Abstract
Identifying predictors of inadequate response to methotrexate (MTX) in rheumatoid arthritis (RA) is key to move from a “trial and error” to a “personalized medicine” treatment approach where patients less likely to adequately respond to MTX monotherapy could start combination therapy at an earlier stage. This study aimed to identify potential predictors of inadequate response to MTX in RA patients naïve to disease modifying anti-rheumatic drugs.
Data from a real-life cohort of newly diagnosed RA patients starting MTX (baseline, T0) as first-line therapy were analyzed. Outcomes, assessed after 6 months (T1), were defined as failure to achieve a disease activity score 28 (DAS28) low disease activity (LDA) or a good/moderate response to MTX, according to the European League Against Rheumatism (EULAR) response criteria. Logistic regression was used to assess the associations between baseline variables and the study outcomes.
Overall, 294 patients (60.5% females, median age 54.5 years) with a median disease duration of 7.9 months were recruited. At T1, 47.3% of subjects failed to achieve LDA, and 29.3% did not have any EULAR-response. In multivariate analysis, significant associations were observed between no LDA and current smoking (adjusted odds ratio [adjOR] 1.79, P = .037), female gender (adjOR 1.68, P = .048), and higher DAS28 (adjOR 1.31, P = .013); and between no EULAR-response and current smoking (adjOR: 2.04, P = .019), age (adjOR: 0.72 per 10-years increases, P = .001), and higher erythrocyte sedimentation rate (adjOR: 0.49; P = .020). By contrast, there were no associations between past smoker status and study outcomes.
In summary, in our real-life cohort of disease modifying anti-rheumatic drug naïve RA patients, current smoking habit independently predicts inadequate response to MTX. This, together with other independent predictors of response to treatment identified in our study, might assist with personalized monitoring in RA patients. Further studies are required to investigate whether smoking quitting strategies enhance the therapeutic response to MTX.
Keywords: methotrexate, precision medicine, rheumatoid arthritis, smoking
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease primarily involving the synovial joints and potentially resulting in irreversible damage, disability, impaired quality of life, and inability to work.[1]
There is very good evidence that prompt treatment initiation and early achievement of remission are among the major predictors of long-term outcomes in RA.[2] Accordingly, the European League Against Rheumatism (EULAR) recommends commencing treatment with disease modifying anti-rheumatic drugs (DMARDs) as soon as the diagnosis is made to achieve remission or, at least, reasonable control of disease activity.[3]
In the current therapeutic algorithms for RA, methotrexate (MTX) is a first-line DMARD thanks to its widely documented efficacy and safety, also in reducing complications and mortality.[3–8] However, up to two third of patients treated with MTX monotherapy fail to achieve treatment targets and require a combination therapy with other conventional synthetic (csDMARDs) or biologic DMARDs (bDMARDs).[9,10]
Identifying predictors of inadequate response to MTX is key to move from a “trial and error” to a “personalized medicine” treatment approach where patients less likely to adequately respond to MTX monotherapy could start combination therapy at an earlier stage.[11] This would have, in turn, immediate benefits in terms of reduced patient exposure to disease activity, ineffective drugs and damage accrual, and reduced healthcare costs.
The aim of this study was to identify potential predictors of inadequate response to MTX as first-line treatment in RA patients naïve to DMARDs.
2. Methods
2.1. Patients
We retrospectively studied a real-life, monocentric, inception cohort of Caucasian RA patients followed up at the tertiary Rheumatology Unit of the AOU University Clinic of Cagliari, from January 2006 to December 2018.
Eligible patients were:
-
(1)
≥18 years old,
-
(2)
initially diagnosed with RA in our Centre according to the 1987 American College of Rheumatology or 2010 ACR/EULAR criteria (depending on the first assessment year),[12,13]
-
(3)
with moderate to high disease activity score 28 joints (DAS28),
-
(4)
naïve to any DMARDs at baseline and prescribed with MTX as first-line therapy, with or without glucocorticoids (GC),
-
(5)
followed up for at least 6 months.
Patients previously treated with other DMARDs were excluded. Given the observational nature of the study, the MTX starting dose was based on individual physicians’ choice rather than pre-determined trial-based protocols. Patients who stopped MTX treatment before 6 months, due to side effects or other reasons, were also excluded.
The study was conducted in accordance with the declaration of Helsinki and approved by the local AOU University Clinic of Cagliari (Italy) Ethics Committee (PG/2017/3272). Patients were enrolled after signing an informed consent.
2.2. Data collection
The following data were collected from medical records and clinical files at baseline (T0), before MTX initiation: age at disease onset, age at diagnosis, disease duration, body mass index, smoking habits, positivity for rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA), MTX dosage. Current smokers were those reporting active smoking at T0. Past smokers were those who had stopped smoking before T0. Data on smoking habits duration and average number of cigarettes per day were also collected. Tender joint count and swollen joint count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), overall 3 DAS28-ESR (14), and GC dosage were assessed both at T0 and after 6 months (T1).
2.3. Definition of outcomes
The study outcomes were assessed after 6 months (T1). The primary outcome was the inadequate response to MTX after 6 months, defined as failure to achieve low disease activity (LDA) expressed by a DAS28 score ≤3.2. The secondary outcome was the failure to attain a good or moderate response to MTX (no EULAR-response), according to the EULAR-response criteria (DAS28 improvement ≤ 0.6 or DAS28 improvement > 0.6 but ≤1.2 and 6-month DAS28 > 5.1).[14]
Subjects undergoing therapeutic modification (i.e., increasing oral GC dose, intravenous, intra-muscular or intra-articular injection of GC, combination with or switch to other cs- o b-DMARDs) for persistently high disease activity before T1 were classified as inadequate responders both for the primary and the secondary outcome.
2.4. Statistical analysis
The variables are reported as mean ± standard deviation (SD) and median with interquartile range, for continuous variables; or frequencies and percentages, for categorical variables.
Age, sex, disease duration, body mass index, status of current and past smoker, baseline tender joint count, swollen joint count, ESR, CRP and DAS28, RF and/or ACPA positivity, MTX starting dose, ongoing GC, and cumulative prednisone dose were analyzed as potential predictors of failure to achieve LDA or EULAR-response at T1 through univariate and multivariate logistic regression. Variables showing associations, P values ≤ .10, in univariate analysis were included in multivariate analysis. Multivariate logistic regression models were further adjusted by classification criteria, 1987 or 2010, as an independent variable. The Statistical significance was set for P < .05. Statistical analyses were performed using SPSS software (version 24.0, Armonk, NY).
3. Results
3.1. Patients
Two hundred ninety-four patients were recruited according to the inclusion criteria. Overall, our cohort represented a fairly typical population of newly diagnosed RA patients with median duration of symptoms of 7.9 (3.9–18.6) months. Out of the 294 recruited patients, 178 (60.5%) were females and the median (interquartile range) age at enrollment was 54.5 (43.5–64.7) years. The median DAS28 was 5.3 (4.5–6.1). A total of 88 (29.9%) and 69 (23.4%) patients were respectively classified as current and past smokers. The most frequent MTX starting dosages were 10 and 15 mg/week (in 47.3% and 45.9% of patients, respectively). The dosage was gradually increased according to individual disease characteristics (up to a maximum dose of 20 mg/week within the first 3 months in all patients). The median cumulative dose of prednisone (PDN) or equivalent was 0.83 (0.31–1.17) g.
Baseline characteristics of recruited patients are described in Table 1.
Table 1.
Baseline characteristics of the study cohort.
| No. of recruited patients | 294 |
| Gender, n (%) female | 178 (60.5%) |
| Age at enrollment, median (IQR) (yrs) | 54.5 (43.5–64.7) |
| Disease duration, median (IQR) (mo) | 7.9 (3.9–18.6) |
| BMI, median (IQR) (kg/m2)∗ | 23.7 (21.7–26.0) |
| Current smokers, n (%) | 88 (29.9%) |
| Past smokers, n (%) | 69 (23.4%) |
| ACPA positive, n (%)∗ | 158 (54.3%) |
| RF positive, n (%)† | 155 (53.1) |
| TJC28, median (IQR) | 9.0 (5.0–14.5) |
| SJC28, median (IQR) | 5.0 (2.0–10.0) |
| ESR (mm/first hour), median (IQR) | 35.0 (21.0–57.0) |
| DAS28-ESR, median (IQR) | 5.3 (4.5–6.1) |
| PDN prescribed at baseline, n (%) | 217 (73.8%) |
| PDN at 6 mo, n (%) | 164 (55.5%) |
| MTX dosage, mean (SD) (mg/wk) | 12.4 (2.6) |
3.2. Predictors of no-LDA achievement
A total of 139 (47.3%) patients failed to achieve LDA at T1. In univariate analysis, factors significantly associated with no-LDA achievement included female gender (odds ratio [OR] 1.87, 95% confidence interval [CI] 1.16–3.02, P = .010), younger age at disease onset (OR 0.81, 95%CI 0.69–0.95 per 10-year increase, P = .009), and diagnosis (OR 0.81, 95%CI 0.69–0.95 per 10-year increase, P = .008), current smoking (OR 1.85, 95%CI 1.12–3.06, P = .017), past smoking (OR 0.43, 95%CI 0.24–0.76, P = .004), cumulative PDN dose (OR 1.38, 95%CI 1.01–1.87 per 1 g increases, P = .041), and baseline DAS-28 (OR 1.34, 95%CI 1.10–1.64, P = .003) (Table 2).
Table 2.
Univariate analysis for factors associated with no LDA and no EULAR-response, at 6 mo from MTX initiation.
| No LDA | No EULAR-response | |||
| Candidate predictor | OR (95%CI) | P | OR (95%CI) | P |
| Female | 1.87 (1.16–3.02) | .010 | 1.32 (0.78–2.22) | .303 |
| Onset age, per 10-yrs increases | 0.81 (0.69–0.95) | .009 | 0.73 (0.62–0.87) | <.001 |
| Diagnosis age, per 10-yrs increases | 0.81 (0.69–0.95) | .008 | 0.73 (0.61–0.87) | <.001 |
| Disease duration, per 1 mo increases | 0.98 (0.96–0.99) | .969 | 1.00 (1.00–1.01) | .670 |
| BMI | 0.99 (0.93–1.05) | .989 | 0.96 (0.90–1.02) | .956 |
| Current smoking | 1.85 (1.12–3.06) | .017 | 1.87 (1.10–3.17) | .022 |
| Past smoking | 0.43 (0.24–0.76) | .004 | 0.48 (0.25–0.94) | .032 |
| ESR ↑ | 1.52 (0.86–2.70) | .151 | 0.50 (0.28–0.90) | .020 |
| CRP ↑ | 0.88 (0.51–1.52) | .651 | 0.50 (0.28–0.90) | .020 |
| TJC-28 | 1.02 (0.99–1.05) | .291 | 0.97 (0.93–1.01) | .090 |
| SJC-28 | 1.03 (1.00–1.07) | .097 | 0.99 (0.95–1.03) | .988 |
| DAS-28 | 1.34 (1.10–1.64) | .003 | 0.74 (0.59–0.91) | .005 |
| ACPA+ | 1.33 (0.84–2.12) | .224 | 1.18 (0.71–1.96) | .535 |
| RF+ | 1.10 (0.70–1.75) | .682 | 0.99 (0.60–0.91) | .975 |
| ACPA and/or RF+ | 1.20 (0.74–1.93) | .464 | 1.15 (0.71–1.96) | .613 |
| ACPA and/or RF HT | 1.29 (0.81–2.07) | .283 | 0.95 (0.57–1.58) | .847 |
| MTX dose | 1.12 (0.34–3.71) | .851 | 0.67 (0.19–0.24) | .527 |
| PDN ongoing at 6 mo | 0.73 (0.46–1.17) | .187 | 1.18 (0.70–1.99) | .529 |
| Cum. PDN dose, per 1 g increases | 1.38 (1.01–1.87) | .041 | 1.10 (1.00–1.01) | .054 |
In multivariate analysis, only current smoking (adjusted odds ratio [adjOR] 1.79, 95%CI 1.04–3.08, P = .037), female gender (adjOR 1.68, 95%CI 1.01–2.81, P = .048), and higher DAS-28 at baseline (adjOR 1.31, 95%CI 1.06–1.62, P = .013) remained independently associated with failure to achieve LDA (Fig. 1a).
Figure 1.
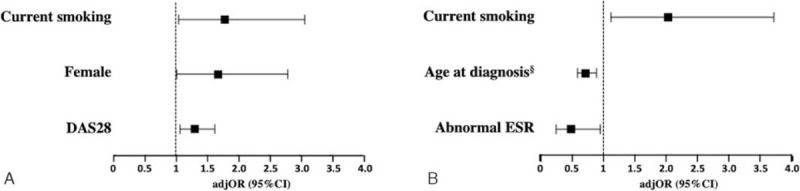
Factors independently associated in multivariate analysis with failure to achieve LDA (a) and a good/moderate EULAR-response to treatment (b), at 6 mo from MTX initiation. Associations are presented as adjusted odds ratios (adjOR) with 95% confidence intervals (95%CI). § OR for 10-years increments. MTX = methotrexate.
3.3. Predictors of no EULAR-response
A total of 86 (29.3%) patients were non-responders, according to the EULAR-response criteria. In univariate analysis, baseline factors significantly associated with no EULAR-response included younger age at the onset of symptoms (OR 0.73, 95%CI 0.62–0.87 per 10-year increase, P < .001) and diagnosis (OR 0.73, 95%CI 0.61–0.87 per 10-year increase, P < .001), current (OR 1.87, 95%CI 1.10–3.17, P = .022) and past smoking (OR 0.48, 95%CI 0.25–0.94, P = .032), higher ESR (OR 0.50, 95%CI 0.28–0.90, P = .020) and CRP (OR 0.50, 95%CI 0.28–0.90, P = .020), and lower DAS-28 (OR 0.74, 95%CI 0.59–0.91, P = .005) (Table 2).
In multivariate analysis, current smoking (adjOR: 2.04, 95%CI: 1.12–3.71; P = .020), age at diagnosis (adjOR: 0.72, 95%CI 0.59–0.88 per 10-year increase, P = .001), and higher ESR (adjOR: 0.49, 95%CI: 0.25–0.95; P = .036), remained independently associated with no EULAR-response to MTX (Fig. 1b).
Notably, the MTX starting dosage did not predict the study endpoints (P > .05). Similarly, the duration of exposure to tobacco and the number of cigarettes per day were not significantly associated with the study outcomes, both in current and past smokers (P > .05).
4. Discussion
In this real-life cohort of DMARD naïve RA patients, after 6 months of MTX treatment as monotherapy, 47.3% of subjects failed to achieve LDA, and 29.3% had no EULAR-response. Significant associations were observed between no LDA achievement and current smoking, female gender and higher DAS28; as well as between no EULAR-response and current smoking, normal ESR and younger age at diagnosis. The reported association between current smoking, a modifiable risk factor, and inadequate response to MTX is potentially of clinical relevance, particularly given that it was independent of phenotypical RA features, such as ACPA positivity, and that the same relationship was not recorded for past smoking. Past smokers had, in fact, similar response to MTX when compared with never smokers.
A robust comparison with the results of previous studies is difficult because of the wide heterogeneity in terms of outcome measures and follow-up, the different study design (e.g., clinical trial, observational cohort studies) and the variable assessment of MTX as mono- or combined therapy. In particular, smoking has been investigated as a potential predictor of response to MTX in several studies, but with inconsistent results. Indeed, a significant association between smoking and inadequate response to MTX was reported by several[9,10,15–19] but not all authors[20,21] (Table 3). In this context, our results provide strong evidence on the key role of smoking in independently predicting an inadequate response to MTX. Differently from previous reports, our findings stem from a population-based study specifically designed to identify the predictors of responsiveness to MTX in a real-life setting, which facilitates the translation of our results into routine clinical management. Further strengths of our study are represented by the selection of therapeutic strategies, follow-up time points, and outcome measures which are based on current EULAR recommendations for RA and therefore immediately transferable to patient care. Moreover, to reduce a potential source of bias, all enrolled patients were DMARDs naïve and received first-line MTX treatment as monotherapy. Finally, the status of both current and past smokers was separately assessed as predictors of inadequate response to methotrexate.
Table 3.
Previous studies searching for potential predictors of response to methotrexate (MTX): focus on the role of smoking.
| References | Data source | N pts | Therapy | Primary endpoint | Time (mo) | Results |
| Saevarsdottir et al (2011)[10] | Clinical trial | 405 | MTX | EULAR-response | 3–4 | • Current smoking associated with lower EULAR-response (OR 0.35, 95%CI 0.20–0.63), as well as other outcome measures, such as SDAI and CDAI. |
| • Past smoking did not associate with the study outcomes. | ||||||
| • Other associated factors: HAQ, age, prednisolone, gender, and symptom duration. | ||||||
| Saevarsdottir et al (2011)[15] | Cohort study | 626 | MTX | Good EULAR-response | 3, 6 | • Current smoking inversely associated with good EULAR-response to MTX both at 3 (adjOR 0.60, 95%CI 0.39–0.940) and 6 mo (analysis on 436 patients) (adjOR 0.58, 95%CI 0.39–0.94). A significant association was also recorded with EULAR remission at 6 mo (OR 0.41, 95%CI 0.24–0.71). |
| • Packs/day and past smoking did not associate with the study outcomes. | ||||||
| • Other associated factors: not separately assessed. | ||||||
| Rojas-Serrano J et al (2011)[9] | Cohort study | 144 | MTX + SFZ | No-ACR 50 | 6 | • Current smoking associated with no-ACR50 achievement (OR 3.58, 95%CI 1.23–11.22; P < 0.008). |
| • Other associated factors: none. | ||||||
| Soderlin et al (2011)[16] | Cohort study | 1787 | DMARDs (MTX 40%) | Good EULAR-response | 3, 6, 12 | • Current smoking inversely associated with good EULAR-response both at 3 (OR 0.64, 95%CI 0.47–0.87, P = .004), 6 (OR 0.66, 95%CI 0.48–0.91, P = .011), and 12 mo (OR 0.69, 95%CI 0.50–0.93, P = .02). |
| • Past smoking did not associate with the study outcomes. | ||||||
| Teitsma MX et al (2018)[17] | Clinical trial | 108 | MTX + HCQ | No-remission (DAS28 > 2.6 SJC ≤ 4, for 24 wk | 12 | • Current smoking (ORadj 3.02, 95%CI 1.1–8.0, P = .027) was associated with failure to achieve remission within 12 mo. |
| • Other associated factors: alcohol consumption. | ||||||
| de Rotte et al (2018)[18] | Clinical trial | 270 | MTX | No-LDA | 3 | • Current smoking (OR 2.01, 95%CI 1.19–3.41, P < .001) associated with insufficient response. |
| • Other associated factors: higher DAS28, higher HAQ score, and higher BMI. | ||||||
| Sergeant et al (2018)[19] | Cohort study | 1050∗ | MTX | No EULAR-response | 6 | • Current smoking associated with no EULAR-response in univariable (OR 1.78, 95%CI 1.28–2.48, P < .001). |
| • Past smoker did not associate with no EULAR-response. | ||||||
| • Other associated factors: lower DAS28, RF negative, higher TJC and HAQ, higher Hospital Anxiety and Depression score. | ||||||
| Maska et al (2012)[20] | Clinical trial | 412 | MTX | Delta-DAS28 | 6 | • Current smoking: no differences in the mean DAS28 score between 48 and 102 wk based on smoking status for the overall group (P = .881) or by specific treatment assignment. |
| Vesperini et al (2013)[21] | Cohort study | 641 | DMARD (MTX 62%) | Good EULAR-response | 12 | • Current smoking: status had no influence EULAR-response and EULAR remission. Inversely, association with reduced 1-year radiographic disease progression was described. |
| • Other associated factors: male sex, HLA–DRB1 shared epitope. Factors associated with DAS28 remission at 1 yr included male sex and older age. | ||||||
| Floris et al (present study) | Cohort study | 294 | MTX | No LDA | 6 | • Current smoking associated with no LDA achievement (OR 1.79 95%CI 1.04–3.08, P = .037). Association also with no EULAR-response achievement (OR: 2.04, 95%CI: 1.12–3.71; P = .019). |
| • Past smoking and duration of smoking exposure did not associate with the study outcomes. | ||||||
| • Other associated factors: female gender and higher DAS28 associated to no-LDA; increased ESR and diagnosis age inversely associated to no EULAR-response. |
Increasing epidemiologic and biological evidence supports the role of smoking in RA susceptibility and phenotypical presentation. Indeed, smoking is the best-established environmental risk factor for RA, with a likelihood of disease development that is approximately twice as high for smokers than for non-smokers.[22] Furthermore, smoking status is associated with ACPA positive RA and, albeit not consistently, higher levels of disease activity and increased severity in terms of damage accrual.[23] Notably, in our study, the association between smoking status and inadequate response to MTX was independent of other demographic and clinical characteristics, including ACPA and/or RF positivity and baseline disease activity.
Smoking may contribute to the persistence of the systemic pro-inflammatory state by different mechanisms, including enhanced oxidative stress, induction of epigenetic changes, and increased expression of CRP, adhesion molecules (e.g., ICAM ed E-selectine), matrix metalloproteinases, and pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6), all key factors in the pathogenesis of RA.[24] Further potential mechanisms explaining the reduced likelihood of response to MTX in RA smokers are related to the drug's pharmacokinetics. Stamp et al showed that smoker RA patients had lower concentrations of MTX polyglutamates, the active metabolites retained in cells that have been reported to correlate with clinical response.[25] Metsios et al[26] found that RA patients who smoke have higher basal metabolic rates than non-smokers, which might also lead to excessive metabolism of anti-rheumatic drugs.
Notably, in our study as well as others,[10,15] past smokers had similar response to MTX when compared with never smokers, suggesting that smoking discontinuation at treatment initiation may enhance response. Pending the results of adequately designed studies, this hypothesis is of potential clinical relevance and further supports the benefits of smoking quitting strategies in the management of RA patients.
The identification of the other factors that were significantly associated with inadequate response to MTX in our cohort is in line with the results of previous studies. Indeed, a negative correlation, although not always statistically significant, was reported between female gender and higher DAS28 and failure to achieve remission in several cohorts of MTX treated patients.[11] Similarly, younger age was described to negatively correlate with the likelihood of MTX response by different authors.[11] The influence of these factors on MTX response may be at least partially due to the their widely described association with higher disease severity. This, in turn, may explain why the same factors have been frequently identified as potential predictors of inadequate response to other treatments.[27,28] Finally, the negative association between lower ESR and EULAR-response, described also in other studies,[11] may be due to the fact that lower baseline ESR (included in the DAS28 calculation) may impair the sensitivity to detect significant reductions of disease activity from baseline.
The main limitations of our study are its retrospective and monocentric design. Further, the lack of data on possible smoking cessation over the 6 months might have affected our results.
In conclusion, in a cohort of newly diagnosed DMARDs naïve patients, current, but not past smokers, had an increased likelihood of inadequate response to MTX, as assessed by established definitions. Pending additional studies on the effects of smoking quitting strategies on MTX response, smoking status, together with other well-known predictors of response identified in our study, might assist with personalized treatment selection and monitoring in RA patients.
Acknowledgments
The authors would like to thank all the patients participating in this research.
Author contributions
Conceptualization: Alberto Floris, Alessandro Mathieu, Matteo Piga.
Data curation: Daniela Perra, Ignazio Cangemi, Mattia Congia, Elisabetta Chessa.
Formal analysis: Alberto Floris, Matteo Piga.
Investigation: Mattia Congia, Maria Maddalena Angioni.
Methodology: Arduino Aleksander Mangoni, Gian Luca Erre, Alberto Cauli.
Supervision: Alessandro Mathieu, Alberto Cauli.
Validation: Arduino Aleksander Mangoni, Gian Luca Erre.
Visualization: Daniela Perra, Ignazio Cangemi, Mattia Congia, Elisabetta Chessa, Maria Maddalena Angioni, Arduino Aleksander Mangoni, Gian Luca Erre, Alessandro Mathieu, Matteo Piga, Alberto Cauli.
Writing – original draft: Alberto Floris, Daniela Perra, Ignazio Cangemi, Mattia Congia, Elisabetta Chessa, Maria Maddalena Angioni, Arduino Aleksander Mangoni, Gian Luca Erre, Alessandro Mathieu, Matteo Piga, Alberto Cauli.
Writing – review & editing: Alberto Floris, Daniela Perra, Ignazio Cangemi, Mattia Congia, Elisabetta Chessa, Maria Maddalena Angioni, Arduino Aleksander Mangoni, Gian Luca Erre, Alessandro Mathieu, Matteo Piga, Alberto Cauli.
Footnotes
Abbreviations: ACPA = anti-citrullinated peptide antibodies, adjOR = adjusted odds ratio, CI = confidence interval, CRP = C-reactive protein, DAS28 = disease activity score 28 joints, DMARDs = disease modifying anti-rheumatic drugs, ESR = erythrocyte sedimentation rate, EULAR = European League Against Rheumatism, GC = glucocorticoids, IQR = interquartile range, LDA = low disease activity, MTX = methotrexate, OR = odds ratio, RA = rheumatoid arthritis, RF = rheumatoid factor, TJC = tender joint count.
How to cite this article: Floris A, Perra D, Cangemi I, Congia M, Chessa E, Angioni MM, Mangoni AA, Erre GL, Mathieu A, Piga M, Cauli A. Current smoking predicts inadequate response to methotrexate monotherapy in rheumatoid arthritis patients naïve to DMARDs: results from a retrospective cohort study. Medicine. 2021;100:17(e25481).
This study was supported by an unconditioned Research grant from Pfizer Inc, which had no role in the study design, writing of the manuscript, or decision to submit it for publication.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
ACPA = anti-citrullinated peptide antibodies; BMI = body mass index; DAS-28 = disease activity score on 28 joints; ESR = erythrocyte sedimentation rate; IQR = interquartile range; MTX = methotrexate; RF = rheumatoid factor; SJC = swollen joint count; TJC = tender joint count.
Value referred to a total of 291 patients who had available data.
Value referred to a total of 292 patients who had available data.
ACPA = anti-citrullinated peptide antibody; BMI = body mass index; CI = confidence interval; CRP = C-reactive protein; Cum. PDN = cumulative prednisolone or equivalents; DAS28 = disease activity score 28; ESR = erythrocyte sedimentation rate; EULAR = European League Against Rheumatism; LDA = low disease activity; MTX = methotrexate; OR = odds ratio; RF = rheumatoid factor; SJC = swollen joint count; TJC = tender joint count.
adjOR = adjusted odds ratio; ACR = American College of Rheumatology criteria; BMI = body mass index; CDAI = Clinical Disease Activity Index; CI = confidence interval; DAS28 = disease activity score 28 joints; DMARDs = disease modifying anti-rheumatic drugs; ESR = erythrocyte sedimentation rate; EULAR = European League Against Rheumatism; HAQ = Health Assessment Questionnaire; HCQ = hydroxychloroquine; LDA = low disease activity; OR = odds ratio; RF = rheumatoid factor; SDAI = Simple Disease Activity Index; SLZ = sulfasalazine; TJC = tender joint count; TNFì = TNF inhibitors.
Including undifferentiated polyarthritis.
References
- [1].Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. [DOI] [PubMed] [Google Scholar]
- [2].Monti S, Montecucco C, Bugatti S, et al. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open 2015;1:e000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- [4].Salliot C, Heijde D, van der. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 2009;68:1100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Erre GL, Cadoni ML, Meloni P, et al. Methotrexate therapy is not associated with increased liver stiffness and significant liver fibrosis in rheumatoid arthritis patients: a cross-sectional controlled study with real-time two-dimensional shear wave elastography. Eur J Intern Med 2019;69:57–63. [DOI] [PubMed] [Google Scholar]
- [6].Mangoni AA, Zinellu A, Sotgia S, et al. Protective effects of methotrexate against proatherosclerotic cytokines: a review of the evidence. Mediators Inflamm 2017;2017:9632846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choi HK, Hernán MA, Seeger JD, et al. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 2002;359:1173–7. [DOI] [PubMed] [Google Scholar]
- [8].Mangoni AA, Tommasi S, Zinellu A, et al. Methotrexate and vasculoprotection: mechanistic insights and potential therapeutic applications in old age. Curr Pharm Des 2019;25:4175–84. [DOI] [PubMed] [Google Scholar]
- [9].Rojas-Serrano J, Pérez LL, García CG, et al. Current smoking status is associated to a non-ACR 50 response in early rheumatoid arthritis. A cohort study. Clin Rheumatol 2011;30:1589–93. [DOI] [PubMed] [Google Scholar]
- [10].Saevarsdottir S, Wallin H, Seddighzadeh M, et al. Predictors of response to methotrexate in early DMARD naïve rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis 2011;70:469–75. [DOI] [PubMed] [Google Scholar]
- [11].Roodenrijs NMT, Goes MC, van der, et al. Is prediction of clinical response to methotrexate in individual rheumatoid arthritis patients possible? A systematic literature review. Joint Bone Spine 2020;87:13–23. [DOI] [PubMed] [Google Scholar]
- [12].Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [13].Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- [14].Riel PLCM, van Renskers L. The disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol 2016;34:S40–4. [PubMed] [Google Scholar]
- [15].Saevarsdottir S, Wedrén S, Seddighzadeh M, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum 2011;63:26–36. [DOI] [PubMed] [Google Scholar]
- [16].Söderlin MK, Petersson IF, Bergman S, et al. Smoking at onset of rheumatoid arthritis (RA) and its effect on disease activity and functional status: experiences from BARFOT, a long-term observational study on early RA. Scand J Rheumatol 2011;40:249–55. [DOI] [PubMed] [Google Scholar]
- [17].Teitsma XM, Jacobs JWG, Welsing PMJ, et al. Inadequate response to treat-to-target methotrexate therapy in patients with new-onset rheumatoid arthritis: development and validation of clinical predictors. Ann Rheum Dis 2018;77:1261–7. [DOI] [PubMed] [Google Scholar]
- [18].de Rotte MCFJ, Pluijm SMF, Jong PHP de, et al. Development and validation of a prognostic multivariable model to predict insufficient clinical response to methotrexate in rheumatoid arthritis. PLoS One 2018;13:e0208534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sergeant JC, Hyrich KL, Anderson J, et al. Prediction of primary non-response to methotrexate therapy using demographic, clinical and psychosocial variables: results from the UK Rheumatoid Arthritis Medication Study (RAMS). Arthritis Res Ther 2018;20:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maska LB, Sayles HR, O’Dell JR, et al. Serum cotinine as a biomarker of tobacco exposure and the association with treatment response in early rheumatoid arthritis. Arthritis Care Res 2012;64:1804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vesperini V, Lukas C, Fautrel B, et al. Association of tobacco exposure and reduction of radiographic progression in early rheumatoid arthritis: results from a French multicenter cohort. Arthritis Care Res 2013;65:1899–906. [DOI] [PubMed] [Google Scholar]
- [22].Sugiyama D, Nishimura K, Tamaki K, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2010;69:70–81. [DOI] [PubMed] [Google Scholar]
- [23].Costenbader KH, Feskanich D, Mandl LA, et al. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med 2006;119:503.e1–e9. [DOI] [PubMed] [Google Scholar]
- [24].Chang K, Yang SM, Kim SH, et al. Smoking and rheumatoid arthritis. Int J Mol Sci 2014;15:22279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stamp LK, O’Donnell JL, Chapman PT, et al. Determinants of red blood cell methotrexate polyglutamate concentrations in rheumatoid arthritis patients receiving long-term methotrexate treatment. Arthritis Rheum 2009;60:2248–56. [DOI] [PubMed] [Google Scholar]
- [26].Metsios GS, Stavropoulos-Kalinoglou A, Nevill AM, et al. Cigarette smoking significantly increases basal metabolic rate in patients with rheumatoid arthritis. Ann Rheum Dis 2008;67:70–3. [DOI] [PubMed] [Google Scholar]
- [27].Sokka T, Toloza S, Cutolo M, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther 2009;11:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ma MH, Ibrahim F, Walker D, et al. Remission in early rheumatoid arthritis: predicting treatment response. J Rheumatol 2012;39:470–5. [DOI] [PubMed] [Google Scholar]


