox4) is essential for mesangial cell migration during embryogenesis (9) and is a pivotal mediator of diabetic kidney disease (10). Therefore, we defined the role of Nox4 in the setting of streptozotocin (STZ)-induced diabetes in the absence and presence of gadolinium-based contrast agent treatment as well as the mediatory role of Nox4 in gadolinium-induced disease.
MATERIALS AND METHODS
Animals
All experimental protocols and procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Male C57 black mice (stock no. 000664, Jackson Laboratory) were acclimatized and randomized to control (n = 10) or STZ treatment (n = 10, 50 mg/kg intraperitoneally daily 5 times). Fifteen weeks after STZ, mice in each group (n = 5) were randomized to untreated or gadolinium (Omniscan, General Electric HealthCare, Little Chalfont, UK) treatment (2.5 mmol/kg intraperitoneally daily, 20 doses over 4 wk) (6, 7). Age- and weight-matched Nox4-deficient mice (B6.129-Nox4tm1Kkr/J, stock no. 022996, Jackson Laboratory) were randomized to no treatment (n = 5) or STZ (n = 5) or gadolinium-based contrast agent treatment (n = 5). End points were 1) weight loss of 10%, 2) dermatological findings, or 3) completion of 4 wk of contrast treatment. Signs of systemic fibrosis were surveyed daily (1, 4–7).
Inductively Coupled Plasma Mass Spectroscopy
Flash-frozen tissues were processed as previously described (6) via the Northwestern University Quantitative Bio-element Imaging Center.
Histology, Immunofluorescence, and Immunochemistry
Lumbar dorsal skin sections were dissected off the underlying fascia and processed as previously described (1, 4–7). Sections were divided and either flash frozen (for immunoblot, immunofluorescence, and gadolinium quantitation) or fixed in neutral-buffered formalin overnight. The renal capsule was removed from the remnant kidney followed by a butterfly section. A portion was fixed in 10% neutral-buffered formalin, and the cortex of the remnant was flash frozen (for immunoblot, immunofluorescence, metabolomics, or gadolinium quantification).
Immunofluorescence
Sections (7 µm) of paraffin-embedded tissues were placed on glass slides, deparaffinized in xylene, and rehydrated as previously described (6, 7). Immunofluorescence involved staining for the indicated antigens followed by fluorescent secondary antibodies (Table 1). Goat secondary antibodies to mouse and rabbit immunoglobulins were Alexa Fluor 594 and Alexa Fluor 488 (ThermoFisher Scientific Life Sciences, Waltham, MA). Mountant for the coverslips contained 4,6-diamidino-2-phenylindole (DAPI).
Table 1.
Immunofluorescence stain synopsis
| Marker | Dilution | Company | City, State |
|---|---|---|---|
| Collagen type IV | 1:100 | Abcam | Cambridge, MA |
| Fibronectin (F3648) | 1:100 | Sigma-Aldrich | St. Louis, MO |
| CD45RO (MA5-11532) | 1:25 | ThermoFisher Scientific Life Sciences | Waltham, MA |
| 3-Nitrotyrosine (06-284) | 1:100 | Millipore | Darmstadt, Germany |
Immunohistochemistry
Frozen tissues (5 µm) were fixed with acetone (10 min), blocked with hydrogen peroxidase (5 min), and then stained with the indicated antibodies. Goat-on-rodent RP polymer (GP626G) and rabbit-on-rodent horseradish peroxidase polymer antibodies were from Biocare Medical (San Francisco, CA). Immunoreactivity was visualized with 3-3′-diaminobenzidine (Biocare Medical).
Dihydroethidium Staining
Flash-frozen tissue was cut (5 µm) and placed on collagen type I-coated coverslips (22 mm round, no. 1.5 thickness, German class, BD, Franklin Lakes, NJ) as previously described (1). After being mounted in Attofluor cell chambers (A7816, Invitrogen, Grand Island, NY), tissues were air-dried for 5 min and then incubated with dihydroethidium (DHE; 10 µM, Invitrogen) in phosphate-buffered saline. Imaging with confocal laser microscopy (FV1000, Olympus, Tokyo, Japan) used excitation and emission wavelengths of 515 and 580 nm, respectively.
Immunoblot Analysis
As previously described (1, 4, 5), tissues were homogenized in radioimmunoprecipitation assay buffer. Antibodies for fibronectin (F3648) and glyceraldehyde-3-phosphate dehydrogenase (sc-25778) were from Sigma-Aldrich (St. Louis, MO) and Santa Cruz Biotechnology (Dallas, TX), respectively.
Statistics
Multigroup comparisons were analyzed using analysis of variance with Tukey’s honestly significant difference post hoc testing using the statistical software R (version 3.6.1) (RStudio version 1.1.414).
RESULTS
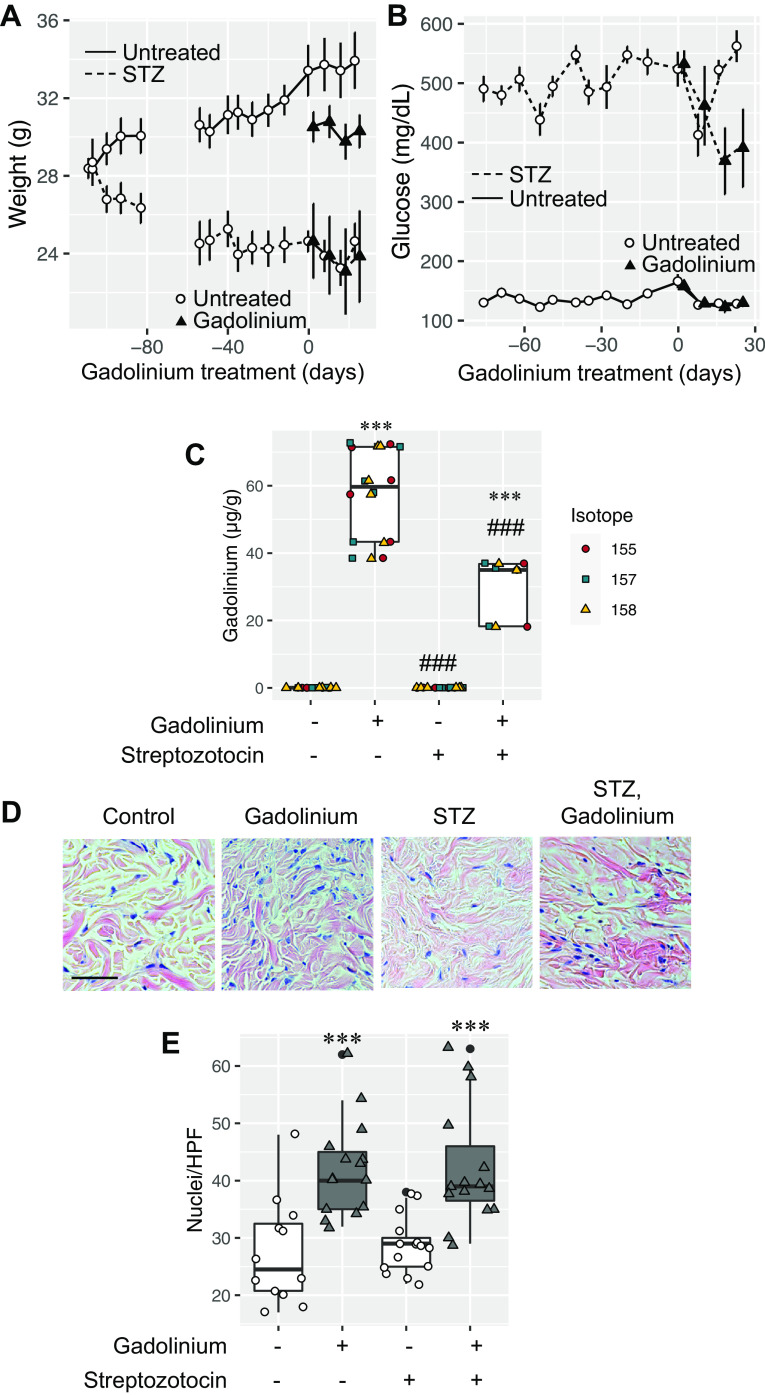
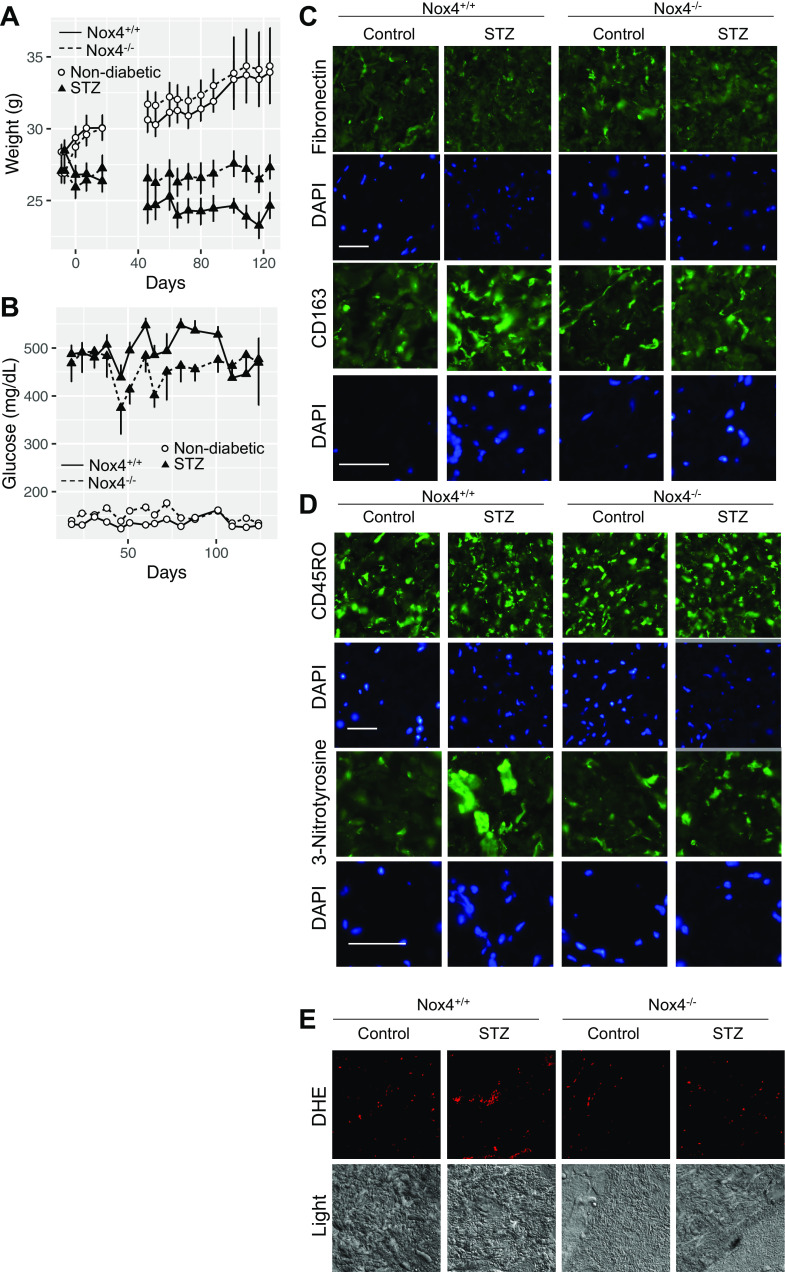
Gadolinium-based contrast agents are not biologically inert in vitro (4) or in vivo (1, 5–7, 11). Not every patient, even with chronic kidney disease, acquires systemic fibrosis after exposure to gadolinium-based contrast agents. Therefore, there are undefined risk factors (8). Systemic treatment with gadolinium-based contrast agents induces glycolytic switching, hypercholesterolemia, hypertriglyceridemia, and insulin resistance (7). We examined the impact of preexisting diabetes mellitus on gadolinium-induced systemic fibrosis. Mice (20 wk of age) were randomized to control or experimentally induced diabetes (STZ) groups (Fig. 1A). Within 23 days of STZ treatment, random blood glucose levels in the diabetic group were elevated relative to the control group (Fig. 1B). Mice were treated with a gadolinium-based contrast agent (Omniscan, 2.5 mmol/kg intraperitoneally daily; see Supplemental Material; all Supplemental Material is available at https://digitalrepository.unm.edu/kinm/2 and https://digitalrepository.unm.edu/kinm/3) for 20 doses over 4 wk to model systemic fibrosis (1, 4–7). At the end point, gadolinium content in flash-frozen skin was elevated only in contrast agent-treated groups (Fig. 1C). As we have previously reported (6), gadolinium-based contrast agent treatment induced skin fibrosis and increased dermal cellularity (Fig. 1, D and E). Although gadolinium content was slightly less in the contrast-treated diabetic group (Fig. 1C, lane 4), dermal cellularity was equal to the gadolinium-treated, nondiabetic group (Fig. 1E, lanes 2 and 4).
Figure 1.
A: wild-type mice (C57 black, 146 days old) were randomized to control (n = 10) or streptozotocin (STZ) treatment (n = 10, 50 mg/kg ip daily ×5). Fifteen weeks after the STZ, mice in each group (n = 5) were randomized to untreated or gadolinium-treated (2.5 mmol/kg ip daily, 20 doses over 4 wk) groups (6, 7). Mean weights ± SE are depicted. B: blood glucose levels in nondiabetic and diabetic animals. C: skin gadolinium concentrations after 4 wk of gadolinium treatment. Frozen tissue was used, and inductively coupled plasma mass spectroscopy was performed. D: gadolinium treatment induced dermal hypercellularity and fibrosis in nondiabetic and diabetic animals. Hematoxylin and eosin-stained, fixed, embedded tissue was used. Calibration bar = 0.05 mm. E: nuclei per high-power field (HPF). Analysis of variance and Tukey honestly significant difference by post hoc testing (first and second lanes, respectively, ***P < 0.001 and ###P < 0.001).
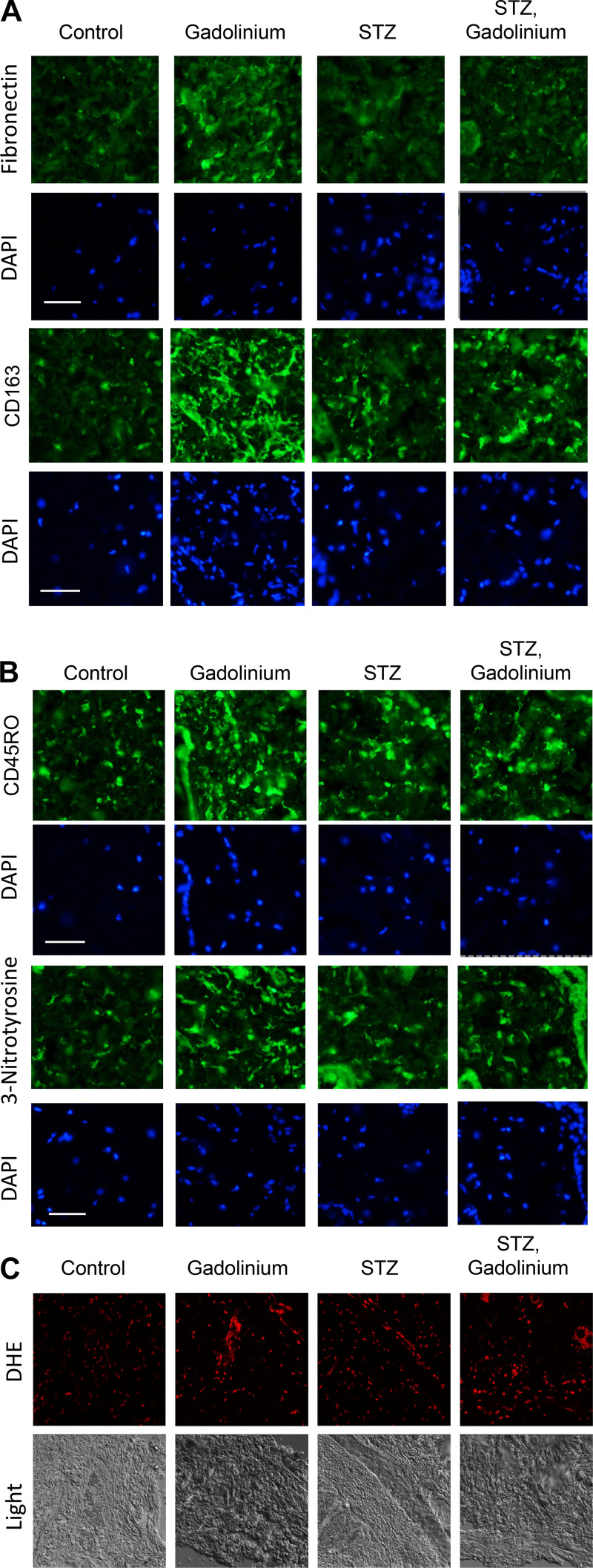
Systemic gadolinium-based contrast agent treatment is known to induce dermal fibronectin accumulation (1), CD163-positive macrophages (5), infiltration of CD45RO-positive bone marrow-derived fibrocytes (5, 6), and oxidative stress from Nox4 (1). Immunofluorescence staining of the skin was used to assess the augmentation of gadolinium-based contrast agent-induced markers by experimental diabetes (Fig. 2). Gadolinium treatment and experimental diabetes did increase markers of fibrosis, infiltration by CD163-positive macrophages (Fig. 2A), and CD45RO-positive cells (Fig. 2B). There was a similar nitrosylation increase in gadolinium treatment and experimental diabetes groups (Fig. 2B) and an increase in reactive oxygen species generation (Fig. 2C). These data demonstrate that similar phenomena are occurring within both gadolinium-induced pro-fibrotic processes in the skin and the state of experimental diabetes.
Figure 2.
Augmentation of gadolinium-based contrast agent-induced dermal pathology by experimental diabetes. A: gadolinium-based contrast agent treatment reproducibly induced fibronectin expression and CD163, a marker of alternatively activated macrophages (5). B: experimental diabetes and gadolinium-based contrast agent treatment-induced infiltration of CD45RO-positive fibrocytes and increased 3-nitrotyrosine in the dermis. Immunofluorescence was performed on paraffin-embedded skin. Calibration bars = 0.05 mm. C: experimental diabetes and gadolinium-based contrast agent treatment increased oxidative stress in the skin. Dihydroethidium (DHE) was used with confocal microscopy. Original magnification: ×200. STZ, streptozotocin.
Other than renal insufficiency, risk factors for gadolinium-based contrast agent-induced systemic fibrosis are entirely unexplored. Worldwide, diabetes is among the most common cause of chronic and end-stage kidney disease. The superimposition of experimental diabetes was compared with systemic gadolinium-based contrast agent treatment (Fig. 3). There was a trend for gadolinium-based contrast agent treatment to increase the kidney weight relative to the body weight. Experimental diabetes increased kidney/body weight by nearly 1.5-fold from control (11 ± 2 versus 7 ± 1 mg/g, respectively, mean ± SD; Fig. 3A). Experimental diabetes increased renal retention of gadolinium nearly twofold (Fig. 3B) from 215 ± 13 to 390 ± 129 µg/g (mean ± SD). Histology revealed that gadolinium-based contrast agent treatment induced renal proximal tubular damage, vacuolization, and mesangial expansion (Fig. 3C). Renal pathology was augmented by the combination of experimental diabetes and gadolinium-based contrast agent treatment. Qualitatively, contrast agent treatment, experimental diabetes, and the combination of the two increased mesangial sclerosis markers. An increase in reactive oxygen species accompanied these changes (Fig. 3D).
Figure 3.
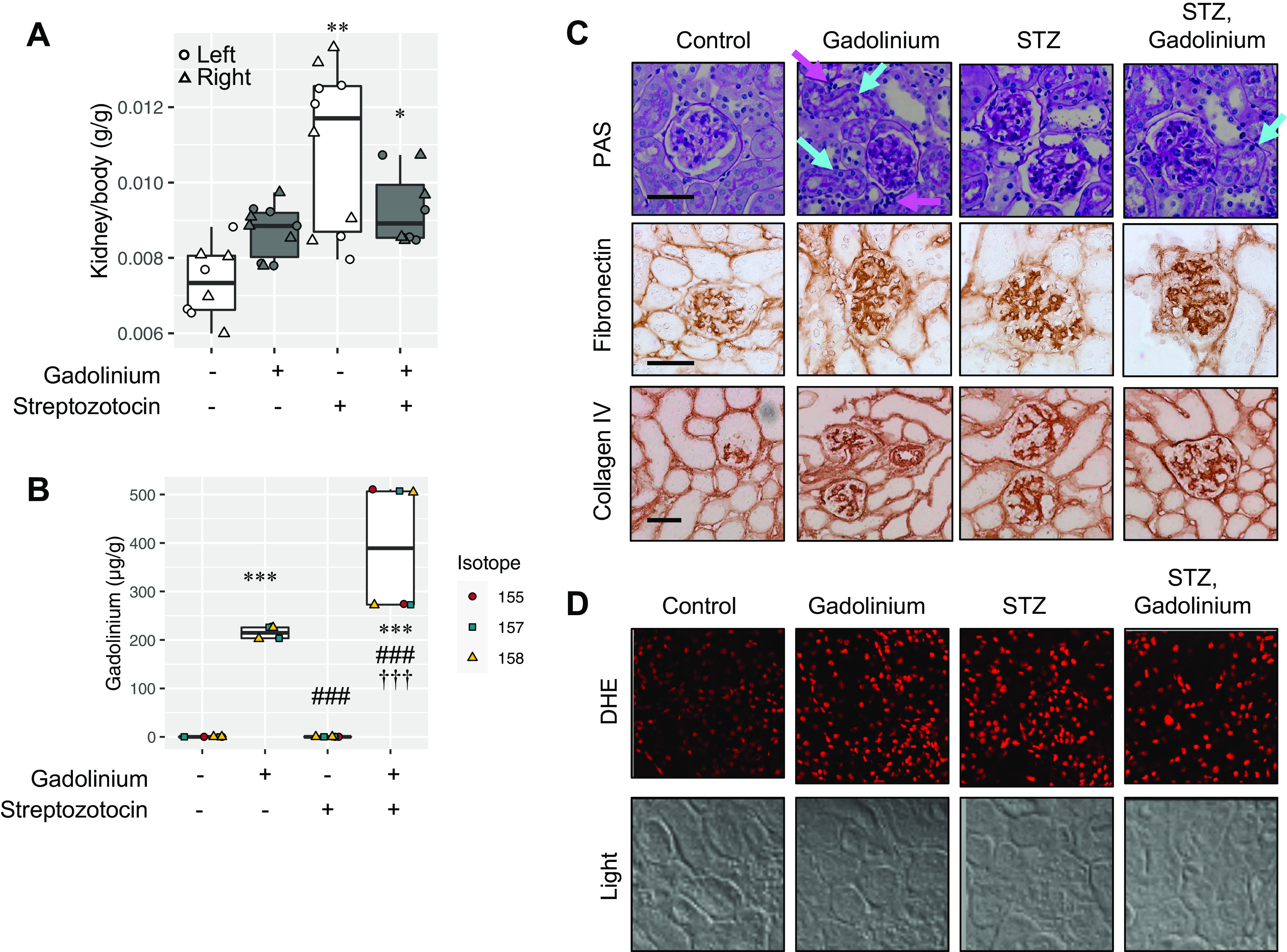
Superimposition of streptozotocin (STZ)-induced experimental diabetes and gadolinium-based contrast agent treatment in the kidney. A: kidney weights for untreated (n = 8), gadolinium (n = 10), STZ (n = 10), and the combination of STZ and gadolinium (n = 8). Left kidney (circle), right kidney (triangle). B: experimentally induced diabetes increased gadolinium retention in the kidney. Analysis of variance and Tukey honestly significant difference by post hoc test (*P < 0.05, **P < 0.01, and ***P < 0.001 from the first lane, ###P < 0.001 from the second lane, and †††P < 0.001 from third lane). C: impact of gadolinium-based contrast agent treatment and experimental diabetes in the renal cortex. Top, gadolinium-based contrast agent treatment-induced tubular damage (cyan arrows) and increased interstitial cells with dense nuclei (magenta arrows) in addition to increased glomerular mesangial matrix. The combination of experimental diabetes and contrast treatment was characterized by mesangial expansion and acute tubular damage (with heterogenous proximal tubular nuclei, cyan arrows). Periodic acid-Schiff (PAS) staining is shown. Middle, fibronectin expression. Immunohistochemistry was performed. Bottom, collagen type IV staining. Immunohistochemistry was performed. Calibration bars = 0.05 mm. D: gadolinium-based contrast agent treatment and experimental diabetes increased renal cortical reactive oxygen species generation. DHE, dihydroethidium. Overall magnification: ×200.
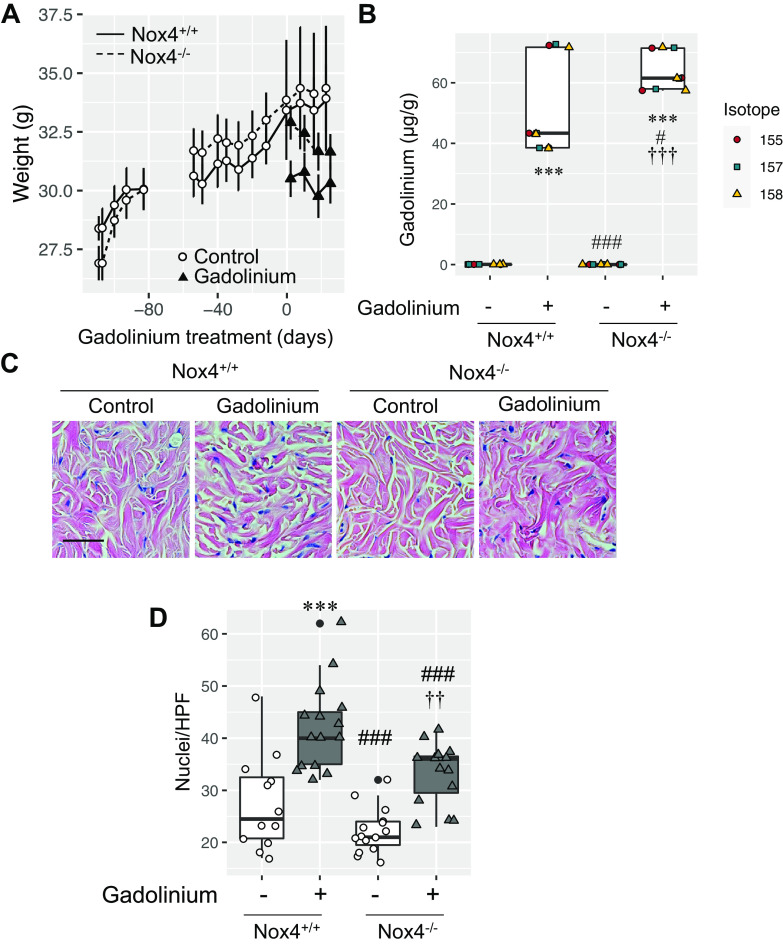
Gadolinium-based contrast agents induce skin fibrosis in a redox-dependent manner in a rat model of systemic fibrosis (1). The degree of reactive oxygen species generation and fibrosis correlated directly with Nox4 expression. We compared the impact of gadolinium-based contrast agent administration in wild-type and Nox4-deficient (Nox4−/−) mice (Fig. 4). Wild-type and Nox4−/− mice were randomized to untreated or gadolinium-treated groups at 35 wk of age (Fig. 4A). Skin gadolinium concentrations were slightly higher in the contrast-treated Nox4−/− group compared with the contrast-treated wild-type group (Fig. 4B). Skin from the contrast-treated Nox4−/− group demonstrated less clefting and less infiltration with spindle-shaped, dense nuclear cells than the treated wild-type group (Fig. 4C). Quantitatively, dermal cellularity of the gadolinium-treated Nox4−/− group did not differ from the untreated wild-type control group but was less than the gadolinium-treated wild-type group (Fig. 3D). In total, these data support the role of Nox4-derived reactive oxygen species generation in mediating gadolinium-based contrast agent-induced systemic fibrosis.
Figure 4.
NADPH oxidase 4 (Nox4) mediates gadolinium-based contrast agent-induced systemic fibrosis. A: wild-type (solid line) and Nox4-deficient (dashed line) mice were randomized to untreated (open symbols) or gadolinium-treated (solid symbols) groups (n = 5 each) at 35 wk of age. Values are presented as mean weights ± SE. B: skin gadolinium concentrations after 4 wk of gadolinium treatment. Frozen tissue was used. Inductively coupled plasma mass spectroscopy was performed. C: Nox4 deficiency abrogated gadolinium-induced dermal fibrosis and cellularity. Hematoxylin and eosin-stained, fixed, embedded tissue was used. Calibration bar = 0.05 mm. D: Nox4 deficiency abrogated gadolinium-based contrast agent-induced dermal hypercellularity. Analysis of variance and Tukey honestly significant difference by post hoc testing (***P < 0.001 from first lane; #P < 0.05 and ###P < 0.001 from the second lane, ††P < 0.01 and †††P < 0.001 from the third lane, respectively). HPF, high-power field.
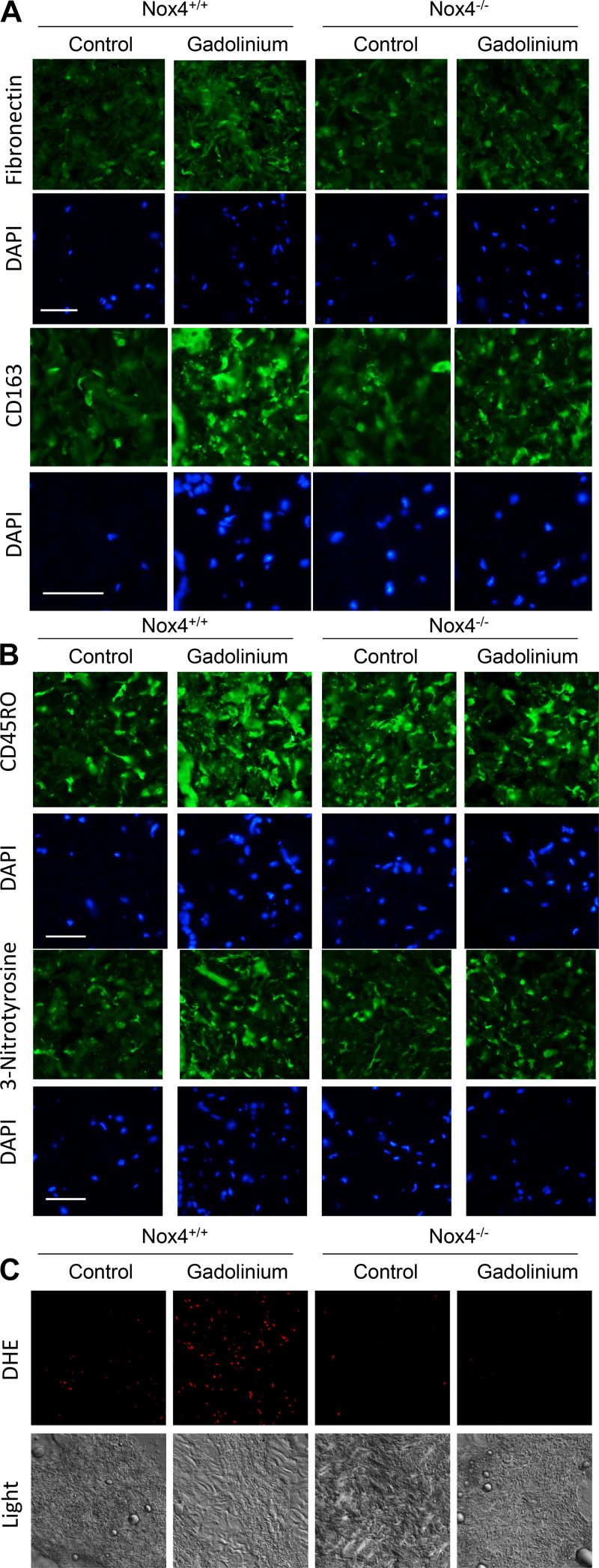
Nox4 deletion abrogated gadolinium-based contrast agent-induced dermal disease (Fig. 5). Absence of Nox4 diminished gadolinium-based contrast agent-induced dermal fibronectin accumulation, infiltration of CD163-positive alternatively activated macrophages (Fig. 5A), infiltration of CD45RO-positive fibrocytes and 3-nitrotyrosine (Fig. 5B). Gadolinium-based contrast agent-induced reactive oxygen species formation in the dermis, as assessed by DHE, was similarly tempered in Nox4-deficient animals (Fig. 5C). In total, these data demonstrate that Nox4 is an essential mediator of gadolinium-based contrast agent-induced skin disease (despite gadolinium concentrations being slightly greater in the skin of gadolinium-treated Nox4−/− animals).
Figure 5.
NADPH oxidase 4 (Nox4) deletion reduces gadolinium-based contrast agent-induced disease in the skin. A: fibronectin expression and CD163-positive alternatively activated macrophages were lessened in Nox4−/− animals treated with gadolinium-based contrast agents. B: Nox4 deficiency abrogated gadolinium-based contrast agent-induced CD45RO-positive cellular infiltration in the dermis relative to treated wild-type animals. This correlated with decreased gadolinium-based contrast agent-induced 3-nitrotyrosine staining. Immunofluorescence was performed on paraffin-embedded skin. Calibration bars = 0.05 mm. C: deficiency of Nox4 reduced gadolinium-based contrast agent-induced oxidative stress in the dermis. DHE, dihydroethidium. Overall magnification: ×200.
Deletion of Nox4 had no impact on renal cortical gadolinium retention (Fig. 6A). Nox4 deficiency abrogated gadolinium-induced mesangial sclerosis and glomerulomegaly (Fig. 6B) as well as the generation of reactive oxygen species (Fig. 6C). Expression of fibronectin was elevated in the renal cortices from the gadolinium-treated group and abrogated in the Nox4-deficient group (Fig. 6D). These data suggest that gadolinium stimulates accumulation of the extracellular matrix in a Nox4-dependent manner.
Figure 6.
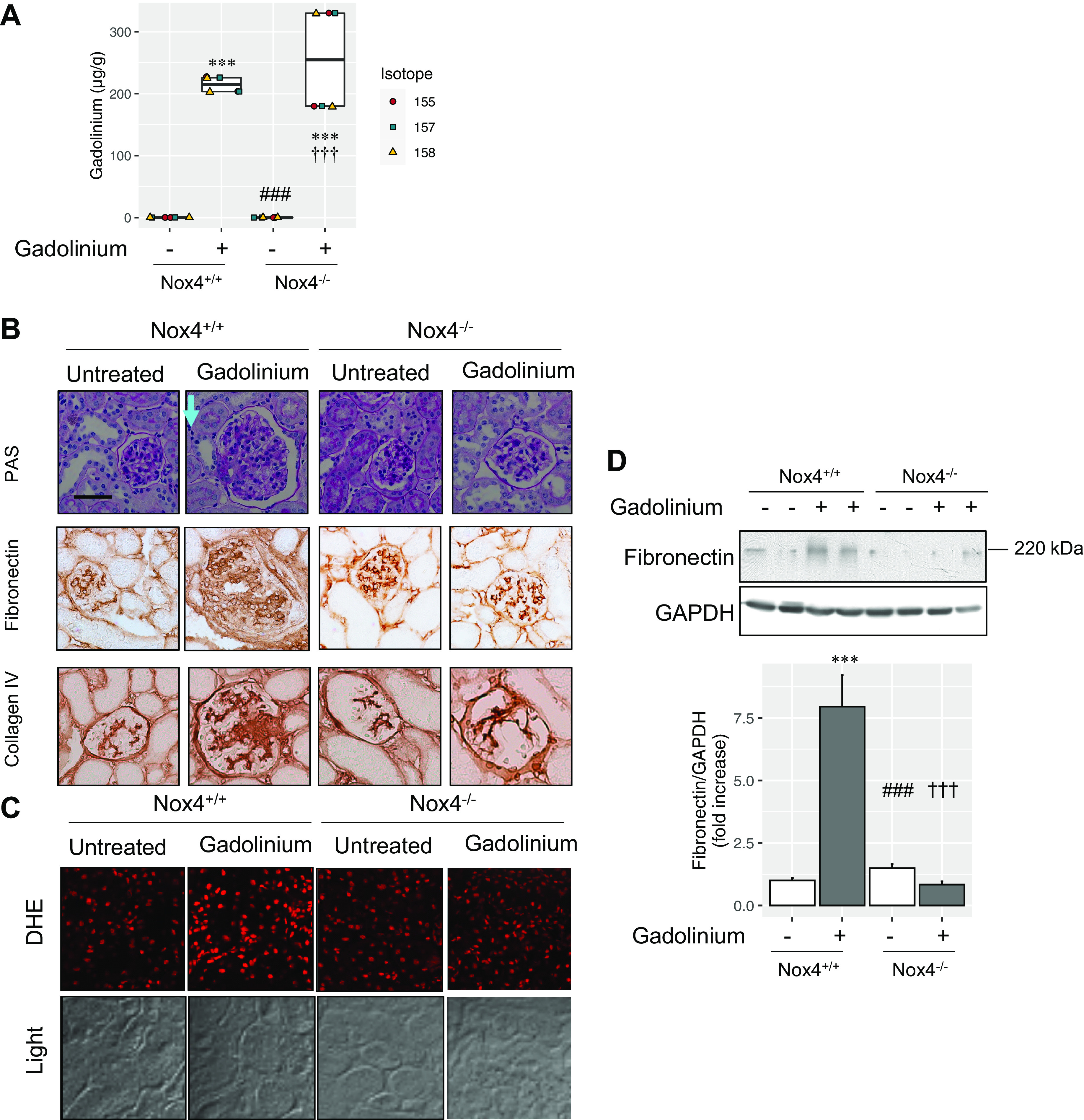
NADPH oxidase 4 (Nox4) mediates gadolinium-induced renal fibrosis. A: gadolinium concentrations from the flash-frozen renal cortex. Inductively coupled plasma mass spectroscopy was performed. B: Nox4 deficiency abrogated gadolinium-based contrast agent-induced renal pathology. Top, gadolinium-based contrast agent treatment induced mesangial expansion, glomerulomegaly, and an increase in interstitial cellularity (cyan arrow). Periodic acid-Schiff (PAS) staining was performed. Middle, fibronectin. Immunohistochemistry was performed. Bottom, collagen type IV. Immunohistochemistry was performed. Calibration bars = 0.05 mm. C: deficiency of Nox4-abrogated gadolinium-induced renal cortical reactive oxygen species. Confocal microscopy was performed. DHE, dihydroethidium. Overall magnification: ×200. D: gadolinium-based contrast agent-induced renal fibronectin accumulation was abrogated by the absence of Nox4. Immunoblot analysis was peformed. The accompanying bar chart depicts the pixel densities of fibronectin relative to the loading control, GAPDH. Analysis of variance and Tukey honestly significant difference by post hoc testing (***P < 0.001 from first lane, ###P < 0.001 from second lane, †††P < 0.001 from third lane, respectively).
Wild-type and Nox4-deficient mice were randomized to STZ-induced experimental diabetes or nondiabetic groups. Weights (Fig. 7A) and blood glucose levels (Fig. 7B) were tracked. At 20 wk of experimental diabetes, the skin demonstrated little change in fibronectin in any group (Fig. 7C). Still, there was a diminution of infiltrative CD163-positive cells in the dermis from diabetic wild-type mice compared with diabetic Nox4−/− mice. There were no differences in CD45RO expression among the groups (Fig. 7D). In wild-type animals, there was evidence of reactive oxygen species generation by both 3-nitrotyrosine staining (Fig. 7D) and DHE staining (Fig. 7E). Nox4 deficiency abolished the increase in 3-nitrotyrosine staining and reactive oxygen species generation in the setting of experimental diabetes. These data indicate that Nox4 is a mediator of CD163 cellular infiltration and protein nitrosylation in the lesions and that Nox4 is the mediator of the reactive oxygen species generation.
Figure 7.
Dermal impact of NADPH oxidase 4 (Nox4) deficiency in experimental diabetes. A: wild-type (solid lines) or Nox4-deficient (dashed lines) mice were randomized to streptozotocin (STZ)-induced experimental diabetes (▴) or control (○) groups. Values are presented as mean weights ± SE. B: blood glucose levels in wild-type (solid lines) and Nox4−/− (dashed lines) mice in untreated (○) and experimental diabetes (▴) groups. C: fibronectin expression and CD163-positive alternatively activated macrophages were lessened in Nox4−/− animals treated with experimental diabetes. D: CD45RO-positive cellular infiltration in the dermis and dermal staining for 3-nitrotyrosine in wild-type and experimental animals. Immunofluorescence was performed on paraffin-embedded skin. Calibration bars = 0.05 mm. E: deficiency of Nox4 reduced diabetes-induced oxidative stress in the dermis. Confocal microscopy was performed. DHE, dihydroethidium. Overall magnification: ×200.
In a rat model, Nox4 mediates hypertrophy and fibronectin expression in the diabetic kidney (10). Similarly, mice with experimental diabetes demonstrated renal hypertrophy (Fig. 8A). Nox4 deficiency eliminated the diabetes-induced effect (Fig. 8A, lane 4). The mesangial sclerosis concomitant with markers of glomerulosclerosis induced by experimental diabetes was suppressed by Nox4 deficiency (Fig. 8B). Diabetes-induced renal pathologies correlated with the generation of reactive oxygen species, which was reduced by Nox4 deficiency (Fig. 8C). Again, protein expression of fibronectin induced by diabetes was normalized in the setting of Nox4 deficiency (Fig. 8D). Similar to gadolinium treatment, Nox4 mediates diabetes-induced mesangial sclerosis.
Figure 8.
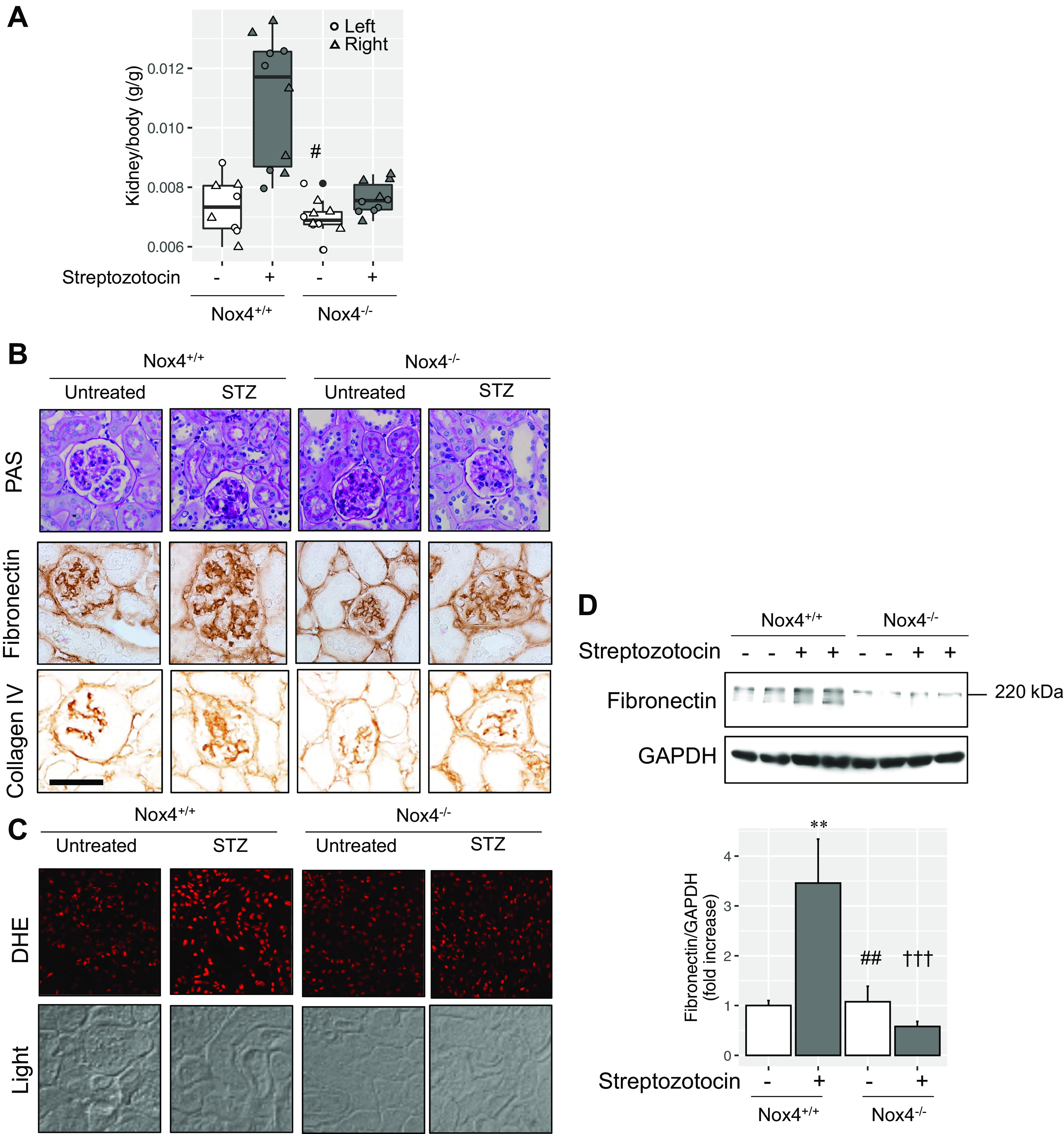
Impact of NADPH oxidase 4 (Nox4) on diabetes-induced renal pathology. A: kidney-to-body weight by analysis of variance and Tukey honestly significant difference post hoc testing (#P < 0.05 from second lane). Left kidney, circle; right kidney, triangle. B, top: experimental diabetes induced mesangial expansion and tubular damage relative to the untreated wild-type group. These changes were abrogated by Nox4 deficiency. PAS, Periodic acid-Schiff. Middle, fibronectin expression. Immunohistochemistry was performed. Bottom, collagen type IV staining. Immunohistochemistry was performed. Calibration bars = 0.05 mm. D: the increase in renal cortical reactive oxygen species by experimental diabetes was blunted in the absence of Nox4. DHE, dihydroethidium. Overall magnification: ×200. D, top: renal cortical fibronectin expression in the renal cortex. GAPDH was used as a loading control. Immunoblot analysis was performed. Bottom, quantification of fibronectin relative to GAPDH by pixel densitometry. ANOVA and Tukey honestly significant difference by post hoc testing was used (**P < 0.01 from first lane, ###P < 0.001 from second lane, †††P < 0.001 from third lane, respectively).
DISCUSSION
Rare earth elements, such as gadolinium, are integral to contemporary society. The increasing use of gadolinium for diagnostic imaging has proven that this metal is a staple of modern medicine. Rising gadolinium levels as salient micropollutant markers indicate environmental contamination due to medical facility use (2). For instance, gadolinium concentrations have risen in the San Francisco Bay over 14-fold in the last 20 yr (12). Gadolinium is now detectable in tap water-derived soft drinks in Germany (13). Despite tens of millions of patients exposed annually, there is a vast knowledge gap concerning the long-term biological consequences of gadolinium-based contrast agents from intravenous administration or ingestion via contaminated water and food supplies (14).
Gadolinium-based contrast agent treatment has a profound impact on renal physiology (7). Repetitive treatment leads to elevations in serum creatinine, proximal tubular vacuolization, interstitial and glomerular fibrosis, and podocyte loss (7). Gadolinium-rich nanostructures form in the glomerulus and inside lipid-laden proximal tubular vacuoles (6, 7). Metabolomic analysis demonstrated that the impact of gadolinium-based contrast agent treatment parallels that of a high fat-induced obesity model (and the combination of gadolinium and high fat augment the fibrosis) (6).
Because gadolinium-induced symptoms seem to be on the increase in patients with or without renal disease, and the startling revelation that gadolinium-based contrast agents enter the cerebrospinal fluid within minutes of administration (15), the United States Food and Drug Administration convened a special meeting of the Medical Imaging Drugs Advisory Committee on September 8, 2017 (16).
Some patients develop iatrogenic systemic fibrosis after just a single dose of a gadolinium-based contrast agent (17–21). At-risk patients may have moderate (chronic kidney disease stage G3) renal insufficiency (21). Recently, a patient developed iatrogenic systemic fibrosis (from an American College of Radiology group II agent, MultiHance) with a normal estimated glomerular filtration rate (22). It can take years before gadolinium-induced systemic fibrosis manifests (23, 24).
Medical science cannot revoke its responsibility for complications that occur from the use of gadolinium-based contrast agents. There are undiscovered factors that put certain individuals at risk for gadolinium-induced diseases (7, 8). We discovered that gadolinium-based contrast agents have profound systemic metabolic effects, such as the induction of hypertriglyceridemia, hypercholesterolemia, and insulin resistance: pillars of metabolic syndrome (7). Furthermore, gadolinium-based contrast agent treatment induced similar renal lesions as did a high-fat diet-induced obesity model, and the combination was additive (7). Data presented herein demonstrate that there are features of both gadolinium-induced fibrosis and diabetes mellitus that overlap in the skin or kidney.
Too many diagnosticians and clinicians harbor the misbelief that these agents are inert and eliminated within 24 h of administration. Recently, investigators have demonstrated that among 16 healthy subjects (normal kidney function), none of them eliminated gadolinium even after 30 days (25). After an intravenous dose, gadolinium partitions into many compartments immediately (every vital organ, including the brain). The clearance of the gadolinium-based contrast agent follows a multiple compartment model (11).
With these experiments, we demonstrated that Nox4 is critical in the generation of reactive oxygen species in the skin and the subsequent recruitment of myeloid cells to fibrotic lesions, supporting our prior models (1). Additionally, Nox4 deficiency diminished gadolinium-based contrast agent-induced dermal fibrosis and mesangial sclerosis. Nox4 mediates diabetic kidney disease (10). This work, however, is the first to establish the role of Nox4 in gadolinium-induced disease in the skin and in gadolinium-induced kidney disease. Furthermore, the profibrotic systemic effects of pharmaceutical-grade gadolinium-based contrast mimic the profibrotic state of experimental diabetes. This finding is consistent with our recent discovery that gadolinium-based contrast agents have a similar systemic and profibrotic effect as a high-fat diet-induced experimental model of obesity: both increase serum cholesterol and triglycerides (7). Gadolinium-based contrast agents and experimentally induced obesity increased insulin resistance. Both induce renal fibrosis, cortical oil red O staining, and aberrations in monoacylglycerol, diacylglycerol, and phospholipid metabolism while perturbing the energetics of the cortex (i.e., inducing a Warburg effect). Not every high-risk (chronic kidney disease/end-stage renal disease) patient develops gadolinium-induced systemic fibrosis following gadolinium-based contrast agent treatment. Therefore, there are latent risk factors that mediate susceptibility to the disease. Our results indicate that these factors involve metabolic states. This study further demonstrates the mechanisms by which gadolinium-based contrast agents induce systemic diseases. Our discoveries indicate that Nox4 is an attractive prophylactic and therapeutic target for gadolinium-induced diseases.
SUPPLEMENTAL DATA
All Supplemental Material is available at https://digitalrepository.unm.edu/kinm/2 and https://digitalrepository.unm.edu/kinm/3.
GRANTS
The work was supported Veterans Administration Merit Award Grant I01 BX001958 (B.W.), National Institutes of Health (NIH) Grant R01DK-102085 (B.W.), and the DCI/Kidney Institute of New Mexico. B.W. is an Associate Member to the University of New Mexico Health Sciences Center Autophagy, Inflammation, and Metabolism (AIM) Center of Biomedical Research Excellence AIM CoBRE, supported by National Institutes of Health Grant P20GM121176. This project was additionally supported in part by Dedicated Health Research Funds of the University of New Mexico School of Medicine allocated to the Signature Program in Cardiovascular and Metabolic Disease, the School of Medicine Research Allocation Committee/New Mexico Medical Trust Grant C-2459-RAC, the University of New Mexico Brain and Behavioral Health Institute Grants BBHI 2020-21-002 and BBHI 2019-1008, a metabolomics voucher from the AIM Center, National Institutes of Health Grant P20GM121176, and Center for Integrated Nanotechnologies User Agreement 2019AU0120.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.B., C.D., D.Y.L., C.T., and B.W. conceived and designed research; F.B., C.D., D.Y.L., and C.T. performed experiments; F.B., J.D., C.D., D.Y.L., C.T., G.P.E., and B.W. analyzed data; J.D., C.D., G.P.E., and B.W. interpreted results of experiments; J.D., G.P.E., and B.W. prepared figures; J.D. and B.W. drafted manuscript; J.D. and B.W. edited and revised manuscript; J.D. and B.W. approved final version of manuscript.
REFERENCES
- 1.Wagner B, Tan C, Barnes JL, Ahuja S, Davis TL, Gorin Y, Jimenez F. Nephrogenic systemic fibrosis: Evidence for bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol 181: 1941–1952, 2012. doi: 10.1016/j.ajpath.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Rogowska J, Olkowska E, Ratajczyk W, Wolska L. Gadolinium as a new emerging contaminant of aquatic environments. Environ Toxicol Chem 37: 1523–1534, 2018. doi: 10.1002/etc.4116. [DOI] [PubMed] [Google Scholar]
- 3.Nörenberg D, Schmidt F, Schinke K, Frenzel T, Pietsch H, Giese A, Ertl-Wagner B, Levin J. Investigation of potential adverse central nervous system effects after long term oral administration of gadolinium in mice. PLoS One 15: e0231495, 2020. doi: 10.1371/journal.pone.0231495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do C, Barnes JL, Tan C, Wagner B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am J Physiol Renal Physiol 307: F844–F855, 2014. doi: 10.1152/ajprenal.00379.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drel VR, Tan C, Barnes JL, Gorin Y, Lee DY, Wagner B. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J 30: 3026–3038, 2016. doi: 10.1096/fj.201500188R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Do C, Drel V, Tan C, Lee D, Wagner B. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol 139: 2134–2143e2, 2019. doi: 10.1016/j.jid.2019.03.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do C, Ford B, Lee DY, Tan C, Escobar P, Wagner B. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol 375: 32–45, 2019. doi: 10.1016/j.taap.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens 28: 154–162, 2019. doi: 10.1097/MNH.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner B, Ricono JM, Gorin Y, Block K, Arar M, Riley D, Choudhury GG, Abboud HE. Mitogenic signaling via platelet-derived growth factor beta in metanephric mesenchymal cells. J Am Soc Nephrol 18: 2903–2911, 2007. doi: 10.1681/ASN.2006111229. [DOI] [PubMed] [Google Scholar]
- 10.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 11.Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol 311: F1–F11, 2016. doi: 10.1152/ajprenal.00166.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatje V, Bruland KW, Flegal AR. Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco Bay over a 20 year record. Environ Sci Technol 50: 4159–4168, 2016. doi: 10.1021/acs.est.5b04322. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt K, Bau M, Merschel G, Tepe N. Anthropogenic gadolinium in tap water and in tap-water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ 687: 1401–1408, 2019. doi: 10.1016/j.scitotenv.2019.07.075. [DOI] [PubMed] [Google Scholar]
- 14.Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem 26: 1877–1885, 2011. doi: 10.1016/j.apgeochem.2011.06.011. [DOI] [Google Scholar]
- 15.Robert P, Frenzel T, Factor C, Jost G, Rasschaert M, Schuetz G, Fretellier N, Boyken J, Idee JM, Pietsch H. Methodological aspects for preclinical evaluation of gadolinium presence in brain tissue: critical appraisal and suggestions for harmonization: a joint initiative. Invest Radiol 53: 499–517, 2018. doi: 10.1097/RLI.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA. Medical Imaging Drugs Advisory Committee Meeting. Gadolinium retention after gadolinium based contrast magnetic resonance imaging in patients with normal renal function. Briefing document. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM572848.pdf.
- 17.Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 188: 586–592, 2007. doi: 10.2214/ajr.06.1094. [DOI] [PubMed] [Google Scholar]
- 18.Collidge TA, Thomson PC, Mark PB, Traynor JP, Jardine AG, Morris ST, Simpson K, Roditi GH. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology 245: 168–175, 2007. doi: 10.1148/radiol.2451070353. [DOI] [PubMed] [Google Scholar]
- 19.Lauenstein TC, Salman K, Morreira R, Tata S, Tudorascu D, Baramidze G, Singh-Parker S, Martin DR. Nephrogenic systemic fibrosis: center case review. J Magn Reson Imaging 26: 1198–1203, 2007. doi: 10.1002/jmri.21136. [DOI] [PubMed] [Google Scholar]
- 20.Marckmann P, Skov L, Rossen K, Heaf JG, Thomsen HS. Case-control study of gadodiamide-related nephrogenic systemic fibrosis. Nephrol Dial Transplant 22: 3174–3178, 2007. doi: 10.1093/ndt/gfm261. [DOI] [PubMed] [Google Scholar]
- 21.Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 243: 148–157, 2007. doi: 10.1148/radiol.2431062144. [DOI] [PubMed] [Google Scholar]
- 22.Lohani S, Golenbiewski J, Swami A, Halalau A. A unique case of nephrogenic systemic fibrosis from gadolinium exposure in a patient with normal eGFR. BMJ Case Rep, 2017: bcr2017221016, 2017. doi: 10.1136/bcr-2017-221016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis 18: 188–198, 2011. doi: 10.1053/j.ackd.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Wiginton CD, Kelly B, Oto A, Jesse M, Aristimuno P, Ernst R, Chaljub G. Gadolinium-based contrast exposure, nephrogenic systemic fibrosis, and gadolinium detection in tissue. AJR Am J Roentgenol 190: 1060–1068, 2008. doi: 10.2214/AJR.07.2822. [DOI] [PubMed] [Google Scholar]
- 25.Alwasiyah D, Murphy C, Jannetto P, Hogg M, Beuhler MC. Urinary gadolinium levels after contrast-enhanced MRI in individuals with normal renal function: a pilot study. J Med Toxicol 15: 121–127, 2019. doi: 10.1007/s13181-018-0693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]