Abstract
Background:
Interstitial lung disease (ILD) has a poor prognosis and lacks specific biomarkers for early diagnosis, assessment of disease severity, and prognosis. YKL-40 levels were found to be elevated in patients with ILD, but these results are inconsistent. Therefore, we conducted a systematic review and meta-analysis to accurately study the relation between YKL-40 and ILD.
Methods:
We performed a systematic literature search in many databases (PubMed, Embase, the China National Knowledge Infrastructure, and Wanfang databases) and commercial Internet search engines to identify studies involving the role of YKL-40 in patients with ILD. The weighted mean difference with its 95% confidence interval were used to investigate the effect sizes. If obvious heterogeneity was found in the meta-analysis, the level of YKL-40 was directly compared by the Mann-Whitney test.
Results:
Sixteen eligible articles were finally identified. The results showed that the serum YKL-40 levels of patients with idiopathic pulmonary fibrosis, connective tissue-related ILD, sarcoidosis, cryptogenic tissue pneumonia, asbestosis-ILD, and idiopathic nonspecific interstitial pneumonia were higher than those in controls, but there was no increase in patients with pulmonary alveolar proteinosis. We also found that there are certain differences in the serum YKL-40 levels in patients with different types of ILD. The results showed that the bronchoalveolar lavage fluid YKL-40 levels of patients with idiopathic pulmonary fibrosis were significantly higher than that in controls. A systematic review indicated that there were correlations between the serum YKL-40 levels and lung function in patients with different ILD. In addition, YKL-40 may be used as a valuable biomarker for survival, with risk ratios ranging from 1.006 to 10.9.
Conclusions:
This study suggests that YKL-40 may be a useful biomarker for the diagnosis and prognosis of ILD.
Keywords: biomarker, interstitial lung disease, meta-analysis, YKL-40
1. Introduction
Interstitial lung disease (ILD) is regarded as a heterogeneous disease that shows marked differences in the clinical process, radiological patterns, treatment, and prognosis.[1] There are many diseases or etiologies that can lead to ILD, such as connective tissue disease (CTD), occupational exposure, drugs, radiotherapy, or allergens (allergic pneumonia). Some ILD have no identified cause, such as idiopathic interstitial pneumonia.[2] The major ILD include idiopathic pulmonary fibrosis (IPF), idiopathic nonspecific interstitial pneumonia (NSIP), hypersensitivity pneumonitis, sarcoidosis, cryptogenic tissue pneumonia (COP), pneumoconiosis, and connective tissue disease-related ILD (CTD-ILD).[3–5] The overall prognosis of ILD is poor, including low life quality, high mortality and disability rates, and high medical cost burden.[6,7] Although recent advances have contributed to a better understanding of the etiology of ILD, there has not been a breakthrough in the treatment of ILD, and there is a lack of drugs that can slow the progress of the disease, as well as a lack of specific biomarkers to evaluate its prognosis.[8,9]
YKL-40 is a member of the mammalian chitinase-like protein family, which is coded by a gene located on chromosome 1q32.1.[10] It is produced by a variety of cell types, including airway epithelial cells, macrophages, neutrophils, monocytes, vascular smooth muscle cells, and chondrocytes.[11] The detailed function of YKL-40 is not fully clear, but as an inflammatory glycoprotein, YKL-40 has been found to be involved in many normal and pathological conditions, including cell proliferation and survival, migration, recombination, and tissue remodeling.[12,13] Previous studies have found that serum YKL-40 levels were increased in patients with ILD.[14,15] The results also showed that the serum YKL-40 levels were closely related to the deterioration of lung function and prognosis of patients with ILD.[16] However, sample sizes in most studies are small, and a single study may not be powerful enough to investigate the potentially small effect of the YKL-40 levels on ILD. In addition, it is unclear whether the serum YKL-40 levels in patients with different types of ILD are similar or different, and whether they can be used as a biomarker (the measurable change associated with a physiological or pathophysiological process) for differential diagnosis of ILD. In order to solve those limitations and to better explore the possible role of YKL-40 in ILD, we performed a meta-analysis and systematic review to study the relationship between YKL-40 and ILD patients.
2. Methods
2.1. Literature search
Our retrieval strategy is similar to previously published study.[17] We performed a systematic literature search in PubMed, Embase, the China National Knowledge Infrastructure (CNKI) (www.cnki.net), and Wanfang databases (www.wanfangdata.com.cn) to identify studies involving the role of YKL-40 in patients with ILD, with the most recent search having been conducted on July 1, 2020. The key search terms were as follows: (YKL-40 OR chitinase-3-like-1 protein OR CHI3L1) AND (interstitial lung diseases OR ILD OR pulmonary fibrosis OR interstitial pneumonitis OR collagen vascular disease OR organizing pneumonia OR sarcoidosis OR asbestosis OR pneumosilicosis OR connective tissue disease). The language was restricted to English or Chinese. Moreover, we used the same keywords to search literatures in multiple academic Internet search engines (such as Google and Baidu scholars). All analyses in the current meta-analysis were based on previously published studies; therefore, ethical approval is not required.
2.2. Study selection
The inclusion criteria were defined as follows:
-
(1)
a study involving the role of YKL-40 in ILD designed as a case-control study;
-
(2)
a primary study provided available data (e.g., mean, median, standard deviation [SD], standard error) for counting weighted mean difference (WMD) with a 95% CI; and
-
(3)
if there was duplication of data, only the most complete and recent study was included.
The exclusion criteria were similar to the previously published study,[17] as follows:
-
(1)
study was not designed as a case-control study;
-
(2)
study did not provide useful data for pooling the effects;
-
(3)
study was missing other essential information; and
-
(4)
review, abstract, or overlapping study.
2.3. Data extraction
Like the previous study,[17] two independent authors (XT and YM) extracted the detailed information and data from each primary study using a predesigned data extraction Excel form. If there was any disagreement or doubt, the third author (TL) further reviewed these articles. The extractions included the following: first author, year of publication, age of the participant, sample sizes of patients and controls, YKL-40 levels in serum and bronchoalveolar lavage fluid (BALF), and correlation coefficient (r). According to validated methods provided in the literature,[18] if a study provided medians and ranges (or interquartile ranges, IQR), we can convert the data into approximate means and SD for meta-analysis.
2.4. Statistical methods
The statistical methods used in this study were similar to those of the previous study.[17] After appropriate conversion, the WMD with a 95% confidence interval (CI) was used to compare the YKL-40 levels of serum or BALF in the patients with ILD with the levels in controls. In addition, the study also compared the differences in serum YKL-40 levels between patients with different types of ILD. The existing exact data[19] shows that the random effect model will be more conservative when it is used to merge data, and it leads to a lower type I error rate and a wider CI of effects, when compared with the fixed-effect model. The between-study heterogeneity was investigated by the chi-square (χ2)-based Q-test and I-square (I2) statistics test. The I2 value of > 50% or P value of < .10 suggested statistically significant heterogeneity. If a notable heterogeneity was found, we used a Mann-Whitney test to directly compare the levels of YKL-40 in patients with ILD with the levels in controls and reported the P value of this analysis. In the current study, all data analyses were performed using STATA 12.0 (Stata Corp., College Station, TX, USA) and (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Study characteristics
All 101 articles were found in our initial search of Embase, PubMed, CNKI, and Wanfang databases, and academic internet search engines. As shown in the flowchart (Fig. 1), 64 studies were deleted because they were duplicated across databases. When we carefully read the title and abstract, 11 articles were excluded because their content was not relevant to YKL-40 in relation to ILD. The remaining 26 articles were full-text browsed. Two articles were excluded because they investigated the association between cystic fibrosis and YKL-40. Two studies were eliminated since they did not have control individuals. One article was deleted because it only studied the relationship between the level of YKL-40 in sputum and patients with ILD. Another five articles were excluded because they were reviews. Finally, 16 eligible articles were included in this study.[14–16,20–32]
Figure 1.
The flow diagram of included and excluded studies.
Of these articles, 6 studied the relationship between YKL-40 and CTD-ILD,[20,21,23,25,27,28] four reported the relationship between YKL-40 and IPF,[14,16,20,22] and 8 explored the association between COP, sarcoidosis, asbestosis-ILD, pulmonary alveolar proteinosis (PAP), and NSIP and YKL-40.[15,20,22,24,26,29,30,32] Among them, 15 studies reported the relationship between serum YKL-40 levels and ILD, and 3 articles reported the relationship between BALF YKL-40 levels and IPF. In addition, some studies have sporadically reported the correlations between serum YKL-40 and lung function, and the diagnostic value of YKL-40 as a biomarker. Nine studies were from Europe and six were from Asia (Table 1).
Table 1.
Characteristics of studies reporting the association between serum/BALF YKL-40 levels and ILD.
| Authors | Age | Case (ng/mL) | Control (ng/mL) | |||||||
| Serum | Year | Cases/Controls | Type | Case/control | Mean | SD | N | Mean | SD | N |
| Bonella F | 2017 | 34/50 | PAP | 49 ± 2/42 ± 2 | 286 | 27 | 34 | 39 | 4 | 50 |
| Chen S | 2019 | 23/87 | CTD-ILD | NA6/59.6 ± 8.6 | 76.5 | 10.8 | 23 | 27.4 | 5.5 | 87 |
| Corradi M | 2013 | 16/66 | Asbestosis-ILD | 72.2 ± 8.7/61.6 ± 10 | 121 | 131.7 | 16 | 86.3 | 60.6 | 66 |
| Furuhashi K | 2010 | 63/41 | IPF | 70.2 ± 7.8/67.5 ± 8.5 | 245.8 | 180.2 | 63 | 116 | 58.3 | 41 |
| Furukawa T | 2019 | 18/17 | CTD-ILD | 63.3 ± 13.8/59.6 ± 13.9 | 54.3 | 26.7 | 18 | 23.9 | 17.1 | 17 |
| Hozumi H | 2017 | 69/34 | CTD-ILD | 53.8 ± 9.5/51.0 ± 10.5 | 86.9 | 49.0 | 69 | 31.1 | 16.0 | 34 |
| Jiang L | 2019 | 41/44 | CTD-ILD | 52.6 ± 10.5/45.6 ± 12.1 | 72.2 | 60.7 | 41 | 15.9 | 9.0 | 44 |
| Johansen J | 2005 | 27/173 | Sarcoidosis | 42.5 ± 10.0/39.0 ± 7.4 | 486.5 | 223.8 | 27 | 124.7 | 115.1 | 173 |
| Korthagen NM | 2014 | 185/124 | IPF | 63.8 ± 10.7/51.6 ± 7.7 | 134.1 | 96.1 | 185 | 47.9 | 25.1 | 124 |
| 38/124 | CTD-ILD | 57.8 ± 12.5/51.6 ± 7.7 | 100.7 | 84.7 | 38 | 47.9 | 25.1 | 124 | ||
| 27/124 | COP | 60.8 ± 12.2/51.6 ± 7.7 | 185.5 | 125 | 27 | 47.9 | 25.1 | 124 | ||
| 25/124 | NISP | 66.3 ± 9.5/51.6 ± 7.7 | 172.4 | 124.2 | 25 | 47.9 | 25.1 | 124 | ||
| Kruit A | 2007 | 75/333 | Sarcoidosis | 39.0 ± 11.2/40.1 ± 11.5 | 181.3 | 288.7 | 75 | 49.1 | 16.9 | 333 |
| Li Y | 2014 | 31/25 | PAP | 47 ± 8/45 ± 7 | 69.7 | 32.3 | 31 | 37.6 | 25.5 | 25 |
| Long X | 2017 | 45/60 | IPF | 71 ± 1/40 ± 2 | 214.0 | 20 | 45 | 39 | 4 | 60 |
| 21/60 | COP | 61 ± 3/40 ± 2 | 213 | 33 | 21 | 39 | 4 | 60 | ||
| 34/60 | NSIP | 68 ± 2/40 ± 2 | 184 | 21 | 34 | 39 | 4 | 60 | ||
| Nordenbaek C | 2005 | 27/70 | CTD-ILD | NA/54.0 ± 12.4 | 215.3 | 119.0 | 27 | 114 | 45.4 | 70 |
| Vaananen T | 2017 | 19/28 | Asbestosis-ILD | 68.5 ± 6.0/62.2 ± 6.6 | 79.2 | 82.5 | 19 | 38.5 | 28.0 | 28 |
| BALF | ||||||||||
| Korthagen NM | 2011 | 60/43 | IPF | 65 ± 10/32 ± 16 | 12.53 | 7.43 | 60 | 5.43 | 4.68 | 43 |
| Furuhashi K | 2010 | 63/41 | IPF | 70.2 ± 7.8/67.5 ± 8.5 | 17.8 | 19.1 | 18 | 0.3 | 0.9 | 16 |
| Long X | 2017 | 45/60 | IPF | 71 ± 1/40 ± 2 | 9 | 2 | 42 | 3 | 1 | 10 |
3.2. The association between serum YKL-40 levels and patients with IPF and CTD-ILD
The meta-analysis results indicated that the levels of serum YKL-40 of IPF and CTD-ILD patients were significantly higher than those of healthy subjects (WMD = 130.55, 95% CI = 60.77–200.34, P < .001; WMD = 50.53, 95%CI = 40.59–60.47, P < .001; respectively) (Fig. 2). A significant heterogeneity between studies was observed (I2 = 98.4%, I2 = 60.8%, respectively). Therefore, we compared the levels of serum YKL-40 in patients with IPF and CTD-ILD with the levels in controls using the Mann-Whitney test. The results showed that the serum YKL-40 levels of CTD-ILD patients were significantly different from that of the control group (P = .037), and the serum YKL-40 levels of patients with IPF were slightly statistically different from that of the control group (P = .05) (Table 2).
Figure 2.
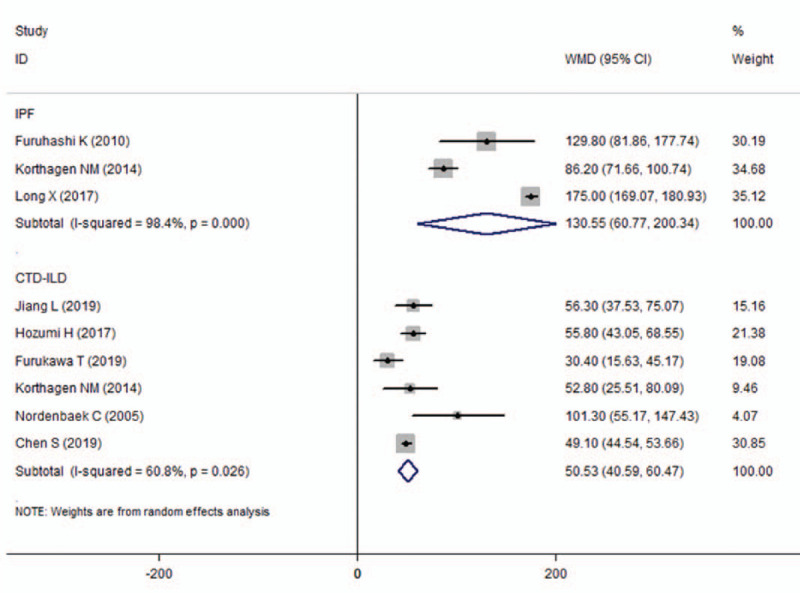
The results of associations between the serum YKL-40 levels and IPF and CTD-ILD. CTD-ILD = connective tissue disease-interstitial lung diseases, IPF = idiopathic pulmonary fibrosis.
Table 2.
The pooled results of the serum/BALF YKL-40 levels in patients with ILD compared with those in healthy controls.
| Diseases | WMD | 95%CI | P value | Mann-Whitney test (P value) |
| Serum | ||||
| IPF | 130.55 | 60.77–200.34 | <.001 | 0.05 |
| CTD-ILD | 50.53 | 40.59–60.47 | <.001 | 0.037 |
| Sarcoidosis | 245.22 | 20.24–470.19 | .033 | NA9 |
| COP | 163.1 | 130.43–195.78 | <.001 | NA |
| Asbestosis-ILD | 39.18 | 5.89–72.47 | .021 | NA |
| NSIP | 144.57 | 137.52–151.63 | <.001 | NA |
| PAP | 139.64 | −421.2 | .194 | NA |
| BALF | ||||
| IPF | 7.43 | 4.60–10.26 | <.001 | 0.05 |
3.3. The association between serum YKL-40 levels and patients with other ILD
The meta-analysis results showed that the serum YKL-40 levels in patients with sarcoidosis, COP, asbestosis-ILD, and NSIP were significantly higher than those in the control group (WMD = 245.22, 95%CI = 20.24–470.19, P = .033; WMD = 163.10, 95%CI = 130.43–195.78, P < .001; WMD = 39.18, 95%CI = 5.89–72.47, P = .021; WMD = 144.57, 95%CI = 137.52–151.63, P < .001, respectively) (Table 2). However, there was no significant difference in serum YKL-40 levels between patients with PAP and the control group (WMD = 139.64, 95%CI = −70.96 to 350.24, P = .194) (Table 2).
3.4. The differences of serum YKL-40 levels between patients with different types of ILD
By comparing the levels of serum YKL-40 in patients with different types of ILD, it was found that patients with sarcoidosis had the highest levels of serum YKL-40 (262.09 ± 303.76 ng/mL), and those with CTD-ILD and asbestosis-ILD had the lowest levels of serum YKL-40 (98.76 ± 81.02 ng/mL; 98.31 ± 108.17 ng/mL, respectively). There was no differences in serum levels of YKL-40 among patients with other ILDs (IPF, 170.39 ± 123.0 ng/mL; PAP, 182.84 ± 112.78 ng/mL; COP, 197.53 ± 96.42 ng/mL; NSIP, 179.08 ± 81.65 ng/mL, respectively).
3.5. The association between BALF YKL-40 levels and patients with IPF
The BALF was collected from 168 patients with IPF and 144 healthy controls to investigate the association between the YKL-40 levels and IPF. The results of this meta-analysis indicated that YKL-40 level in the BALF of patients with IPF was significantly higher than that of the control group (WMD = 7.43, 95%CI = 4.60–10.26, P < .001) (Table 2). We also used the Mann-Whitney test to compare the two groups, and the results showed that YKL-40 in the BALF of patients with IPF was slightly statistically different from the control group (P = .05) (Table 2).
3.6. A systematic review of the correlation between YKL-40 and lung function and prognosis
A total of 6 studies reported the correlation between serum YKL-40 levels and lung function in patients with different ILD. Among them, serum YKL-40 levels were negatively correlated with DLCO in sarcoidosis (two studies; r = −0.40, P = .04; r = 0.27, P = .03; respectively),[15,32] PAP (2 studies; r = −0.53, P = .002; r = −.29, P = .116), IPF (1 study; r = −0.41, P = .003), and CTD-ILD (one study; r = −0.41, P = .01).[14,23,26,29] There was no correlation with FEV/Predict in CTD-ILD (one study; r = −0.18, P = .16).[23] The studies did not find a correlation between BALF YKL-40 levels and other clinical parameters in the patients with ILD. Although cut-off values were different among the studies, the results suggested that YKL-40 could be used as a valuable biomarker for survival analysis, with hazard ratios ranging from 1.006 to 10.9. In addition, although there are few studies on the sensitivity and specificity of YKL-40 in ILD, the results showed that the serum YKL-40 may have high sensitivity and specificity in predicting prognosis.
4. Discussion
The main findings of the current study are as follows:
-
(1)
the serum YKL-40 levels in patients with CTD-ILD were significantly higher than that in the healthy control group by meta-analysis and Mann-Whitney test.
-
(2)
The serum YKL-40 levels in patients with IPF may be higher than that in the healthy control group by meta-analysis and Mann-Whitney test, and the results showed that YKL-40 levels in the BALF of patients with IPF were higher than that of the control group.
-
(3)
The serum YKL-40 levels in patients with sarcoidosis, COP, asbestosis-ILD, and NSIP may be higher than those in the control group, but the levels of YKL-40 in patients with PAP is not significantly higher than that in the control group. However, it is worth noting that only a few studies have explored the relationship between serum YKL-40 levels and these diseases, so the sample size is insufficient, and we need to be cautious in citing these results.
-
(4)
Sarcoidosis patients had the highest serum YKL-40 levels, while CTD-ILD and asbestosis-ILD patients had the lowest serum YKL-40 levels.
In addition, a systematic review showed that the serum YKL-40 levels were negatively correlated with pulmonary function, especially DLCO in patients with different ILD. Interestingly, YKL-40 may be able to predict the prognosis of ILD with high sensitivity and specificity. Unfortunately, the studies did not find a correlation between BALF YKL-40 levels and other clinical parameters in patients with ILD. The small sample sizes of the included studies may explain the negative results. It is necessary to further verify the accurate correlation between serum and BALF YKL-40 levels of patients with ILD and other clinical parameters through large samples in the future.
It is well known that although ILD is a heterogeneous disease, inflammation, tissue remodeling, and fibrosis are common and important pathophysiological features of ILD. A large number of studies in the literature have suggested that YKL-40 may play an important role in inflammatory response.[12,33–35] In addition, YKL-40 increases Th2 inflammatory responses and decreases inflammatory cell apoptosis.[36] Moreover, YKL-40 can regulate a series of signaling pathways,[37–39] which are closely related to the pathogenesis of ILD. Previous studies suggest that YKL-40 is involved in tissue remodeling and fibrosis. Importantly, tissue remodeling and fibrosis are often closely related to lung function and clinical prognosis, which may explain the negative correlation between YKL-40 levels and lung function in patients with ILD found in previous studies. We also found that there may be differences in serum YKL-40 levels in patients with different types of ILD, which may provide a possible reference for differential diagnosis of ILD. Therefore, the current study also suggests that YKL-40 may be a potential biomarker for the diagnosis and prognosis of ILD.
There are some limitations of this study. First of all, language was limited to English and Chinese, so some valuable articles published in other languages may not be included, which may contribute to a bias for overall results. Secondly, we only included published studies in a few databases, and most of them were small in sample sizes, so this may lead to publication bias or overestimation of the effect size. Third, we failed to conduct further subgroup analyses based on risk factors (such as occupation, smoking status, and environmental factors), which may have some affected on the meta-analysis results. However, in spite of these shortcomings, we used a detailed protocol for research screening, data extraction, and statistical analysis to minimize bias and reduce possible errors.
In summary, the present meta-analysis and systematic review suggest that YKL-40 may be a useful biomarker for the diagnosis and prognosis prediction of ILD. Further rigorous and consistent studies are needed to better explore the association between and ILD and to examine whether monitoring the levels of YKL-40 contributes to successful clinical decision-making.
Author contributions
Conceptualization: Xiang Tong, Guihui Wu, Hong Fan.
Data curation: Xiang Tong, Tao Liu, Zhenzhen Li, Sitong Liu.
Formal analysis: Yao Ma.
Methodology: Xiang Tong, Yao Ma, Hong Fan.
Supervision: Guihui Wu, Hong Fan.
Writing – original draft: Xiang Tong, Yao Ma, Tao Liu, Zhenzhen Li.
Writing – review & editing: Xiang Tong, Guihui Wu, Hong Fan.
Footnotes
Abbreviations: BALF = bronchoalveolar lavage fluid, CI = confidence interval, CNKI = China National Knowledge Infrastructure, COP = cryptogenic tissue pneumonia, CTD = connective tissue disease, CTD-ILD = connective tissue disease-related interstitial lung disease, ILD = interstitial lung disease, IPF = idiopathic pulmonary fibrosis, NSIP = idiopathic nonspecific interstitial pneumonia, PAP = pulmonary alveolar proteinosis, WMD = weighted mean difference.
How to cite this article: Tong X, Ma Y, Liu T, Li Z, Liu S, Wu G, Fan H. Can YKL-40 be used as a biomarker for interstitial lung disease? A systematic review and meta-analysis: a systematic review and meta-analysis. Medicine. 2021;100:17(e25631).
This study was supported by National Key R&D Program of China (2017YFC1309703), Project funded by China Postdoctoral Science Foundation (2020M673259), and Post-Doctor Research Project, West China Hospital, Sichuan University (2020HXBH013). We would like to thank Elsevier Language Editing Services for its linguistic assistance during the preparation of this manuscript.
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
BALF = bronchoalveolar lavage fluid, COP = cryptogenic tissue pneumonia, CTD-ILD = connective tissue disease-interstitial lung diseases, IPF = idiopathic pulmonary fibrosis, NA = not available, NSIP = idiopathic nonspecific interstitial pneumonia, PAP = pulmonary alveolar proteinosis, SD = standard deviation.
BALF = bronchoalveolar lavage fluid, CI = confidence interval, COP = cryptogenic tissue pneumonia, CTD-ILD = connective tissue disease-interstitial lung diseases, NSIP = idiopathic nonspecific interstitial pneumonia, IPF = idiopathic pulmonary fibrosis, NA = not available, PAP = pulmonary alveolar proteinosis, WMD = weighted mean difference.
References
- [1].Mikolasch TA, Garthwaite HS, Porter JC. Update in diagnosis and management of interstitial lung disease. Clin Med (London, England) 2016;16: Suppl 6: s71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Park SW, Baek AR, Lee HL, et al. Korean guidelines for diagnosis and management of interstitial lung diseases: Part 1. introduction. Tubercul Respir Dis 2019;82:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rivera-Ortega P, Molina-Molina M. Interstitial lung diseases in developing countries. Ann Global Health 2019;85:04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Giacomelli R, Liakouli V, Berardicurti O, et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int 2017;37:853–63. [DOI] [PubMed] [Google Scholar]
- [6].Vancheri C, Failla M, Crimi N, et al. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 2010;35:496–504. [DOI] [PubMed] [Google Scholar]
- [7].Collard HR, Ward AJ, Lanes S, et al. Burden of illness in idiopathic pulmonary fibrosis. J Med Econ 2012;15:829–35. [DOI] [PubMed] [Google Scholar]
- [8].Alqalyoobi S, Adegunsoye A, Linderholm A, et al. Circulating plasma biomarkers of progressive interstitial lung disease. Am J Respir and Critical Care Medicine 2020;201:250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ (Clin Res Ed) 2016;352:h6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 1997;43:221–5. [DOI] [PubMed] [Google Scholar]
- [11].Duru S, Yuceege M, Ardic S. Chitinases and lung diseases. Tuberk Toraks 2013;61:71–5. [DOI] [PubMed] [Google Scholar]
- [12].Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Danish Med Bull 2006;53:172–209. [PubMed] [Google Scholar]
- [13].Nishikawa KC, Millis AJ. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res 2003;287:79–87. [DOI] [PubMed] [Google Scholar]
- [14].Furuhashi K, Suda T, Nakamura Y, et al. Increased expression of YKL-40, a chitinase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis. Respir Med 2010;104:1204–10. [DOI] [PubMed] [Google Scholar]
- [15].Johansen JS, Milman N, Hansen M, et al. Increased serum YKL-40 in patients with pulmonary sarcoidosis--a potential marker of disease activity? Respir Med 2005;99:396–402. [DOI] [PubMed] [Google Scholar]
- [16].Korthagen NM, van Moorsel CH, Barlo NP, et al. Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respir Med 2011;105:106–13. [DOI] [PubMed] [Google Scholar]
- [17].Tong X, Wang D, Liu S, et al. The YKL-40 protein is a potential biomarker for COPD: a meta-analysis and systematic review. Int J Chronic Obstr Pulmon Dis 2018;13:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Borenstein M, Hedges L, Rothstein H. Meta-analysis: fixed effect vs. random effects. Meta-analysis com. 2007 (access date September 21, 2019). [Google Scholar]
- [20].Korthagen NM, van Moorsel CH, Zanen P, et al. Evaluation of circulating YKL-40 levels in idiopathic interstitial pneumonias. Lung 2014;192:975–80. [DOI] [PubMed] [Google Scholar]
- [21].Jiang L, Wang Y, Peng Q, et al. Serum YKL-40 level is associated with severity of interstitial lung disease and poor prognosis in dermatomyositis with anti-MDA5 antibody. Clin Rheumatol 2019;38:1655–63. [DOI] [PubMed] [Google Scholar]
- [22].Long X, He X, Ohshimo S, et al. Serum YKL-40 as predictor of outcome in hypersensitivity pneumonitis. Eur Respir J 2017;49:1501924. [DOI] [PubMed] [Google Scholar]
- [23].Hozumi H, Fujisawa T, Enomoto N, et al. Clinical Utility of YKL-40 in Polymyositis/dermatomyositis-associated Interstitial Lung Disease. J Rheumatol 2017;44:1394–401. [DOI] [PubMed] [Google Scholar]
- [24].Vaananen T, Lehtimaki L, Vuolteenaho K, et al. Glycoprotein YKL-40 levels in plasma are associated with fibrotic changes on HRCT in asbestos-exposed subjects. Mediat Inflamm 2017;2017:1797512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Furukawa T, Matsui K, Kitano M, et al. Relationship between YKL-40 and pulmonary arterial hypertension in systemic sclerosis. Mod Rheumatol 2019;29:476–83. [DOI] [PubMed] [Google Scholar]
- [26].Bonella F, Long X, He X, et al. Serum YKL-40 is a reliable biomarker for pulmonary alveolar proteinosis. Respirology 2017;22:1371–8. [DOI] [PubMed] [Google Scholar]
- [27].Nordenbaek C, Johansen JS, Halberg P, et al. High serum levels of YKL-40 in patients with systemic sclerosis are associated with pulmonary involvement. Scand J Rheumatol 2005;34:293–7. [DOI] [PubMed] [Google Scholar]
- [28].Chen S, Zhou F. Corralation betwwen serum YKL-40 and polymyositis/dermatomyositis. J Clin Pathol Res (In Chinese) 2019;39:947–51. [Google Scholar]
- [29].Li Y, Tian X, Gui Y, et al. Serum markers in patients with idiopathic pulmonary alveolar proteinosis. Chin J Tubere Respir Dis (In Chinese) 2014;37:497–501. [PubMed] [Google Scholar]
- [30].Corradi M, Goldoni M, Alinovi R, et al. YKL-40 and mesothelin in the blood of patients with malignant mesothelioma, lung cancer and asbestosis. Anticancer Res 2013;33:5517–24. [PubMed] [Google Scholar]
- [31].Gao MZ, Wei YY, Xu QW, et al. Elevated serum YKL-40 correlates with clinical characteristics in patients with polymyositis or dermatomyositis. Ann Clin Biochem 2019;56:95–9. [DOI] [PubMed] [Google Scholar]
- [32].Kruit A, Grutters JC, Ruven HJ, et al. A CHI3L1 gene polymorphism is associated with serum levels of YKL-40, a novel sarcoidosis marker. Respir Med 2007;101:1563–71. [DOI] [PubMed] [Google Scholar]
- [33].Gratchev A, Schmuttermaier C, Mamidi S, et al. Expression of osteoarthritis marker YKL-39 is stimulated by transforming growth factor beta (TGF-beta) and IL-4 in differentiating macrophages. Biomark Insights 2008;3:39–44. [PMC free article] [PubMed] [Google Scholar]
- [34].Recklies AD, Ling H, White C, et al. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem 2005;280:41213–21. [DOI] [PubMed] [Google Scholar]
- [35].Zhu Z, Zheng T, Homer RJ, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004;304:1678–82. [DOI] [PubMed] [Google Scholar]
- [36].Lee CG, Hartl D, Lee GR, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med 2009;206:1149–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Ann Rev Physiol 2011;73:479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Horowitz JC, Rogers DS, Sharma V, et al. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell signal 2007;19:761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shao R, Hamel K, Petersen L, et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009;28:4456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]


