Abstract
Clotting, anticoagulation, platelet consumption, and poor platelet function are major factors in clinical extracorporeal circulation (ECC). We have shown that nitric oxide releasing (NOReL) coatings prevent thrombosis in a rabbit model of ECC without systemic anticoagulation. NOReL prevents platelet adhesion and activation, resulting in preserved platelet count and function. Previous work has shown that activated platelets form platelet-derived microparticles (PMPs). These experiments were designed to determine if PMPs can identify platelet function during ECC. The objective of this study is to investigate the effects of NOReL on platelet activation and PMP formation during ECC. Uncoated ECCs, including with and without systemic heparin, and NOReL coated ECCs, including DBHD/N2O2 and argatroban(AG)/DBHD/N2O2 coated ECCs without systemic heparin, were tested in a 4-hour rabbit thrombogenicity model. Before and after ECC exposure, platelets were stimulated with collagen and PMPs were measured using flow cytometry. The uncoated ECCs clotted within the first hour, while the NOReL coated ECCs circulated for 4 hours. During pre-ECC blood exposure, platelets stimulated with collagen produced PMPs. With post-ECC exposure, platelets from uncoated circuits generated less PMPs than baseline (mean±SD; 23246 ±3611 baseline vs. 1300±523 uncoated post circuit, p=0.018) when stimulated with collagen. However, platelets from the AG/DBHD/N2O2 coated ECCs generated a greater number of PMPs as baseline values (23246±3611 baseline vs. 37040±3263 AG/DBHD/N2O2 post 4 h circuit, p=0.023). Blood exposure during ECC results in platelet activation and clotting in uncoated ECCs. The remaining circulating platelets have lost function, as demonstrated by the low PMP formation in response to collagen. AG/DBHD/N2O2 coated ECCs prevented significant platelet activation and clotting, while DBHD/N2O2 trended towards prevention of platelet activation. In addition, function of the circulating platelets was preserved, as demonstrated by PMP formation in response to collagen. These results indicate that PMPs may be an important measure of platelet activation during ECC. PMPs may provide a simplified way to measure platelet function during clinical ECC.
Keywords: Extracorporeal circulation, thrombogenicity, nitric oxide, rabbit model, platelet microparticles
1. Introduction
Extracorporeal life support (ECLS) used in numerous procedures (i.e. cardiopulmonary bypass, hemodialysis, and hemofiltration) has decreased mortality and morbidity of patients1–5. Although heparin coated components for ECLS are commercially available, clinical procedures using heparin coated tubing have not reduced systemic anticoagulation limitations6. Therefore, systemic anticoagulation is still required in all clinical ECLS procedures7,8. The systemic anticoagulation needed to prevent clotting in devices blocks fibrin formation but does not prevent platelet adhesion7,9. Systemic anticoagulation has many complications, including failure to prevent thrombus and risks associated with heparin use: failure to prevent platelet adhesion, heparin-induced thrombocytopenia (HIT), and increased risk of patient bleeding10. Recent efforts have been devoted to modifying extracorporeal circuit (ECC) surface coating to mimic vascular endothelium and achieve maximum hemocompatibility. Because NO is an endogenous platelet inhibitor and vasodilator, it has been incorporated into polymeric tubing through donor molecules, such as diazeniumdiolated dibutylhexanediamine (DBHD/N2O2)11–13. DBHD/N2O2 has proven efficacious in reducing thrombus formation and platelet activation in animal models; however, small fibrin thrombi are still present.
Heparin efficacy is related to patient plasma antithrombin III levels, but direct thrombin inhibitors, such as argatroban (AG), work independently of patient plasma coagulation factor levels14. AG is a synthetic small molecule that has been clinically shown to provide adequate anticoagulation for patients during ECLS, especially for patients with HIT15,16. While AG coated ECC alone inhibits plasma free and clot bound thrombin, AG/DBHD/N2O2 prevents clot formation and in addition preserves platelet function in animal models17,18.
Shear stress from ECC can activate platelets that shed platelet-derived microparticles (PMPs), which may promote thrombosis2,19. PMPs are 0.1 to 1 micron membrane vesicles that are involved in coagulation, platelet adhesion, and inflammation2,19. The present study was designed to evaluate the effects of nitric oxide releasing (NOReL) coated ECCs on platelet microparticle activation. Understanding the involvement of PMPs with the AG/DBHD/N2O2 in thromboresistance will allow researchers to develop more selective coated medical devices for ECLS patients.
2. Materials and Methods
Materials
Tygon® poly(vinyl chloride) (PVC) tubing was purchased from Fisher Healthcare (Houston, TX). Tetrahydrofuran (THF) was distilled over sodium and benzophenone prior to use. (Z)- 1 -[N-Butyl-N-[6[(N-butylammoniohexl)amino]]-diazen-1-ium-1,2-diolate (diazeniumdiolated dibutylhexanediamine (DBHD/N2O2)) was synthesized by treating N,N′-dibutyl-1,6-hexanediamine (American Custom Chemicals Corp., San Diego, CA) with nitric oxide (NO) (80 psi) at room temperature for 17–24 h as previously described20. The following antibodies were purchased from BioRad (Hercules, CA): mouse anti-pig CD61 (IIIa subunit of GPIIb/IIIa) FITC, mouse anti-human P-selectin glycoprotein (CD62P) PE, mouse isotype controls for IgG1 FITC and IgG1 PE rat anti-mouse integrin Alexa Flour 488, rat anti-mouse CD11b Alexa Flour 488, rat anti-mouse CD14 Alexa Flour 488, mouse anti-human CD14 PE, rat isotype control for IgG2b Alexa Fluor 488, and mouse isotype control for IgG2a PE.The antibody clones for P-selectin, IIb/IIIa receptor, CD11b and CD14 have been previously shown to have cross-reactivity to the rabbit21,22.
Synthesis of argatroban polymeric coatings
Synthesis of AG polymeric coating was performed as previously described18. Briefly, 4 ml of HMDI was slowly added to 2 g of the CarboSil® polymer and stirred for 2 h h at room temperature (RT). The reaction product was precipitated, washed, and air dried for 5 min. After dissolving in 25 ml THF, a 10 μl aliquot was submitted to Fourier transform infrared spectrometer (Nicolet 6700, Thermo Scientific, Madison, WI; FTIR) to determine incorporation of either HMDI or PEG-DI linker into the CarboSil® polymer backbone. The rest of the intermediate product solution was drop wise treated with 30 μmoles AG, precipitated with deionized water, and washed to remove excess AG. After air drying, the resultant AG/CarboSil® linked polymer was dissolved in 25 ml THF and a 10 μl aliquot was submitted to FTIR to determine incorporation of AG to the HMDI linker within the CarboSil® polymer backbone.
In Vitro antithrombin assay
The AG polymeric solution was evaluated for activity using an assay previously described18. Briefly, films of the AG-bound CarboSil® polymer were formed in 96-well plates by adding 100 μl of the modified polymer solution. To determine AG levels in the modified CarboSil® polymer, the antithrombin activity of the films were compared to the antithrombin activity of a free AG standard curve. The chromogenic substrate (i.e., N-p-tosyl-Gly-Pro-Arg-p-nitroanilide acetate) (Sigma–Aldrich, St. Louis, MO) is cleaved by thrombin (BC thrombin, Siemens, Newark, DE) into a residual peptide (i.e., Tosyl-Gly-Pro-Arg-OH) and free p-nitroanilide, which is measured at 405 nm in a Biotek Cytation 3 microplate reader (Winooski, VT).
Preparation of argatroban/NOReI polymer and ECC construction
A plasticized PVC coating containing 25 wt% DBHD/N2O2, a NO donor, was prepared using a method previously reported23. Briefly, triple layers of polymeric coatings which included a base layer, active layer, and top layer were individually coated into the Tygon® tubing that formed the ECC. The base layer was synthesized using 4.2 g CarboSil® in 70 ml THF (solution A). The active layer containing the NO donor was prepared by dissolving 2.69 g CarboSil®, 420 mg 50:50 poly-lactic and glycolic acid (PLGA) in 70 ml THF. 1,050 mg DBHD/N2O2 was added to the polymer (solution B). A third top layer contained a10 μmole AG/CarboSil® polymer (solution C).
The ECC construction used in this study was previously described with some modifications24. The ECC was created, starting at the left carotid artery side, with a 16-gauge angiocatheter (Kendall Monoject Tyco Healthcare Mansfield, MA), one 15 cm length ¼ inch inner diameter (ID) Tygon® tubing, one 8 cm length of 3/8 inch ID Tygon® tubing as the thrombogenicity chamber, another 15 cm length ¼ inch ID tubing, and finally a14-gauge angiocatheter. The angiocatheters were interfaced with tubing using 2 luer-lock PVC connectors. The 3/8-inch ID tubing and the ¼-inch tubing were welded together using THF.
The assembled ECC was coated first with a base coating of solution A, then two coats of the active layer of solution B, and finally a topcoat of solution C. Each coat dried for at least 1 hour and complete ECCs dried under vacuum for 2 days.
NO Release measurements
NO released from Norel coated circuits was measured via a Sievers chemiluminescence NO Analyzer (NOA), model 280 (Boulder, CO) as described previously20. Briefly, a 0.5 cm sample of the circuitry was placed in PBS buffer at 37C. NO was continuously swept from the headspace of the sample vessel and purged from the bathing solution with a nitrogen sweep gas and bubbler into the chemiluminescence detection chamber. The flow rate was set to 200 ml/min with a chamber pressure of 5.4 Torr and an oxygen pressure of 6.0 psi. Using this method, a uniform segment of NORel polymer was tested for NO release prior to and 4 h after blood exposure.
Rabbit thrombogenicity model
The animal handling and surgical procedures were approved by the University Committee on the Use and Care of Animals in accordance with university and federal regulations. A total of 14 New Zealand white rabbits (Covance Princeton, NJ) were used for 2 uncoated ECC groups and 2 NOReL coated ECC groups: one uncoated ECC (n=2), one uncoated ECC with systemic heparin (n=2), DBHD/N2O2 coated ECC (n=4), and AG/DBHD/N2O2 coated ECC (n=4).
Anesthesia:
All rabbits (2.5–3.5 kg) were initially anesthetized with intramuscular injections of 5 mg/kg xylazine injectable (AnaSed® Lloyd Laboratories Shenandoah, Iowa) and 30 mg/kg ketamine hydrochloride (Hospira, Inc. Lake Forest, IL). Maintenance anesthesia was administered via a isoflurane gas inhalation at a rate of 1–3 %. In order to maintain blood pressure stability, IV fluids of Lactated Ringer’s were given at a rate of 10 ml/kg/hr. Mechanical ventilation was performed via a tracheotomy using an ADS 2000 Ventilator (Engler Engineering, Hialeath, FL).
In Vivo hemodynamic monitoring:
For monitoring blood pressure and collecting blood samples, the rabbit right carotid artery was cannulated using a 16-gauge IV angiocatheter (Jelco®, Johnson & Johnson, Cincinnati, OH). Blood pressure and heart rate were monitored with a Powerlab 16 series monitor and Labchart software (AD Instruments Colorado Springs, CO). Temperature was monitored with a rectal probe and maintained at 37°C using a water-jacketed heating blanket. Prior to placement of the arterio-venous (AV) ECC, the rabbit left carotid artery and right external jugular vein were isolated. The following baseline parameters were obtained: hemodynamics, arterial blood gas (blood pH, pCO2, pO2, total hemoglobin and methemoglobin) via an ABL 825 blood gas analyzer (Radiometer Copenhagen Copenhagen, DK)), platelet and total white blood cell (WBC) counts via a Coulter Counter Z1 (Coulter Electronics Hialeah, FL), plasma fibrinogen levels via a BCS XP Coagulation Analyzer (Siemens Malvern, PA), activated clotting time (ACT) via a Hemochron Blood Coagulation System Model 801 (International Technidyne Corp. Edison, NJ), platelet function and microparticulate activation via a Chrono-Log optical aggregometer model 490 (Havertown, PA), and platelet P-selectin and monocyte CD11b expression fluorescent-activated cell sorting (FACS) via a Becton Dickinson FACSCanto II flow cytometer (San Jose, CA).
Artery cannulation:
After baseline parameters were obtained , the AV ECC was placed by cannulating the left carotid artery for ECC inflow and the right external jugular vein for ECC outflow. Flow through the ECC was inititated by unclamping the arterial and venous sides and monitored with an ultrasonic flow probe and flow meter (Transonic HT207 Ithaca, NY).
Euthanasia:
After 4 h on ECC, thrombin clotting time (TCT) was measured via Siemens BCS coagulation analyzer, the circuits were clamped, removed from animal, rinsed with 60 ml of saline, and drained. Any residual thrombus in the thrombogenicity chamber tubing was photographed and quantitated using Image J imaging software from the NIH (Bethesda, MD). After administration of 400 U/kg of sodium , the animals were euthanized using Fatal Plus (130 mg/kg sodium pentobarbital) (Vortech Pharmaceuticals Dearborn, MI).
Blood Sampling
Rabbit whole blood samples were collected in non-anticoagulated 1 cc syringes for ACT, 10% anticoagulant containing 3.2% sodium citrate in 3 cc Vacutainer® tubes (Becton Dickinson, Franklin Lakes, NJ) for cell counts, aggregometry, and FACS analysis, and 1 cc syringes containing 40 U/ml of sodium heparin (APP Pharmaceuticals, LLC Schaumburg, IL) for blood-gas analysis. Following initiation of ECC blood flow, blood samples were collected every hour for 4 h for in vitro measurements and processed within 2 hours of collection.
Platelet aggregometry
Rabbit platelet aggregation was assayed based on Born’s turbidimetric method as previously described9. Citrated blood (1:10 blood to ACD) was collected , centrifuged at 110 xg for 15 minutes to collect platelet-rich plasma (PRP), and centrifuged again at 2,730 x g for 15 minutes to collect platelet-poor plasma (PPP) as ablank for aggregation. PRP was incubated for 10 min at 37°C and then 25 μg/ml collagen (Chrono-PAR #385 Havertown, PA) was added. The percentage of aggregation was determined 3 min after the addition of collagen using Chrono-Log Aggrolink software.
Flow cytometry
To determine platelet P-selectin (CD62P) and CD61 (IIIa subunit of IIb/IIIa fibrinogen receptor) expression, 100 ul diluted blood aliquots (1:100 dilution of blood to Hank’s Balanced Salt Solution (HBSS) without CaCl2 and MgCl2) were directly prepared for cell surface staining of P-selectin and IIIa subunit. , 40 μg/ml collagen (4 μl 1000 μg/ml) was added to two aliquots and 4 μl saline was added to another two aliquots. Saturating concentrations (10 μl) of monoclonal antipig CD61 FITC and monoclonal antihuman CD62P PE antibodies were added to one collagen and one saline treated tube. In the other two tubes containing collagen and saline, 10 μl each of antimouse IgG1 FITC and PE were added as nonbinding isotype controls. All samples were incubated for 15 min at room temperature (RT) in the dark, combined with 700 μl of 1% formaldehyde buffer , and stored at 4°C.
To determine monocyte CD11b, and CD14 expression, saturating concentrations (10 μl) of monoclonal mouse antibody for rat antimouse CD11b Alexa Fluor 488 and CD14 PE antibodies was added to 100 ul of undiluted blood. 5 μl of of anti-rat IgG2b Alexa Fluor 488 and anti-mouse IgG2a PE were added to 100ul of undiluted blood as nonbinding isotype controls. Both samples were incubated for 30 minutes at 4°C in the dark. Red blood cells were lysed with 2 ml of FACSLysing Buffer (Becton Dickinson San Jose, CA), vortexed, and then incubated for 10 minutes at room temperature in the dark. After two rounds of centrifugation at 250 × g for 5 minutes at 4°C, aspirating the supernatant and washing the pellet , the cells were resuspended in 250 ul of 1% formaldehyde buffer and stored at 4°C. After flow cytometry, FACSDiva software was used for data analysis.
Cell populations were identified by gating forward scatter (FSC) and side scatter (SSC) light profiles according to Megamix sized beads that corresponded to different blood components: 3 μm for red and white blood cells, 0.9 μm for whole platelet, and 0.5 μm for microparticles (BioCytex, France)25. For each sample, 30,000 total events were collected. Fluorescence intensity of immunostaining was quantitated by histogram log plot analysis. Mean fluorescent intensity (MFI) was expressed as the geometric mean channel fluorescence minus the appropriate isotype control.
Statistical analysis
Data are expressed as mean ± SEM. Comparison between the NORel and polymer control groups were analyzed by the one-way ANOVA with the multiple comparison of means using Student’s t-test. All statistical analyses were performed using nonparametric statistics in SAS JMP version 13 (SAS Institute Cary, NC). Values of P<0.05 were considered statistically significant.
3. Results
Coating fabrication
The NO release rate (flux) in DBHD/N2O2 alone and AG/ DBHD/N2O2 coating (Figure 1) were between 10 to 20 ×10−10 moles/cm2/min. This flux is comparable to previous synthesis of DBHD/N2O211,12,18,26. The AG topcoat was tested for activity through an in vitro antithrombin assay. Based on the standard curve, the activity was 0.05 to 0.14 μM free AG, which is comparable to a previous synthetic process11.
Figure 1.
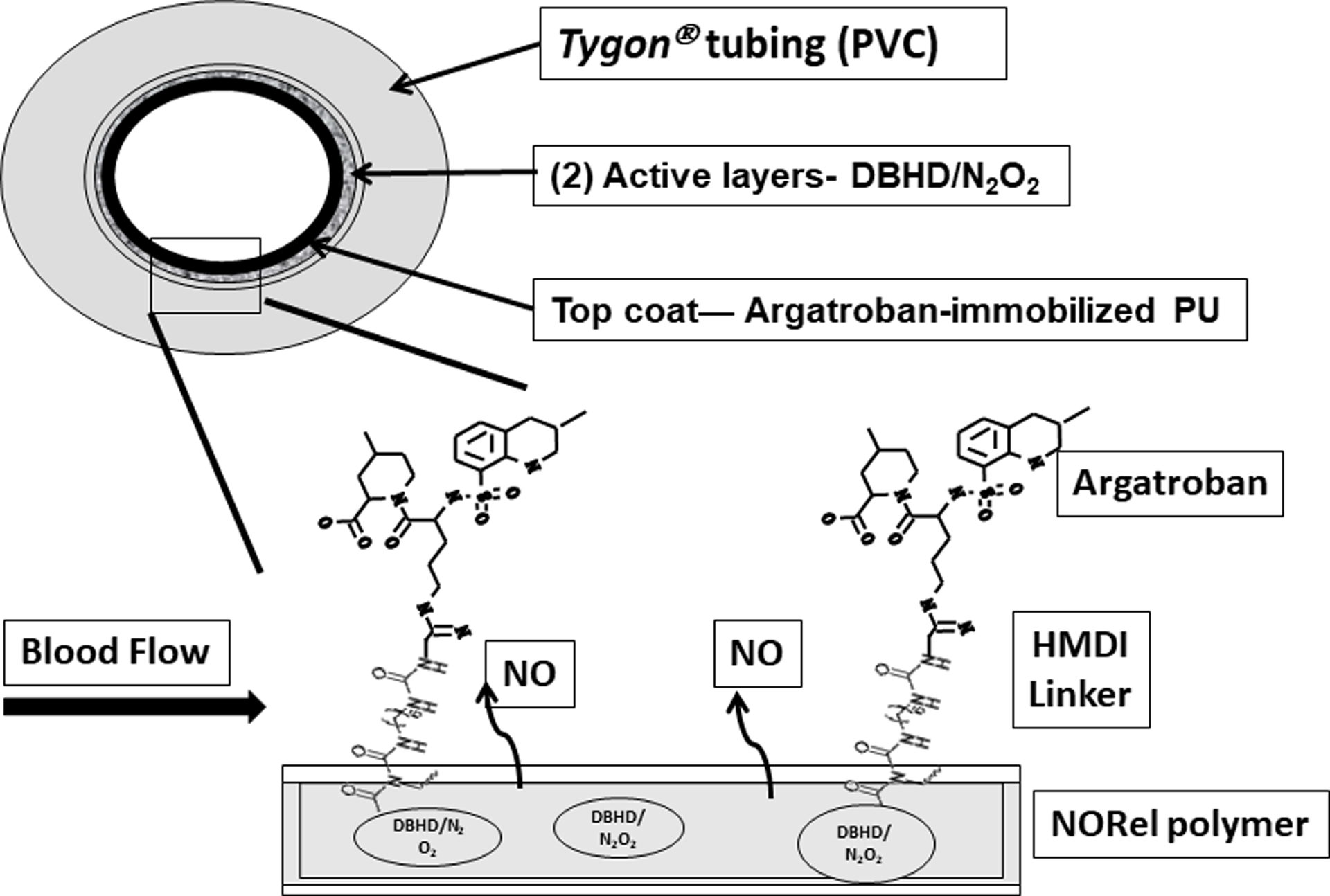
NO releasing (NORel) fabrication and topcoat of argatroban-immobilized CarboSil® polyurethane. Schematic of polymer containing a lipophilic diazeniumdiolated dibutylhexanediamine (DBHD/N2O2) and incorporated at 25 wt % into CarboSil® polymer was coated as active layer on inner surface of PVC tubing. This was coated as an active layer on PVC tubing. The NORel coating was then top coated with the argatroban-immobilized CarboSil® bulk polymer as a single layer (structure of argatroban/hexamethylene diisocyanate (HMDI)/ CarboSil® shown by exploded view of inner coating layers). NO = nitric oxide.
In vivo effects of coated ECCs
Hemodynamics were not significantly different between ECC groups after 4 h of blood exposure (Table 1). The mean arterial blood pressure of coated ECCs significantly fell from baseline levels at 2 h, but thereon maintained a constant level due to continuous IV fluid maintenance. ACT in the DBHD/N2O2 coated ECC group increased from baseline levels likely due to hemodilution. . Blood flow through the ECC was dependent on MAP and area occlusion from thrombi, which is why the uncoated ECC group had a significantly lower flow rate than baseline levels and coated ECCs. To rule out any adverse effects of AG leaching, TCT was measured. There was no significant difference between the TCT of uncoated and coated ECCs. Based on these results, AG was not leaking in any measurable way from the coating; confirming previous work11.
Table 1.
Effect of NO releasing polymer plus top coat of immobilized argatroban on hemodynamics in a rabbit model of extracorporeal circuit.
| Uncoated (n=4) | DBHD (n=5) | AG/DBHD (n=5) | |
|---|---|---|---|
| Mean Blood Pressure (mmHg) | 37.8 ± 13.7 | 36.0 ± 2.1Ŧ | 36.3 ± 4.7Ŧ |
| Heart Rate (beats/min) | 140.0 ± 55.8 | 146.4 ± 43.7 | 133 ± 14.9 |
| Activated Clotting Time (sec) | 156.0 ± 72.5 | 227.2 ± 30.2Ŧ | 210.0 ± 34.9 |
| Fibrinogen | 174.3 ± 65.6 | 151.1 ± 49.5Ŧ | 163.8 ± 24.9Ŧ |
| White Blood Cell Count | 2586.8 ± 709.8Ŧ | 3683.8 ± 1609.7 | 3621.3 ± 565.3 |
| Flow Rate (mL/min) | 23.9 ± 25.1Ŧ* | 63.4 ± 13.4 | 83.0 ± 14.9 |
| Thrombin Clotting Time (sec) | 15.4 ± 1.6 | 15.0 ± 0.5Ŧ | 15.0 ± 0.4Ŧ |
Values are means ± SEM. Wilcoxon/Kruskal Wallis rank nonparametric test with multiple rank sum comparisons was done using SAS JMP 13 (Cary, NC).
p<0.05; between groups.
p<0.05; baseline vs 4 h.
The thrombus area of the combined AG/DBHD/N2O2 ECC group was significantly smaller compared to DBHD/N2O2 and uncoated ECC groups (Figure 2A). The DBHD/N2O2 ECC group had an improvement of 82% over the uncoated ECC group thrombus area, while the AG/DBHD/N2O2 ECC group had almost no thrombus in the thrombogenicity chamber, an improvement of 98% over uncoated ECC thrombus area (Figure 2B). These results confirm that AG shows an additive effect to the antithrombotic properties of DBHD/N2O211,18.
Figure 2.
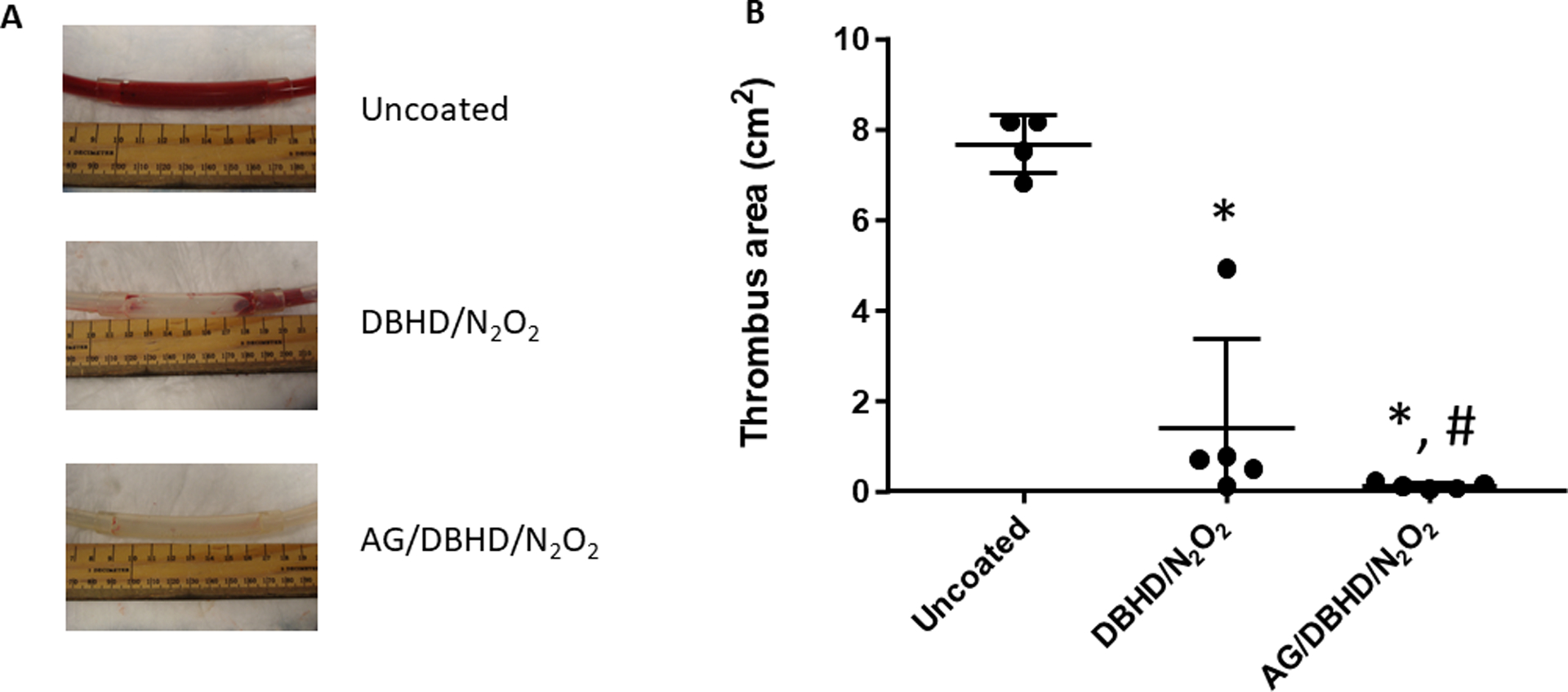
Images (A) and calculated area (B) of thrombus formation in an extracorporeal circuit after 1 hour for the uncoated group to 4 hours for the coated groups of blood exposure in a rabbit model of thrombogenicity. Quantitation of the thrombus area was calculated using Image J software. Values are means ± SEM. * p<0.05; between groups.
Effects of coated ECCs on rabbit WBC
Expression of CD11b, a marker of WBC activation, was measured on monocytes and granulocytes to investigated if the combined AG/DBHD/N2O2 ECC group reduced WBC activation (Figure 3). CD11b remained unchanged from baseline for all ECC groups and confirmed previous cytometric results. Interestingly, the systemic heparin/uncoated ECC group showed a significant increase in CD11b expression after 4 h.
Figure 3.
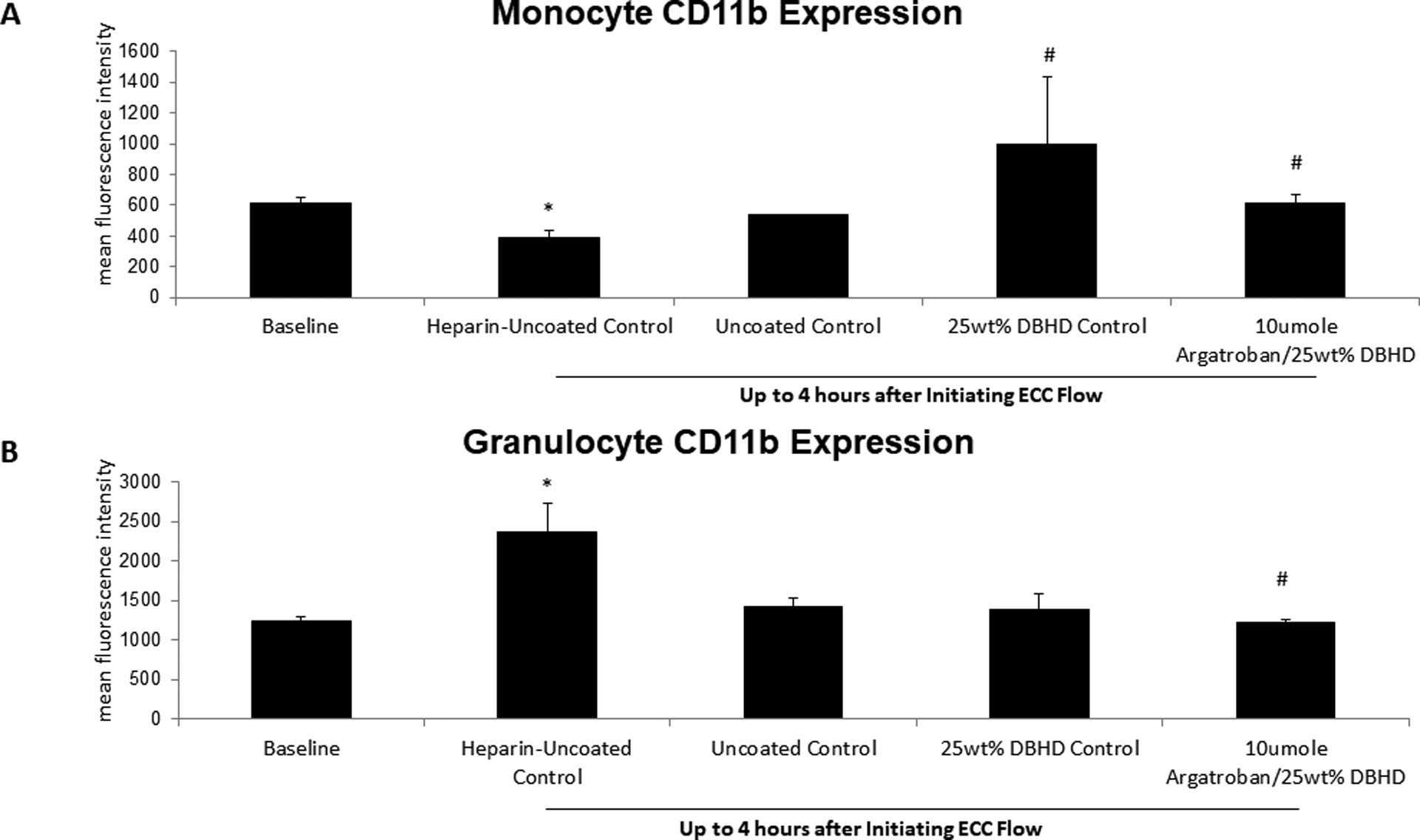
Fluorescent-activated cell sorting (FACS) analysis for circulating monocyte (A) and granulocyte (B) CD11b after 4 h blood exposure with combined argatroban/DBHD/N2O2, DBHD/N2O2 alone, systemic heparin-uncoated and uncoated control ECCs. all control polymer coated ECCs. Monocyte and granulocyte CD11b mean fluorescence intensity (MFI) was quantified after 4 h on ECC in NORel and control ECCs. The data are means ± SEM. * = p < 0.05, for baseline vs heparin/uncoated ECC; # = p < 0.05, for uncoated vs argaroban/DBHD/N2O2 ECC and DBHD/N2O2 alone polymer coatings. The specific MFI data is after the isotype control value was subtracted from each CD11b MFI value. All FACS analyses used the gated FSC/SSC plot for monocytes and granulocytes using 100 μl whole rabbit blood for each determination.
Effects of coated ECCs on rabbit platelets and microparticles
Platelet counts were corrected for hemodilution from additional IV fluids. Platelet counts for the coated ECC groups were maintained at 75% or greater of baseline levels after 4 h of blood exposure (Figure 4A). The uncoated ECC group had a significant 36% drop in platelet count after 1 h of blood exposure. Platelet aggregation remained unchanged for all ECCs compared to baseline levels (Figure 4B). Compared to AG/DBHD/N2O2, the DBHD/N2O2 coated ECC group had significantly higher platelet aggregation levels after 4 h of blood exposure due to variation in baseline levels of platelet aggregation.
Figure 4.
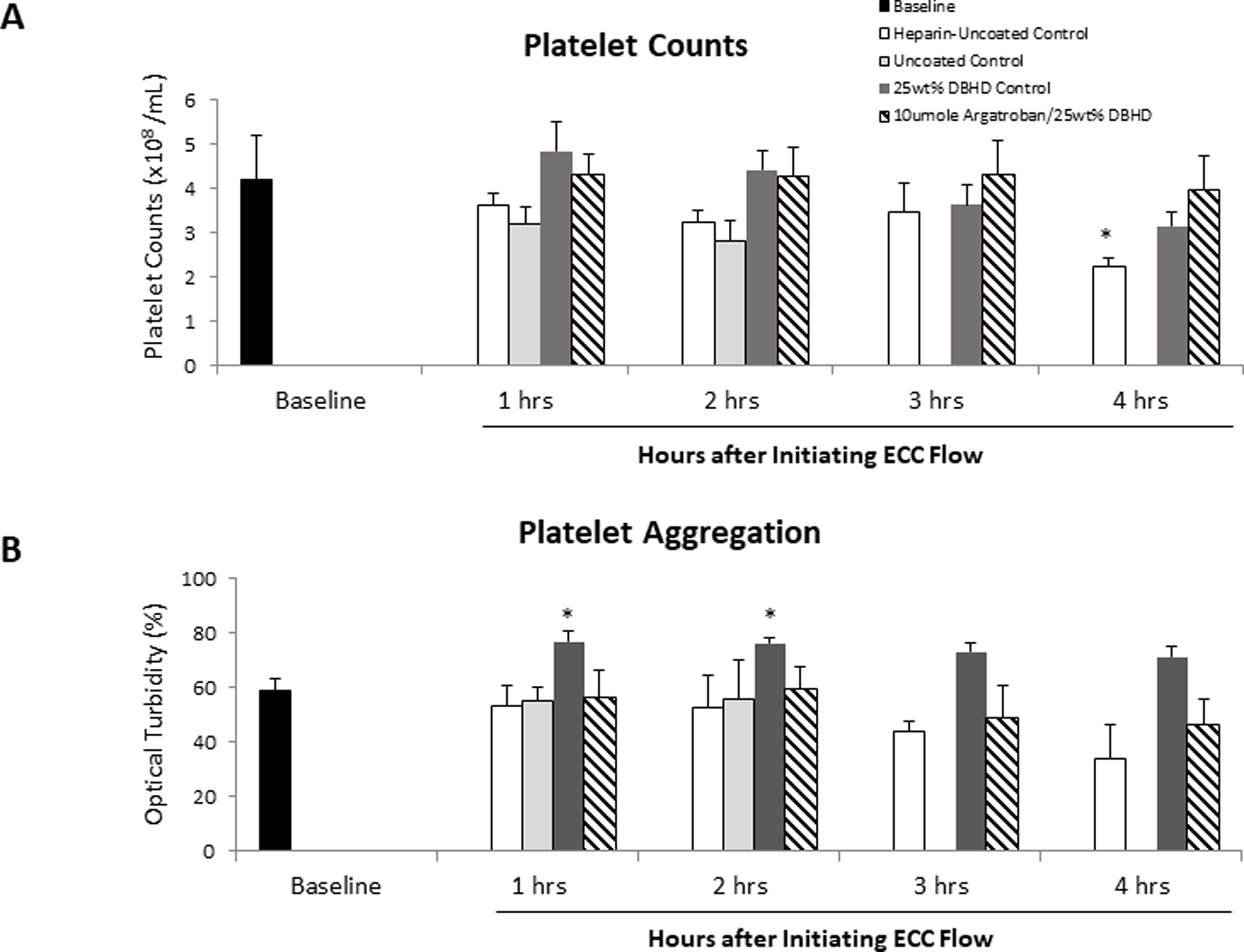
Time-dependent effects of NORel polymer ECC on rabbit platelet count (A) and platelet aggregometry as measured by optical turbidity (B) after 4 h of blood exposure in rabbit thrombogenicity model. Platelet rich plasma was prepared from citrated rabbit blood and the aggregation initiated by 10 mg/ml collagen. Data is the mean ± SEM. * = p < 0.05, baseline vs experimental ECCs.
We were interested in using flow cytometry to evaluate platelet activation and platelet microparticle formation. Based on previous literature, we used 0.5 μm beads to gate for microparticles and 0.9 μm beads to gate for whole platelets (Figure 5)2,19. A limit of 5,000 total events in gated region P1 (Figure 5B) were collected for each sample, which enabled us to compare p-selectin positive platelet and PMP counts between samples. While p-selectin positive platelet counts remained unchanged from baseline for all the groups, the uncoated ECC group had significantly less activated platelets than the AG/DBHD/N2O2 coated group after 4 h of blood exposure (Figure 6A). However, the uncoated ECC group did not have significantly less activated platelets than the DBHD/N2O2 coated group. Following the same trend, p-selectin positive PMP counts remained unchanged from baseline for all the groups. The uncoated ECC group had significantly less activated PMPs than the AG/DBHD/N2O2 coated group after 4 h of blood exposure, but not significantly less than the DBHD/N2O2 coated group (Figure 6B).
Figure 5.

Standard bead sizes (A) were run using flow cytometry to quantify platelets and platelet microparticles (PMP). Bead size of 0.5 μm was gated and this gate used for PMP determination. Bead size of 0.9 μm was gated and this gate used for whole platelet determination. These two gates were used for all subsequent experiments with unstimulated (B) and ex vivo collagen-stimulated platelets (C). P1 gate is for determining whole platelets while P6 and P16 gates (which are the same gate but just different numbers) determines the PMPs. As shown in (B), normal, unstimulated platelets show minimal PMP formation. Collagen-stimulated platelets (C) show a marked increase in PMP count indicating platelet activation.
Figure 6.
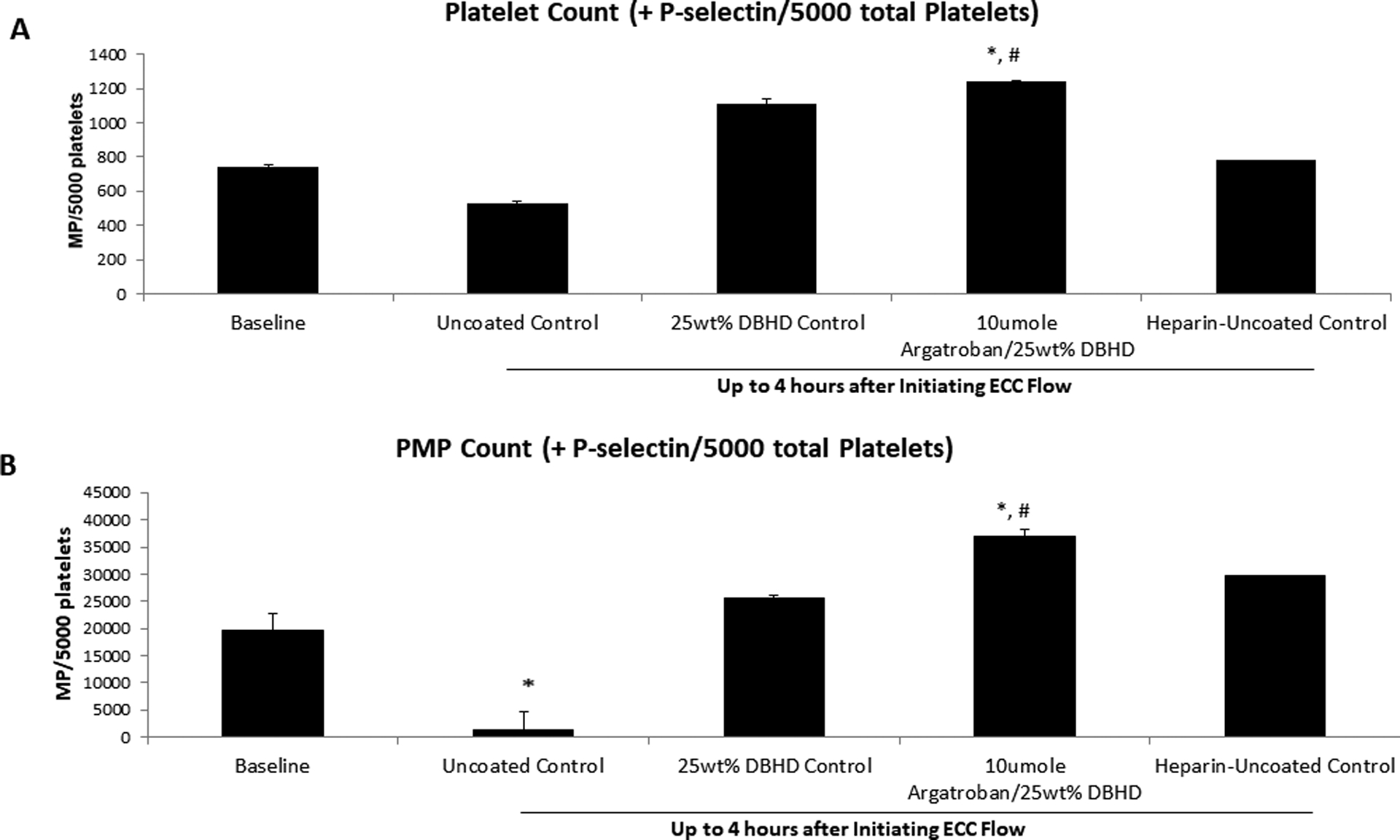
The effects of combined argatroban/ DBHD/N2O2 polymer coated ECC on whole platelet (A) and platelet microparticle (PMP) counts. Uncoated ECC showed significant reduction in both platelet and PMP counts compared to baseline while the combined argatroban/ DBHD/N2O2 coated ECC preserved both platelets and PMPs when compared to baseline values. Values are means ± SEM. * p<0.05; between groups versus baseline; # p<0.05; between groups versus uncoated ECC.
While all the coated ECC group platelets maintained stimulatory capacity after 4 h of blood exposure compared to baseline, the uncoated ECC platelet stimulatory capacity significantly decreased 77% after 2 h of blood exposure compared to baseline capacity (Figure 7A). Although p-selectin expression on PMPs appears to follow the same trend as platelets, the stimulatory capacity of PMPs after blood exposure compared to baseline in all ECC groups was not significant (Figure 7B).
Figure 7.
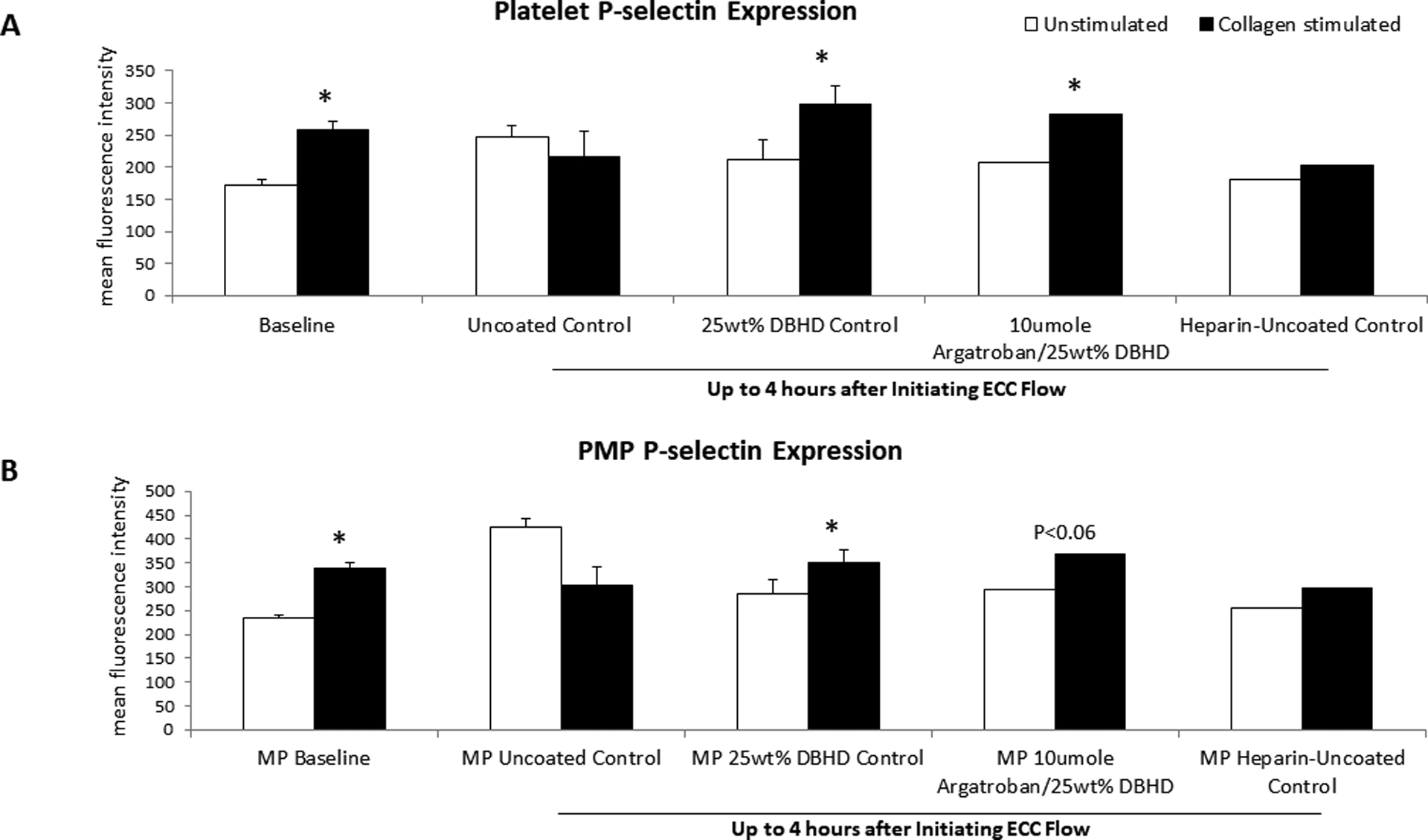
Fluorescent-activated cell sorting (FACS) analysis for circulating platelets (A) and platelet microparticles (PMP) (B) P-selectin after 4 h blood exposure with combined argatroban/DBHD/N2O2, DBHD/N2O2 alone, systemic heparin-uncoated and uncoated control ECCs. Platelet and PMP P-selectin mean fluorescence intensity (MFI) was quantified after 4 h on ECC in NORel and control ECCs. The data are means ± SEM. * = p < 0.05, for baseline vs argaroban/DBHD/N2O2 ECC and DBHD/N2O2 alone polymer coatings. The specific MFI data is after the isotype control value was subtracted from each P-selectin MFI value. All FACS analyses used the gated FSC/SSC plot for platelets and PMPs using 100 μl of diluted whole rabbit blood (1:100) for each determination.
4. Discussion
This study evaluated the effects of NOReL coated circuits, including DBHD/N2O2 and AG/DBHD/N2O2, on platelet activation and subsequent formation of platelet microparticles (PMP) in a 4 h rabbit model of thrombogenicity.
Before delving into the role of PMP formation and thrombosis in this rabbit model, confirmation that NO preserves platelet quiescence and improve thromboresistance in the AG/DBHD/N2O2 coated ECC was established to confirm previous work12,18,26,27. First, the combined AG/DBHD/N2O2 polymer coating produced a similar flux range of 10–20 × 10−10 moles/cm2/min and an antithrombin activity between 0.05 and 0.15 μM of free AG as observed previously18,28. Second, the in vivo results including all hemodynamic parameters were maintained near normal levels under isoflurane gas anesthesia using continuous IV fluids, confirming previous work12,18,28,29. Lastly, AG/DBHD/N2O2 coated ECC had little or no clot in the thrombogenicity chamber compared to the uncoated ECC as previously observed12,18,26,30. This study also confirmed previous results in that the combined polymer significantly reduced the CD11b expression in monocytic and granulocytic WBCs26,27. The interacting effects of the combined AG/DBHD/N2O2 polymer on platelet activation and the subsequent formation of PMPs with the level of coagulopathy in the 4 h exposure of an ECC to circulating blood remains elusive.
PMPs are a subset of a larger population of microparticles that are formed after activation of various circulating blood cells and/or in several disease processes31–36. Diseases with a vascular involvement and hypercoagulability such as disseminated intravascular coagulation, diabetes, immune-mediated thrombosis, kidney diseases, acute coronary syndromes or systemic inflammatory disease, show elevated levels of circulating PMPs. Involvement of procoagulant PMPs in the initiation/dissemination of procoagulant and inflammatory responses are becoming a new challenging front for thrombosis research37. This current study found that platelets and formed PMPs were significantly lower after 4 h of blood exposure in the uncoated ECC compared to the baseline PMP levels. However, the combined AG/DBHD/N2O2 coated ECC preserved platelets and PMPs near baseline levels. In the combined AG/DBHD/N2O2 coated ECC, the collagen-stimulated P-selectin expression for both platelets and PMPs remained near baseline levels while the P-selectin expression in the uncoated and systemic heparin ECCs had significantly reduced expressions compared to baseline. These results indicate that the combined AG/DBHD/N2O2 coated in ECCs can preserve circulating platelet function, as demonstrated by the similar P-selectin response in both the platelet and formed PMPs compared to baseline responses. These results confirm our previous work in that NO releasing polymers do preserve platelets and their response to exogenous stimulation like collagen18,27. However, this current work further demonstrates that activated circulating platelets in the ECC form PMPs and that the level of PMPs are closely related to the extent of platelet activation. Further, the function of the formed PMPs in this work indicates that the combined AG/DBHD/N2O2 coating in ECC elicits thromboresistant responses similarly to circulating platelets when stimulated with agents such as collagen. Even though P-selectin is involved in the overall mechanism of platelet and PMP-induced thrombosis in ECC, the combined AG/DBHD/N2O2 can attenuate that involvement via maintaining initial platelet quiescence. Therefore, PMP formation in ECLS maybe an important biomarker for platelet activation in addition to P-selectin expression Further work is needed to elucidate the degree PMPs regulate thrombosis in ECLS and their mechanism of action in coagulation.
The translation of biological findings derived from animal models to humans should always be preceded with caution. Although this work in a hypercoagulable rabbit model supports the translational capacity of this system to ECMO in human subjects, we recommend the circuit be tested in a larger cohort of rabbits and in a larger animal model for efficacy, given the differences in length of tubing and velocity of blood flow. In addition, the tubing was not clinical grade ECMO material that may have different properties. We also acknowledge that the platelet functional tests and flow cytometry performed are only indirect measures of platelet function outside of the circulatory microenvironment.
5. Conclusion
In summary, this study confirmed previously studied hemocompatibility benefits of an AG/DBHD/N2O2 coated ECC without significantly modifying vital signs or coagulability in a rabbit model of thrombogenicity. The DBHD/N2O2 and AG/DBHD/N2O2 coated ECCs prevented up to 82% and 98% respectively of thrombus formation for at least 4 h in a rabbit model of thrombogenicity. The significant new finding was that PMPs expressing P-selectin followed the P-selectin expression on whole platelets. Whole platelet count was significantly decreased while overall P-selectin expression was significantly increased in the uncoated ECC but maintained at baseline in the DBHD/N2O2 and AG/DBHD/N2O2 coated ECCs. Despite this increase in overall P-selectin expression in the uncoated ECC, the number of p-selectin-expressing platelets and PMPs were fewer in the uncoated ECC compared to the AG/DBHD/N2O2 coated ECC but, maintained at baseline in the DBHD/N2O2 and AG/DBHD/N2O2 coated ECCs. The combined antithrombin and NO release ECC coating appears to maintain PMP expression at baseline levels, delineating a previously unclear microenvironment in a rabbit model of ECLS.
Acknowledgments
The content, including study design, data collection, data analysis, interpretation of data, writing of the report, and the decision to submit the report, is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest and Source of Funding
This work was supported by the National Institutes of Health (NIH) Grant #1R01HLI28337. All authors have no conflicts of interest to declare.
References
- 1.Mikkelsen ME, Woo YJ, Sager JS, Fuchs BD, Christie JD: Outcomes using extracorporeal life support for adult respiratory failure due to status asthmaticus. ASAIO J 55 (1): 47–52, 2009. doi: 10.1097/MAT.0b013e3181901ea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer AD, Gelfond JA, Wiles AA, Freishtat RJ, Rais-Bahrami K: Platelet-derived microparticles generated by neonatal extracorporeal membrane oxygenation systems. ASAIO J 61 (1): 37–42, 2015. doi: 10.1097/MAT.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groom RC, Froebe S, Martin J, et al. : Update on pediatric perfusion practice in North America: 2005 survey. J Extra Corpor Technol 37 (4): 343–50, 2005. [PMC free article] [PubMed] [Google Scholar]
- 4.Haines NM, Rycus PT, Zwischenberger JB, Bartlett RH, Undar A: Extracorporeal Life Support Registry Report 2008: neonatal and pediatric cardiac cases. ASAIO J 55 (1): 111–6, 2009. doi: 10.1097/MAT.0b013e318190b6f7. [DOI] [PubMed] [Google Scholar]
- 5.Pagani FD, Aaronson KD, Dyke DB, Wright S, Swaniker F, Bartlett RH: Assessment of an extracorporeal life support to LVAD bridge to heart transplant strategy. Ann Thorac Surg 70 (6): 1977–84; discussion 1984–5, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Dornia C, Philipp A, Bauer S, et al. : Visualization of thrombotic deposits in extracorporeal membrane oxygenation devices using multidetector computed tomography: a feasibility study. ASAIO J 59 (4): 439–41, 2013. doi: 10.1097/MAT.0b013e3182976eff. [DOI] [PubMed] [Google Scholar]
- 7.Conn G, Kidane AG, Punshon G, Kannan RY, Hamilton G, Seifalian AM: Is there an alternative to systemic anticoagulation, as related to interventional biomedical devices? Expert Rev Med Devices 3 (2): 245–61, 2006. doi: 10.1586/17434440.3.2.245. [DOI] [PubMed] [Google Scholar]
- 8.Frank RD, Muller U, Lanzmich R, Groeger C, Floege J: Anticoagulant-free Genius haemodialysis using low molecular weight heparin-coated circuits. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 21 (4): 1013–8, 2006. doi: 10.1093/ndt/gfi293. [DOI] [PubMed] [Google Scholar]
- 9.Ranucci M, Pazzaglia A, Isgro G, et al. : Closed, phosphorylcholine-coated circuit and reduction of systemic heparinization for cardiopulmonary bypass: the intraoperative ECMO concept. Int J Artif Organs 25 (9): 875–81, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, Registry E: Extracorporeal Life Support Organization Registry Report 2012. ASAIO J 59 (3): 202–10, 2013. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 11.Major TC, Handa H, Annich GM, Bartlett RH: Development and hemocompatibility testing of nitric oxide releasing polymers using a rabbit model of thrombogenicity. J Biomater Appl 29 (4): 479–501, 2014. doi: 10.1177/0885328214538866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Major TC, Handa H, Brisbois EJ, et al. : The mediation of platelet quiescence by NO-releasing polymers via cGMP-induced serine 239 phosphorylation of vasodilator-stimulated phosphoprotein. Biomaterials 34 (33): 8086–96, 2013. doi: 10.1016/j.biomaterials.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren H, Colletta A, Koley D, et al. : Thromboresistant/anti-biofilm catheters via electrochemically modulated nitric oxide release. Bioelectrochemistry 104: 10–6, 2015. doi: 10.1016/j.bioelechem.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cossy J, Belotti D: A short synthesis of argatroban. a potent selective thrombin inhibitor. Bioorg Med Chem Lett 11 (15): 1989–92, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Blum EC, Martz CR, Selektor Y, Nemeh H, Smith ZR, To L: Anticoagulation of Percutaneous Ventricular Assist Device Using Argatroban-Based Purge Solution: A Case Series. J Pharm Pract: 897190017727191, 2017 doi: 10.1177/0897190017727191. [DOI] [PubMed] [Google Scholar]
- 16.Nagakane Y, Tanaka E, Ashida S, et al. : [Safety of Dual Antiplatelet Therapy with Argatroban in Patients with Acute Ischemic Stroke]. Brain Nerve 70 (5): 557–562, 2018. doi: 10.11477/mf.1416201038. [DOI] [PubMed] [Google Scholar]
- 17.Fu X, Ning JP: Synthesis and biocompatibility of an argatroban-modified polysulfone membrane that directly inhibits thrombosis. J Mater Sci Mater Med 29 (5): 66, 2018. doi: 10.1007/s10856-018-6054-4. [DOI] [PubMed] [Google Scholar]
- 18.Major TC, Brisbois EJ, Jones AM, et al. : The effect of a polyurethane coating incorporating both a thrombin inhibitor and nitric oxide on hemocompatibility in extracorporeal circulation. Biomaterials 35 (26): 7271–85, 2014. doi: 10.1016/j.biomaterials.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duvernay M, Young S, Gailani D, Schoenecker J, Hamm HE: Protease-activated receptor (PAR) 1 and PAR4 differentially regulate factor V expression from human platelets. Mol Pharmacol 83 (4): 781–92, 2013. doi: 10.1124/mol.112.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batchelor MM, Reoma SL, Fleser PS, et al. : More lipophilic dialkyldiamine-based diazeniumdiolates: synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric coatings. J Med Chem 46 (24): 5153–61, 2003. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- 21.Brodersen R, Bijlsma F, Gori K, et al. : Analysis of the immunological cross reactivities of 213 well characterized monoclonal antibodies with specificities against various leucocyte surface antigens of human and 11 animal species. Vet Immunol Immunopathol 64 (1): 1–13, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Massaguer A, Engel P, Perez-del-Pulgar S, Bosch J, Pizcueta P: Production and characterization of monoclonal antibodies against conserved epitopes of P-selectin (CD62P). Tissue Antigens 56 (2): 117–28, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Z, Meyerhoff ME: Preparation and characterization of polymeric coatings with combined nitric oxide release and immobilized active heparin. Biomaterials 26 (33): 6506–17, 2005. doi: 10.1016/j.biomaterials.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 24.Skrzypchak AM, Lafayette NG, Bartlett RH, et al. : Effect of varying nitric oxide release to prevent platelet consumption and preserve platelet function in an in vivo model of extracorporeal circulation. Perfusion 22 (3): 193–200, 2007. doi: 10.1177/0267659107080877. [DOI] [PubMed] [Google Scholar]
- 25.Poncelet P, Robert S, Bailly N, et al. : Tips and tricks for flow cytometry-based analysis and counting of microparticles. Transfus Apher Sci 53 (2): 110–26, 2015. doi: 10.1016/j.transci.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Major TC, Brant DO, Reynolds MM, et al. : The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials 31 (10): 2736–45, 2010. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Major TC, Brant DO, Burney CP, et al. : The hemocompatibility of a nitric oxide generating polymer that catalyzes S-nitrosothiol decomposition in an extracorporeal circulation model. Biomaterials 32 (26): 5957–5969, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Brisbois E, Handa H, et al. : The immobilization of a direct thrombin inhibitor to a polyurethane as a nonthrombogenic surface coating for extracorporeal circulation. Journal of Materials Chemistry B [10.1039/C5TB02419F] 4 (13): 2264–2272, 2016. doi: 10.1039/C5TB02419F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH: Hemocompatibility Comparison of Biomedical Grade Polymers Using Rabbit Thrombogenicity Model for Preparing Nonthrombogenic Nitric Oxide Releasing Surfaces. Journal of materials chemistry B, Materials for biology and medicine 2 (8): 1059–1067, 2014. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH: Hemocompatibility Comparison of Biomedical Grade Polymers Using Rabbit Thrombogenicity Model for Preparing Nonthrombogenic Nitric Oxide Releasing Surfaces. J Mater Chem B 2 (8): 1059–1067, 2014. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Said AS, Rogers SC, Doctor A: Physiologic Impact of Circulating RBC Microparticles upon Blood-Vascular Interactions. Front Physiol 8: 1120, 2017. doi: 10.3389/fphys.2017.01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melki I, Tessandier N, Zufferey A, Boilard E: Platelet microvesicles in health and disease. Platelets 28 (3): 214–221, 2017. doi: 10.1080/09537104.2016.1265924. [DOI] [PubMed] [Google Scholar]
- 33.Sibikova M, Zivny J, Janota J: Cell Membrane-Derived Microvesicles in Systemic Inflammatory Response. Folia Biol (Praha) 64 (4): 113–124, 2018. [PubMed] [Google Scholar]
- 34.Pretorius L, Thomson GJA, Adams RCM, Nell TA, Laubscher WA, Pretorius E: Platelet activity and hypercoagulation in type 2 diabetes. Cardiovasc Diabetol 17 (1): 141, 2018. doi: 10.1186/s12933-018-0783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Laval P, Mobarrez F, Almquist T, Vassil L, Fellstrom B, Soveri I: Acute effects of haemodialysis on circulating microparticles. Clin Kidney J 12 (3): 456–462, 2019. doi: 10.1093/ckj/sfy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F, Liao Z, Peng D, Han L: Role of Platelet Microparticles in Blood Diseases: Future Clinical Perspectives. Ann Clin Lab Sci 49 (2): 161–170, 2019. [PubMed] [Google Scholar]
- 37.George FD: Microparticles in vascular diseases. Thromb Res 122 Suppl 1: S55–9, 2008. doi: 10.1016/S0049-3848(08)70020-3. [DOI] [PubMed] [Google Scholar]


